Potential Apoptotic Activities of Hylocereus undatus Peel and Pulp Extracts in MCF-7 and Caco-2 Cancer Cell Lines
Abstract
:1. Introduction
2. Results
2.1. The Phenolic and Flavonoid Contents of H. undatus Peel and Pulp Extracts
2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of the Peel and Pulp Extracts of H. undatus
2.3. Liquid Chromatography Mass Spectrometry Analysis of the Extracts
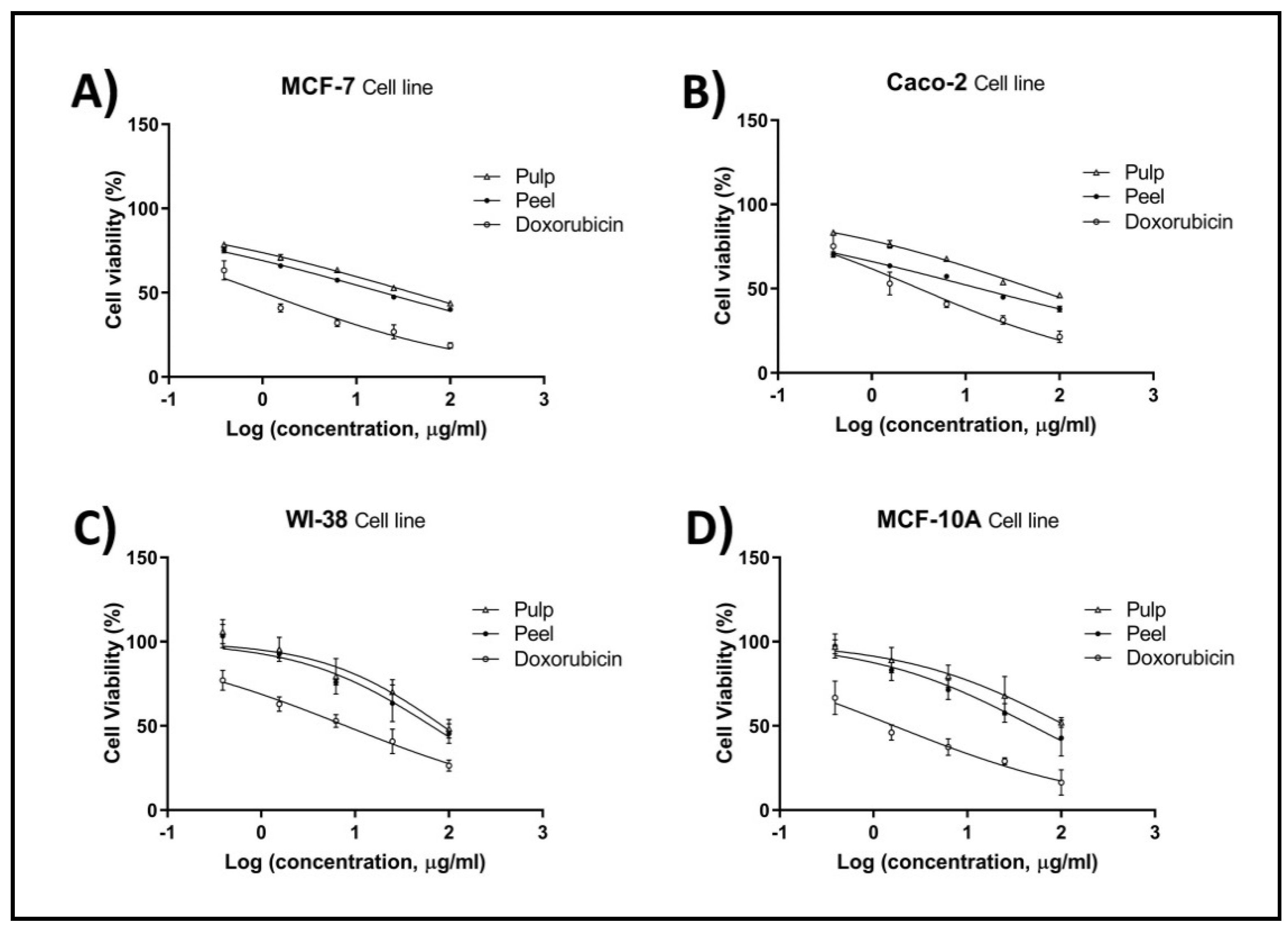
2.4. Cell Proliferation by MTT Assay
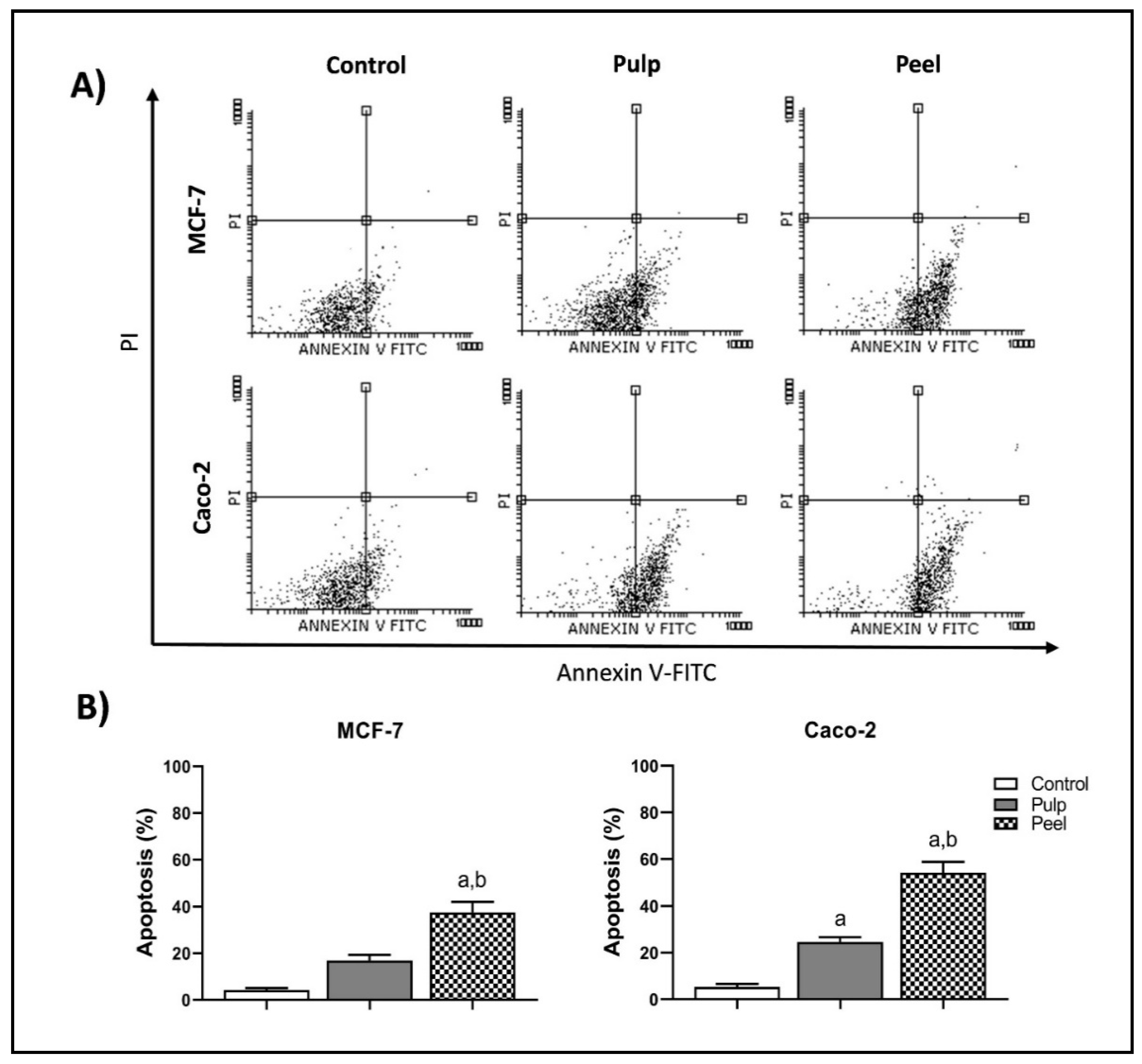
2.5. Annexin-V/PI Double-Staining Assay
2.6. Real-Time PCR Analysis
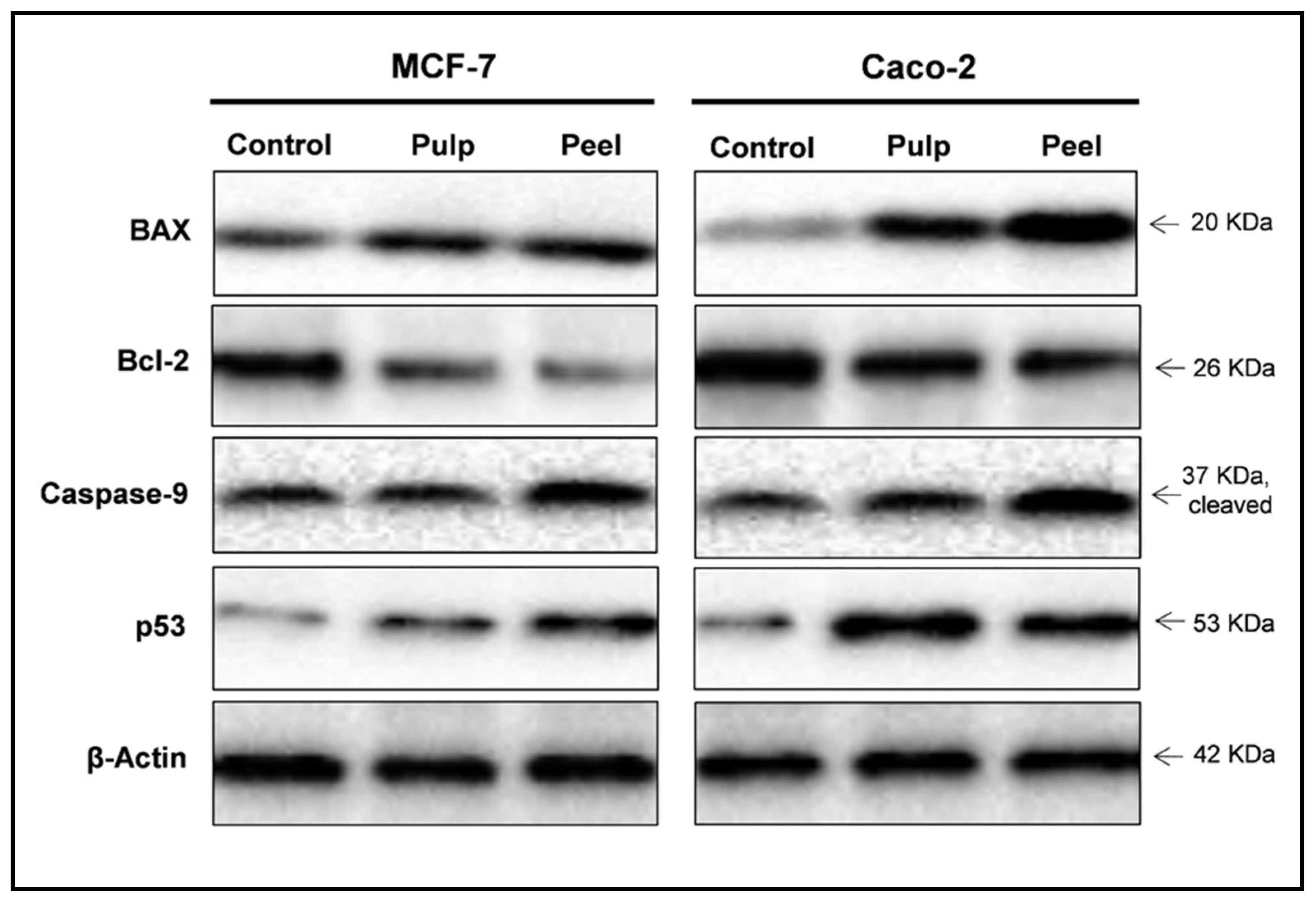
2.7. Western Blot Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of the Plant Samples and Extraction Procedure
4.2. Phytochemical Analysis
4.2.1. Total Phenolic Content (TPC)
4.2.2. Estimation of Total Flavonoid Content
4.2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.2.4. Liquid Chromatography–Mass Spectrometry Analysis of the Extracts
4.3. Cell Lines
4.4. Antiproliferation Assay
4.5. Annexin-V/Propidium Iodide Flow Cytometric Analysis
4.6. Quantitative Real-Time PCR
- Bax F: 5′-CCCGAGAGGTCTTTTTCCGAG-3′Bax R: 5′-CCAGCCCATGATGGTTCTGAT-3′
- Bcl-2 F 5′-CCTGTG GAT GAC TGA GTA CC-3′Bcl-2 R 5′-GAGACA GCC AGG AGA AAT CA-3′
- Caspase-9 F: 5′-CTGAGCCAGATGCTGTCCCAT-3’Caspase-9 R: 5′-CCAAGGTCTCGATGTACCAGGAA-3′
- p53 F 5′-CCCCTCCTGGCCCCTGTCATCTTC-3′p53 R 5′-GCAGCGCCTCACAACCTCCGTCAT-3′
- GAPDH F 5′-GCA AGT TCA ACG GCA CGA TCA AG-3′GAPDH R 5′-CTA CTC AGC ACC AGC ATC ACC-3′
4.7. Western Blot Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arafa, M.A.; Farhat, K. Colorectal cancer in the Arab world-screening practices and future prospects. Asian Pacific J. Cancer Prev. 2015, 16, 7425–7430. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.J.; Al-Shamsi, F.A.; Al-Marzooqi, N.A.; Al-Qasemi, S.S.; Mokdad, A.H.; Khan, G. Burden of breast cancer in the Arab world: Findings from global burden of disease, 2016. J. Epidemiol. Glob. Health 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Czajka, M.L.; Pfeifer, C. Breast cancer surgery. In StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Sukesh, M.; Geraci, S.A.; Kanishka, C. Overview of Cancer Survivorship Care for Primary Care Providers. Cureus 2020, 12, e10210. [Google Scholar]
