Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor
Abstract
:1. Introduction
2. Methods
2.1. Stock Cultivation
2.2. Experimental Design
2.3. Data Collection
2.4. Data Analysis
3. Results
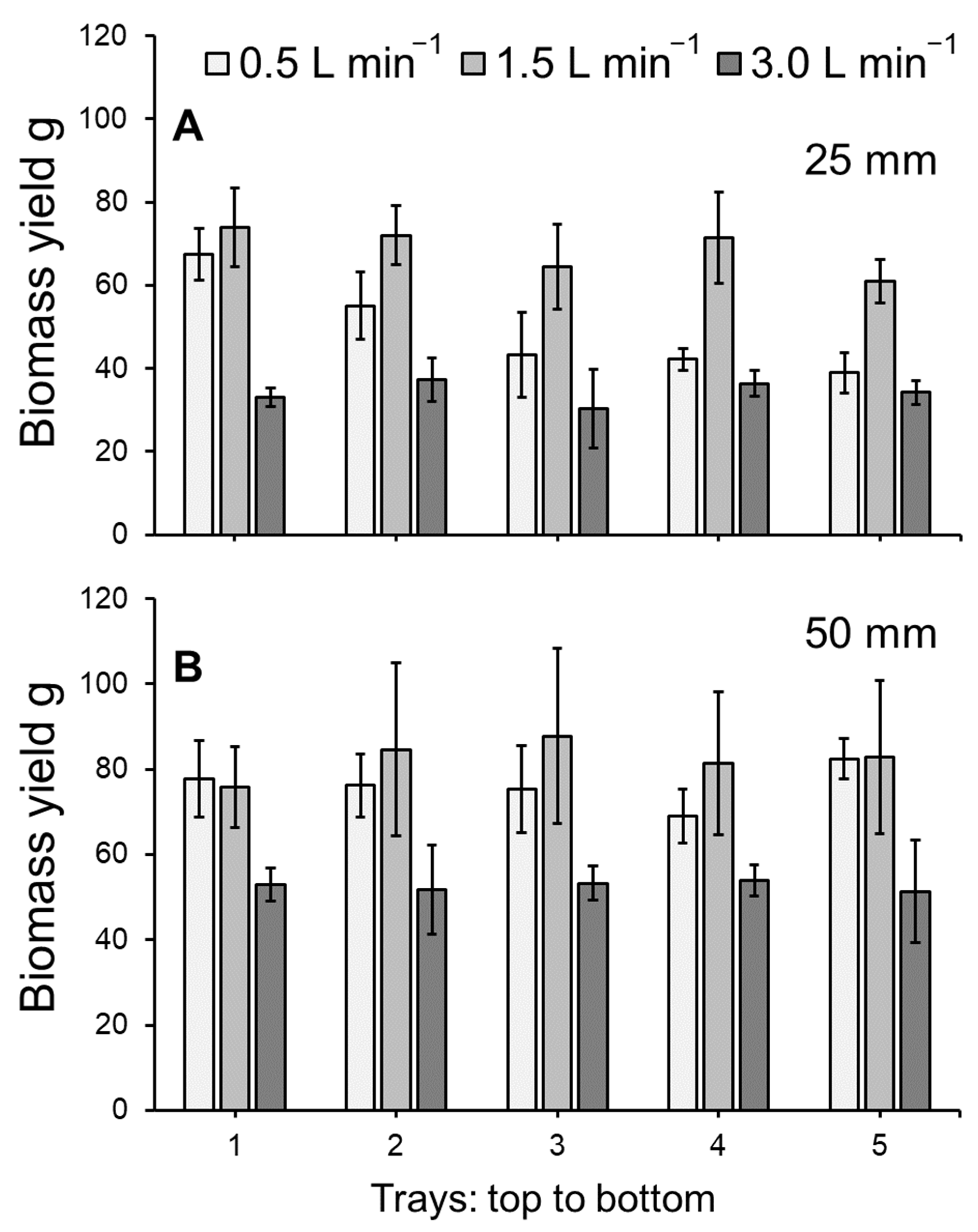
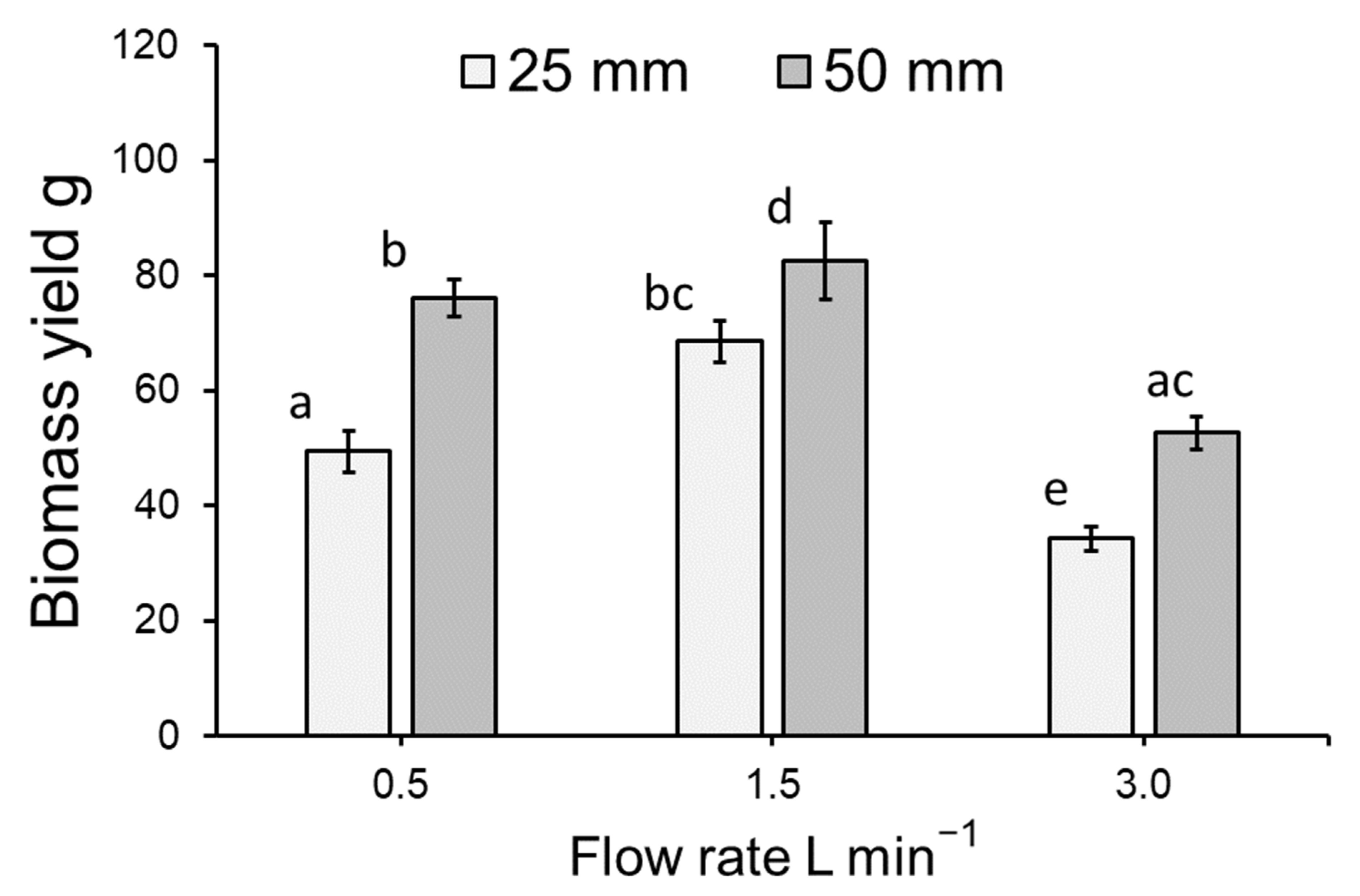
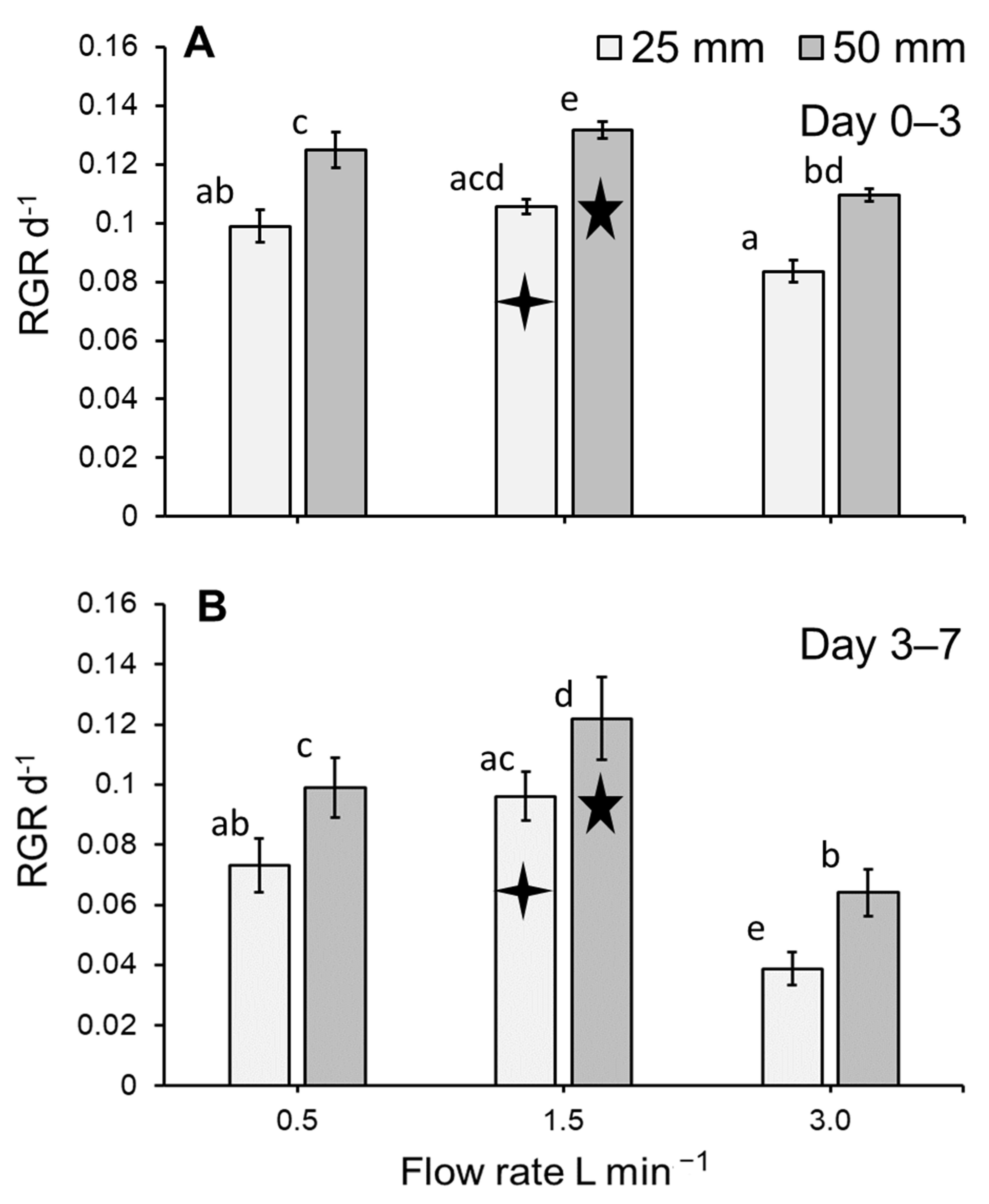