- Mittra, I.; Pal, K.; Pancholi, N.; Shaikh, A.; Rane, B.; Tidke, P.; Kirolikar, S.; Khare, N.K.; Agrawal, K.; Nagare, H. Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin. Ann. Oncol. 2017, 28, 2119–2127. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive effect of curcumin against chemotherapy-induced side-effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Eissa, N.; Althobaiti, F.; Fayad, E.; Abu Almaaty, A.H. Nomad Jellyfish Rhopilema nomadica Venom Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Hepatocellular Carcinoma HepG2 Cells. Molecules 2021, 26, 5185. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural products in cancer therapy: Past, present and future. Nat. Products Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Lowe, H.; Steele, B.; Bryant, J.; Fouad, E.; Toyang, N.; Ngwa, W. Antiviral activity of Jamaican medicinal plants and isolated bioactive compounds. Molecules 2021, 26, 607. [Google Scholar] [CrossRef] [PubMed]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Avilez, D.; Zavala-Hurtado, J.A.; Rendón-Aguilar, B. Damage in Cactaceae, their geographic distribution and new evidences. Bot. Sci. 2019, 97, 551–567. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat affects the chemical profile, allelopathy, and antioxidant properties of essential oils and phenolic enriched extracts of the invasive plant Heliotropium curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef]
- Goettsch, B.; Hilton-Taylor, C.; Cruz-Piñón, G.; Duffy, J.P.; Frances, A.; Hernández, H.M.; Inger, R.; Pollock, C.; Schipper, J.; Superina, M. High proportion of cactus species threatened with extinction. Nat. plants 2015, 1, 15142. [Google Scholar] [CrossRef]
- Bouhrim, M.; Daoudi, N.E.; Ouassou, H.; Benoutman, A.; Loukili, E.H.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Aziz, M.; Bnouham, M. Phenolic content and antioxidant, antihyperlipidemic, and antidiabetogenic effects of Opuntia dillenii seed oil. Sci. World J. 2020, 2020, 5717052. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Luksirikul, P.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Dragon fruits as a reservoir of natural polyphenolics with chemopreventive properties. Molecules 2021, 26, 2158. [Google Scholar] [CrossRef]