3.1. Duckweed Growth
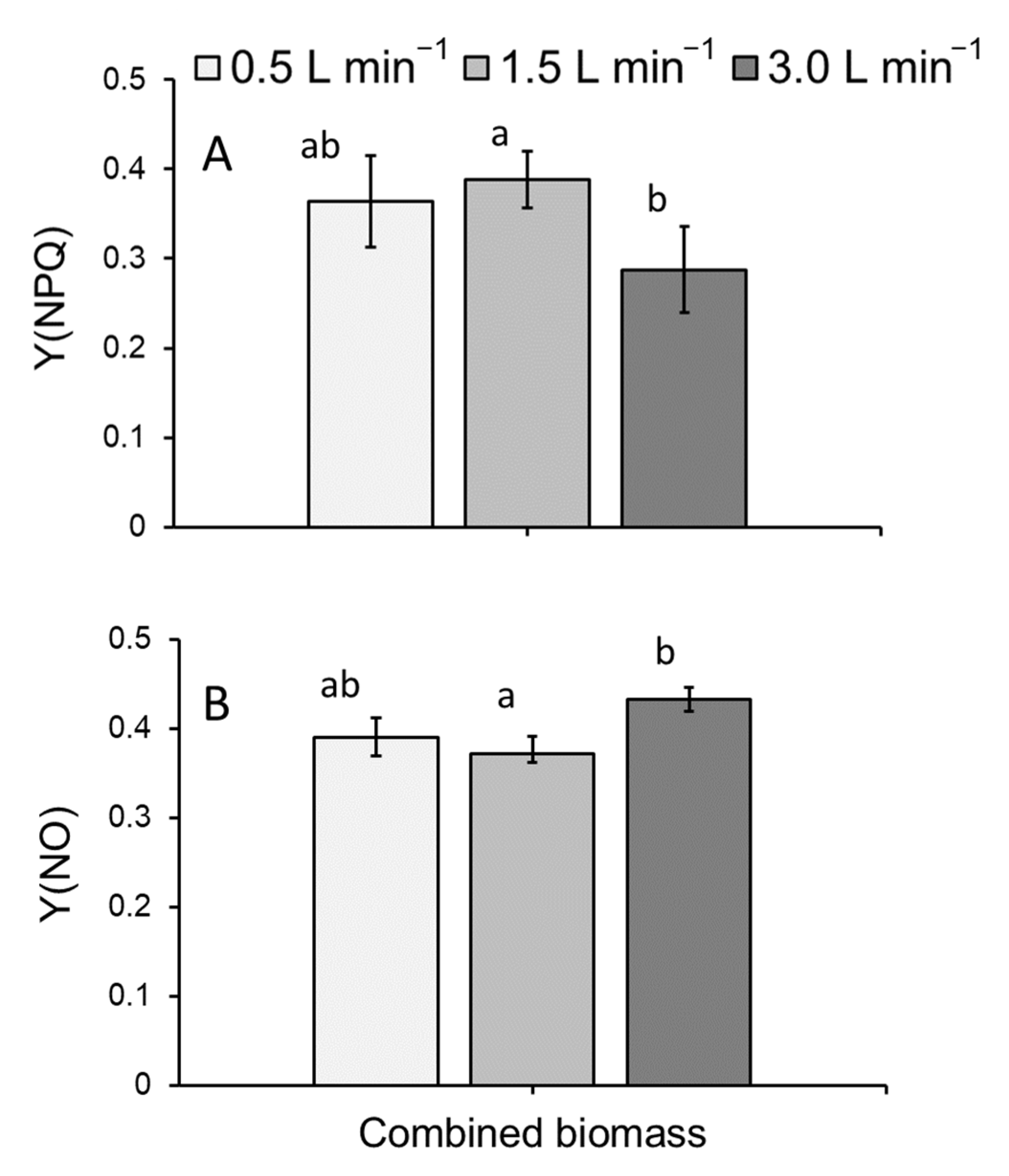
3.2. Chlorophyll a Fluorometry