- Abou-Elella, F.M.; Ali, R.F.M. Antioxidant and anticancer activities of different constituents extracted from Egyptian prickly pear Cactus (Opuntia Ficus-Indica) Peel. Biochem Anal Biochem 2014, 3, 1009–2161. [Google Scholar] [CrossRef]
- R’bia, O.; Chkioua, C.; Hellal, R.; Herchi, W.; Smiti, S.A. Antioxidant and antibacterial activities of Opuntia ficus indica seed oil fractions and their bioactive compounds identification. Turkish J. Biochem. 2017, 42, 481–491. [Google Scholar] [CrossRef]
- Alsaad, A.J.A.; Altemimi, A.B.; Aziz, S.N.; Lakhssassi, N. Extraction and identification of cactus Opuntia dillenii seed oil and its added value for human health benefits. Pharmacogn. J. 2019, 11, 579–587. [Google Scholar] [CrossRef]
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Yang, Y.; Lin, X.; Wang, L.; Li, C.; Yang, R. Phenolic compounds profile and antioxidant capacity of pitahaya fruit peel from two red-skinned species (Hylocereus polyrhizus and Hylocereus undatus). Foods 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Mizrahi, Y. Fruit flesh betacyanin pigments in Hylocereus cacti. J. Agric. Food Chem. 2002, 50, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fra. Molecules 2021, 26, 577. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Almahmoud, S.A.; Arfeen, M.; Srivastava, A.; El-Readi, M.Z.; Ragab, E.A.; Shehata, S.M.; Mohammed, S.A.A.; Mostafa, E.M.; El-khawaga, H.A.; et al. Phytochemical profiling, molecular docking, and in vitro anti-hepatocellular carcinoid bioactivity of Suaeda vermiculata extracts. Arab. J. Chem. 2022, 15, 103950. [Google Scholar] [CrossRef]
- Bender, O.; Atalay, A. Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 311–321. [Google Scholar]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Oskoueian, A.; Jaafar, H.Z.E. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. Molecules 2012, 17, 1203–1218. [Google Scholar] [CrossRef]
- Mohammed, H.A. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef]
- Aziz, S.; Mitu, T.K. Analysis of fatty acid and determination of total protein and phytochemical content of Cassia sophera Linn leaf, stem, flower, and seed. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Tawfik, M.M.; Galal, B.; Nafie, M.S.; El Bous, M.M.; El-Bana, M.I. Cytotoxic, apoptotic activities and chemical profiling of dimorphic forms of Egyptian halophyte Cakile maritima scop. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Taoufik, F.; Zine, S.; El Hadek, M.; Idrissi Hassani, L.M.; Gharby, S.; Harhar, H.; Matthäus, B. Oil content and main constituents of cactus seed oils Opuntia Ficus Indica of different origin in Morocco. Med. J. Nutrition Metab. 2015, 8, 85–92. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly Pear Seed Oil Extraction, Chemical Characterization and Potential Health Benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitroevaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits–A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40, 100888. [Google Scholar] [CrossRef]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e20190347. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G. Chlorogenic acid decreases malignant characteristics of hepatocellular carcinoma cells by inhibiting DNMT1 expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, B.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Identification of phenolic compounds in Australian grown dragon fruits by LC-ESI-QTOF-MS/MS and determination of their antioxidant potential. Arab. J. Chem. 2021, 14, 103151. [Google Scholar] [CrossRef]
- Areche, C.; Hernandez, M.; Cano, T.; Ticona, J.; Cortes, C.; Simirgiotis, M.; Caceres, F.; Borquez, J.; Echeverría, J.; Sepulveda, B. Corryocactus brevistylus (K. Schum. ex Vaupel) Britton & Rose (Cactaceae): Antioxidant, gastroprotective effects, and metabolomic profiling by ultrahigh-pressure liquid chromatography and electrospray high resolution orbitrap tandem mass spectrometry. Front. Pharmacol. 2020, 11, 417. [Google Scholar]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.O.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Yoo, Y.-C.; Shin, B.-H.; Hong, J.-H.; Lee, J.; Chee, H.-Y.; Song, K.-S.; Lee, K.-B. Isolation of fatty acids with anticancer activity fromProtaetia brevitarsis Larva. Arch. Pharm. Res. 2007, 30, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, P.; Muralitharan, G.; Balaji, N.P. Prediction aided in vitro analysis of octa-decanoic acid from Cyanobacterium Lyngbya sp. as a proapoptotic factor in eliciting anti-inflammatory properties. Bioinformation 2017, 13, 301. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.; Moon, J.Y.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Comparative antioxidant and antiproliferative activities of red and white pitayas and their correlation with flavonoid and polyphenol content. J. Food Sci. 2011, 76, C38–C45. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers (Basel). 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Kumar, P.R.; Sakthi Priyadarsini, S.; Meenaxshi, C. In vitro antioxidant and anticancer activity of flavonoid fraction from the aerial parts of Cissus quadrangularis Linn. against human breast carcinoma cell lines. J. Chem. 2013, 2013, 150675. [Google Scholar] [CrossRef]
- Liaudanskas, M.; Žvikas, V.; Petrikaitė, V. The Potential of Dietary Antioxidants from a Series of Plant Extracts as Anticancer Agents against Melanoma, Glioblastoma, and Breast Cancer. Antioxidants 2021, 10, 1115. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Sangpairoj, K.; Settacomkul, R.; Siangcham, T.; Meemon, K.; Niamnont, N.; Sornkaew, N.; Tamtin, M.; Sobhon, P.; Vivithanaporn, P. Hexadecanoic acid-enriched extract of Halymenia durvillei induces apoptotic and autophagic death of human triple-negative breast cancer cells by upregulating ER stress. Asian Pac. J. Trop. Biomed. 2022, 12, 132. [Google Scholar]
- Maungchanburi, S.; Rattanaburee, T.; Sukpondma, Y.; Tedasen, A.; Tipmanee, V.; Graidist, P. Anticancer activity of Piper cubeba L. extract on triple negative breast cancer MDA-MB-231. J. Pharm. Pharmacogn. Res. 2022, 10, 39–51. [Google Scholar] [CrossRef]
- de Guimarães, D.A.B.; de Castro, D.D.S.B.; de Oliveira, F.L.; Nogueira, E.M.; de Silva, M.A.M.; Teodoro, A.J. Pitaya extracts induce growth inhibition and proapoptotic effects on human cell lines of breast cancer via downregulation of estrogen receptor gene expression. Oxid. Med. Cell. Longev. 2017, 2017, 7865073. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.H.; Vigneswari, S.; Ahmad, A.; Mohamad, H.; Muhammad, T.S. Cytotoxic effects and evidence of apoptosis from Avecennia alba extracts on human breast cancer cell line (MCF-7). J. Sustain. Sci. Manag. 2017, 12, 80–88. [Google Scholar]
- Tan, M.L.; Sulaiman, S.F.; Najimuddin, N.; Samian, M.R.; Muhammad, T.S.T. Methanolic extract of Pereskia bleo (Kunth) DC.(Cactaceae) induces apoptosis in breast carcinoma, T47-D cell line. J. Ethnopharmacol. 2005, 96, 287–294. [Google Scholar] [CrossRef]