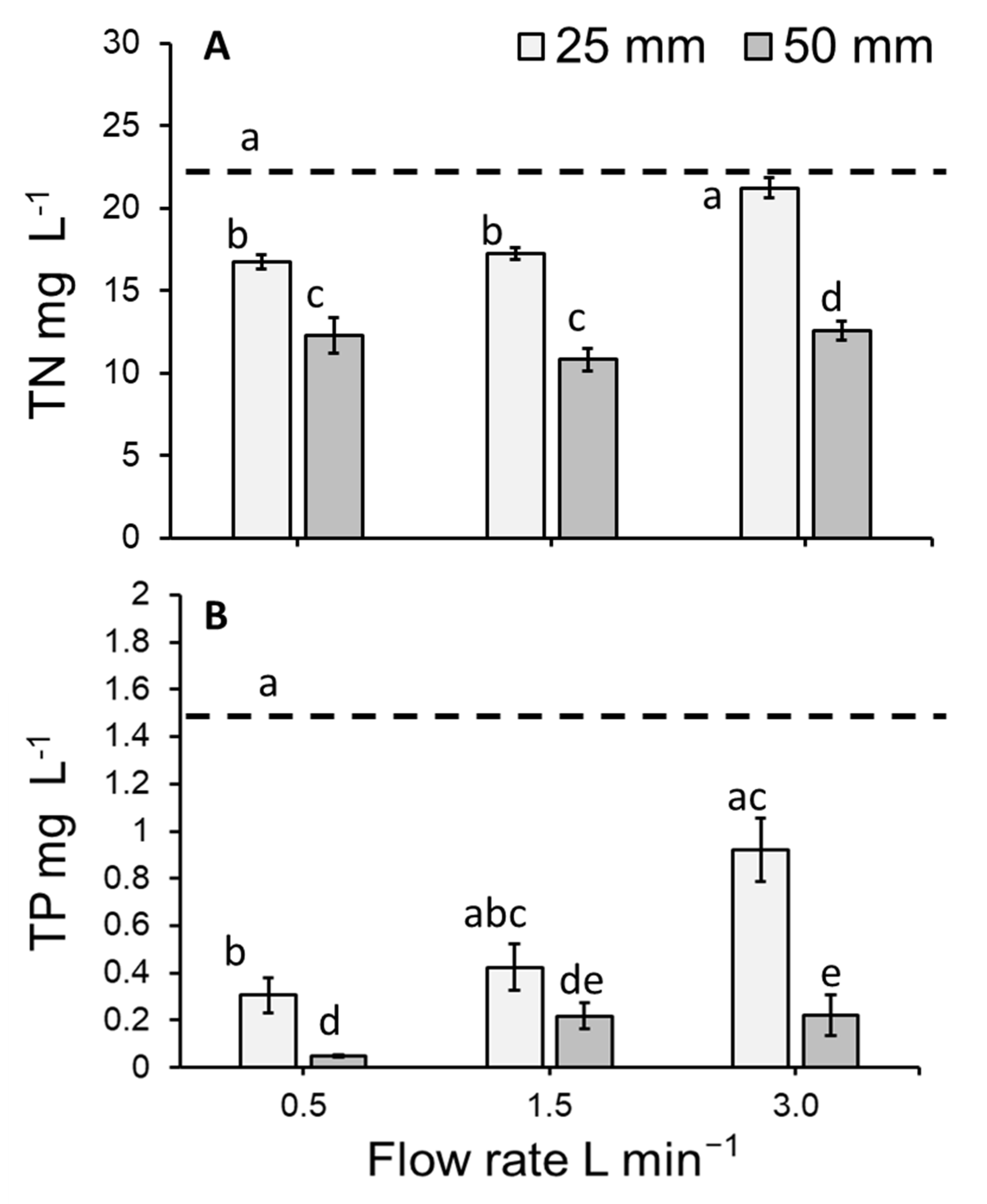
3.3. Total Nitrogen Concentration of the Medium