- Selvaraj, J. Fatty acids and their analogues as anticancer agents. Fat. Acids 2017, 21, 72–86. [Google Scholar]
- Dlugosz, P.J.; Billen, L.P.; Annis, M.G.; Zhu, W.; Zhang, Z.; Lin, J.; Leber, B.; Andrews, D.W. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006, 25, 2287–2296. [Google Scholar] [CrossRef]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta 2009, 1787, 414–420. [Google Scholar] [CrossRef]
- Aziz, F.; Noor, M.M. Ethanol extract of dragon fruit and its effects on sperm quality and histology of the testes in mice. Biomed Res 2010, 21. [Google Scholar]
- Mohammed, H.A.; Al-Omar, M.S.; Khan, R.A.; Mohammed, S.A.A.; Qureshi, K.A.; Abbas, M.M.; Al Rugaie, O.; Abd-Elmoniem, E.; Ahmad, A.M.; Kandil, Y.I. Chemical Profile, Antioxidant, Antimicrobial, and Anticancer Activities of the Water-Ethanol Extract of Pulicaria undulata Growing in the Oasis of Central Saudi Arabian Desert. Plants 2021, 10, 1811. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Mohammed, H.A.; Elsisi, H.A.; Sajid, S.; Almogbel, Y.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharm. J. 2022, 30, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Abdelaal, M.R.; Soror, S.H.; Elnagar, M.R.; Haffez, H. Revealing the Potential Application of EC-Synthetic Retinoid Analogues in Anticancer Therapy. Molecules 2021, 26, 506. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef] [PubMed]
- Burnette, W.N. “Western blotting”: Electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 1981, 112, 195–203. [Google Scholar] [CrossRef]

| TPC (mg/g GAE) | TFC (mg/g QE) | |
|---|---|---|
| Peel extract | 22.8 | 3.5 |
| Pulp extract | 3.0 | 0.11 |
| Peak No. | Retention Time (Minutes) | Compound Name | Peak Area (%) | |
|---|---|---|---|---|
| Peel | Pulp | |||
| 4.02 | 1-propanol, 2-methyl- | 1.05 | NA | |
| 4.19 | Ribitol | 2.26 | NA | |
| 4.44 | 2-methyl malonic acid | 3.34 | NA | |
| 4.54 | 1,3-Propanediol, TBDMS derivative | 8.90 | NA | |
| 4.57 | Propanoic acid, 2-methylpropyl ester | NA | 3.93 | |
| 4.66 | l-Felinine | NA | 4.08 | |
| 5.01 | Pentanoic acid, 4-methyl- | 11.19 | NA | |
| 5.07 | Ethanol, 2-butoxy- | NA | 18.14 | |
| 5.49 | 2,5-Methylene-d,l-rhamnitol | 2.30 | 0.82 | |
| 5.59 | Benzene, 1-ethyl-4-methyl- | 9.98 | NA | |
| 5.66 | Propanoic acid, anhydride | NA | 25.63 | |