3.4. Total Phosphorous Concentration of the Medium
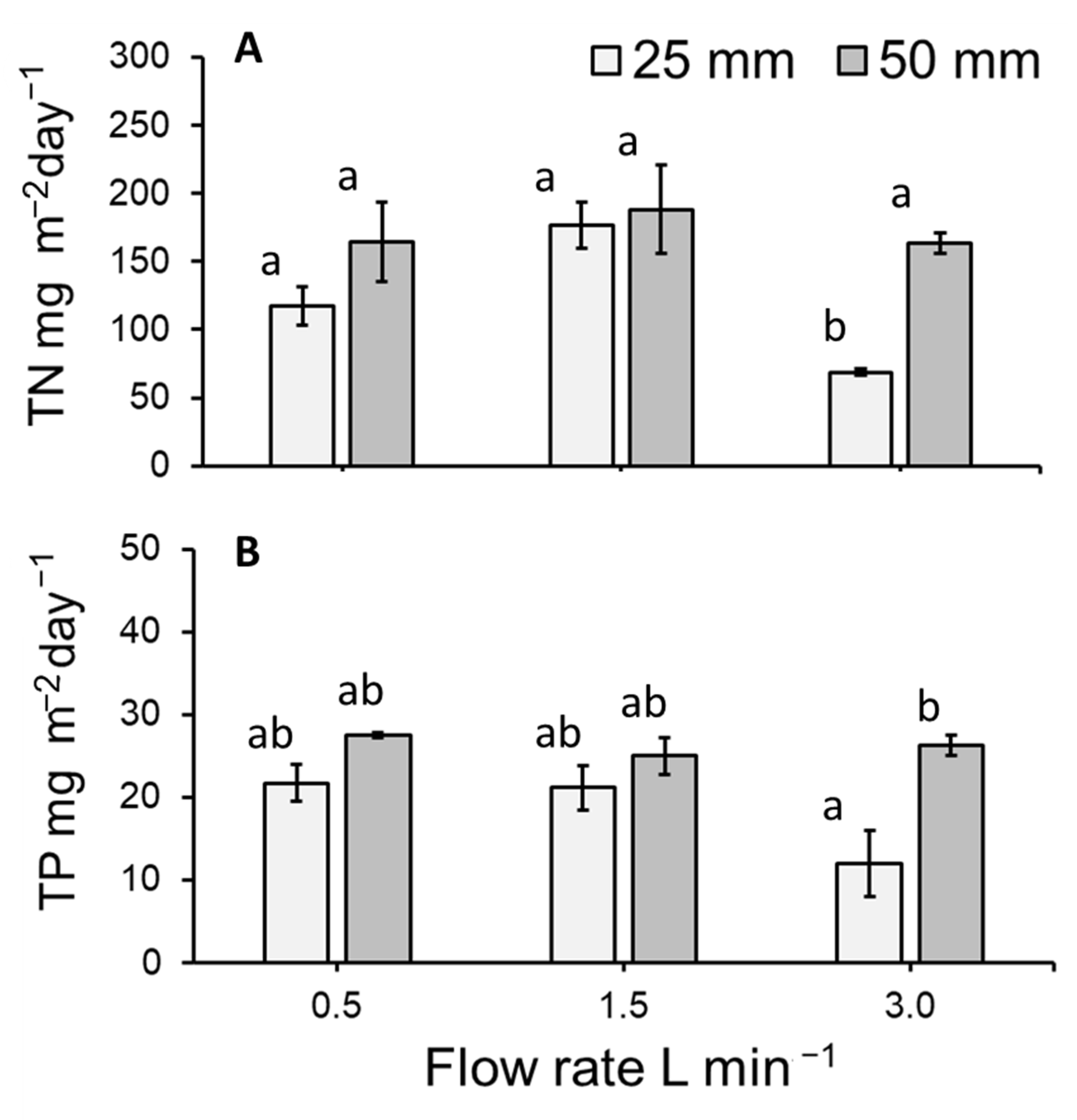