| 5.7 | Benzene, 1,3,5-trimethyl- | 3.23 | 4.08 | |
| 5.85 | 1,1-Cyclobutane dicarboxamide, 2-phenyl-N,N′-bis(1-phenylethyl) | 1.89 | NA | |
| 5.94 | 2,2-Dimethyl-3-phenylpropanoic acid | NA | 1.57 | |
| 6.1 | 1,3,5-trimethylbenzene | 9.32 | 9.11 | |
| 6.57 | Butanoic acid, 2-amino-4-(methylsulfinyl)-, (ñ) | 3.27 | NA | |
| 6.62 | Benzene, 1,3,5-trimethyl- | NA | 1.76 | |
| 6.69 | Citronellal | NA | 1.80 | |
| 6.97 | Acetic acid, Octyl ester | 1.33 | NA | |
| 7.03 | Tetradecane, 1-chloro- | NA | 1.17 | |
| 7.18 | 10,12-Octadecadiynoic acid | NA | 2.35 | |
| 7.76 | Acetic acid, Octyl ester | 1.67 | NA | |
| 7.8 | 1-tetradecanol | NA | 1.63 | |
| 8.25 | Nonanoic acid | NA | 0.66 | |
| 9.09 | 9-octadecenoic acid (z)- | 3.61 | 3.61 | |
| 9.11 | Nonanoic acid | NA | 2.81 | |
| 9.81 | 2-Myristynoyl pantetheine | NA | 0.71 | |
| 15.13 | i-Propyl 9,12,15-octadecatrienoate | 1.27 | 1.62 | |
| 19.68 | Retinal | 3.32 | NA | |
| 20.80 | Alanine, 3-(benzyloxy)-, l- | 2.13 | NA | |
| 23.75 | Oleic acid | NA | 2.92 | |
| 24.2 | 9-octadecenoic acid (z)- | 4.93 | 1.91 | |
| 26.95 | 9-octadecenoic acid (z)- | 13.22 | 8.85 | |
| 27.34 | Oleic acid | 1.90 | 1.10 | |
| 30.05 | 9-Octadecenoic acid (Z)-, 2-hydroxy-1-(hydroxymethyl) ethyl ester | 4.69 | 3.34 | |
| 33.27 | 4H-1-benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-6,8di-á-d-glucopyranosyl-5,7dihydroxy | 1.73 | NA | |
| Compounds | RT | m/z Parent Ion [M-H]- | m/z Daughter Ions | Peel Extract µg/g | Pulp Extract µg/g |
|---|---|---|---|---|---|
| 3.4-Dihydroxybenzoic acid | 5.72 | 153 | 109, 91 | 1.30 | 0.55 |
| Chlorogenic acid | 7.32 | 355 | 163, 145, 135, 117, 89 | 491.03 | 91.80 |
| Caffeic acid | 8.02 | 179 | 161, 135, 105 | 108.94 | 31.78 |
| Coumaric acid | 9.48 | 163 | 119, 93, 71 | 14.31 | 3.63 |
| Vanillin | 9.48 | 151 | 136, 123, 107 | 10.66 | 3.71 |
| Rutin | 9.69 | 609 | 301, 300, 271, 255, 179, 151 | 72.45 | 5.24 |
| Ferulic acid | 10.18 | 192.8 | 178, 149, 134, 117, 89 | 59.86 | 17.26 |
| Quercetin | 13.51 | 301 | 273, 245, 179, 151, 121, 107 | 11.09 | ND |
| Kaempferol | 15.29 | 284.7 | 257, 255, 239, 227, 211, 185, 117, 93 | 0.78 | 0.05 |
| * IC50 (µg/mL) | Corresponding SI | |||||||
|---|---|---|---|---|---|---|---|---|
| MCF-7 | Caco2 | WI-38 | MCF-10A | WI-38/MCF-7 | WI-38/Caco-2 | MCF-10A/MCF-7 | MCF-10A/Caco-2 | |
| Pulp | 39.84 | 52.79 | 86.01 | 113.80 | 2.15 | 1.62 | 2.86 | 2.15 |
| Peel | 19.47 | 14.20 | 65.16 | 49.17 | 3.34 | 4.58 | 2.52 | 3.46 |
| Doxorubicin | 1.03 | 3.20 | 7.97 | 1.66 | 7.73 | 2.49 | 1.61 | 0.52 |
| Viable a | Apoptosis a | Necrosis a | ||
|---|---|---|---|---|
| Early | Late | |||
| MCF-7 | 95.67 ± 0.81 | 4.15 ± 0.42 | 0.13 ± 0.02 | 0.11 ± 0.03 |
| Pulp | 82.85 ± 0.90 | 16.50 ± 2.32 | 0.42 ± 0.07 | 0.20 ± 0.02 |
| Peel | 62.25 ± 4.53 | 37.17 ± 3.49 a,b | 0.38 ± 0.09 | 0.17 ± 0.01 |
| Caco-2 | 94.60 ± 1.31 | 5.15 ± 1.33 | 0.13 ± 0.01 | 0.11 ± 0.02 |
| Pulp | 75.18 ± 2.05 | 24.20 ± 2.01 a | 0.40 ± 0.08 | 0.18 ± 0.01 |
| Peel | 45.61 ± 4.66 | 53.87 ± 4.68 a,b | 0.30 ± 0.07 | 0.18 ± 0.02 |
| Protein Expression (Normalized to β-Actin) a | |||||
|---|---|---|---|---|---|
| BAX | Bcl-2 | BAX/Bcl-2 Ratio | Caspases-9 | p53 | |
| MCF-7 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Pulp extract | 1.49 | 0.66 | 2.22 | 1.25 | 1.44 |
| Peel extract | 2.64 | 0.39 | 6.14 | 2.22 | 2.63 |
| Caco-2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Pulp extract | 2.20 | 0.70 | 3.14 | 1.26 | 2.25 |
| Peel extract | 3.70 | 0.62 | 5.96 | 2.30 | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, H.S.; Tawfik, M.M.; Elnagar, M.R.; Mohammed, H.A.; Zarka, M.A.; Awad, N.S. Potential Apoptotic Activities of Hylocereus undatus Peel and Pulp Extracts in MCF-7 and Caco-2 Cancer Cell Lines. Plants 2022, 11, 2192. https://doi.org/10.3390/plants11172192
Salam HS, Tawfik MM, Elnagar MR, Mohammed HA, Zarka MA, Awad NS. Potential Apoptotic Activities of Hylocereus undatus Peel and Pulp Extracts in MCF-7 and Caco-2 Cancer Cell Lines. Plants. 2022; 11(17):2192. https://doi.org/10.3390/plants11172192
Chicago/Turabian StyleSalam, Hanin S., Mohamed M. Tawfik, Mohamed R. Elnagar, Hamdoon A. Mohammed, Mohamed A. Zarka, and Nabil S. Awad. 2022. "Potential Apoptotic Activities of Hylocereus undatus Peel and Pulp Extracts in MCF-7 and Caco-2 Cancer Cell Lines" Plants 11, no. 17: 2192. https://doi.org/10.3390/plants11172192
APA StyleSalam, H. S., Tawfik, M. M., Elnagar, M. R., Mohammed, H. A., Zarka, M. A., & Awad, N. S. (2022). Potential Apoptotic Activities of Hylocereus undatus Peel and Pulp Extracts in MCF-7 and Caco-2 Cancer Cell Lines. Plants, 11(17), 2192. https://doi.org/10.3390/plants11172192