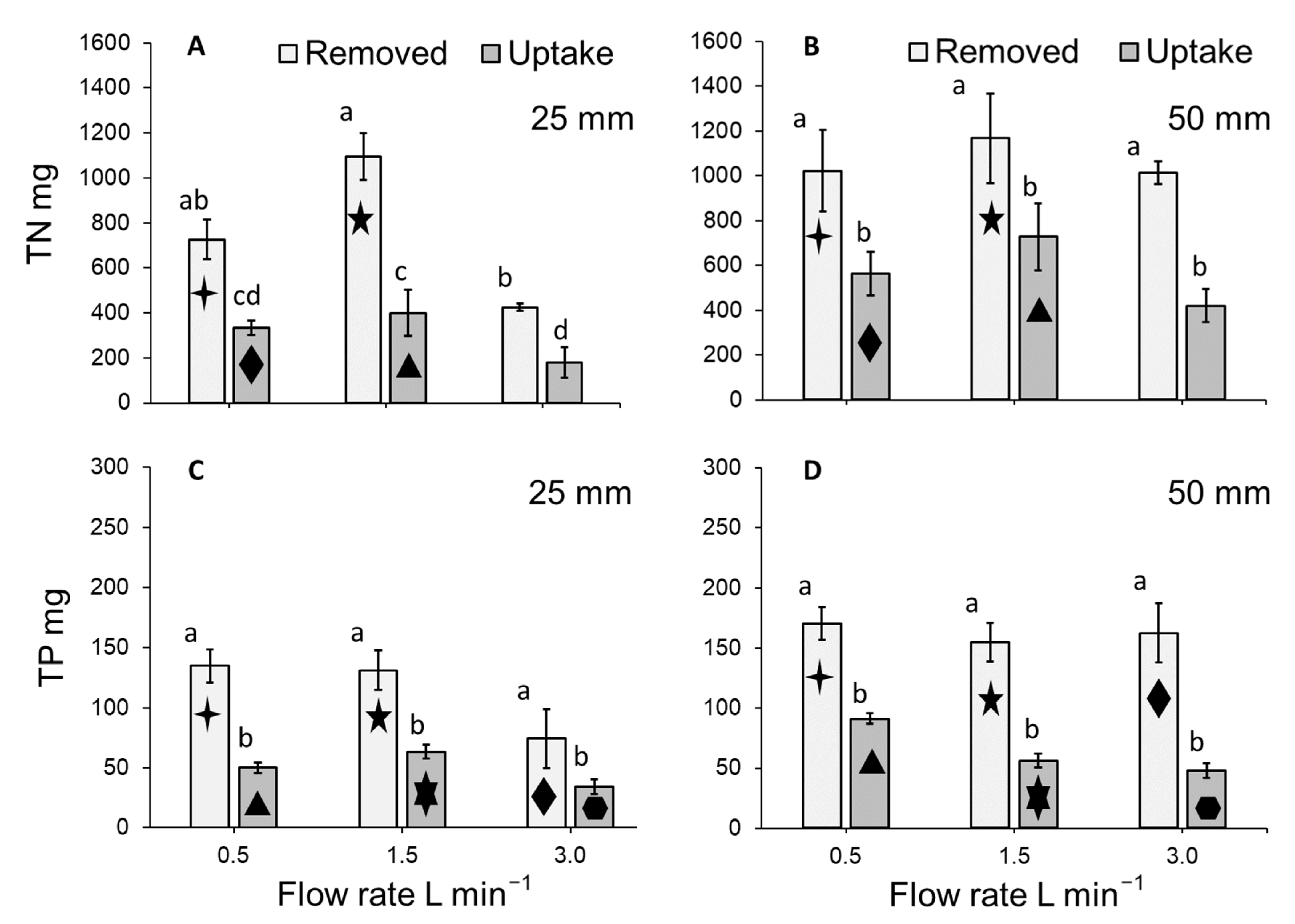
3.5. Total Nitrogen and Total Phosphorous Removal Per m2 of Lemna Minor
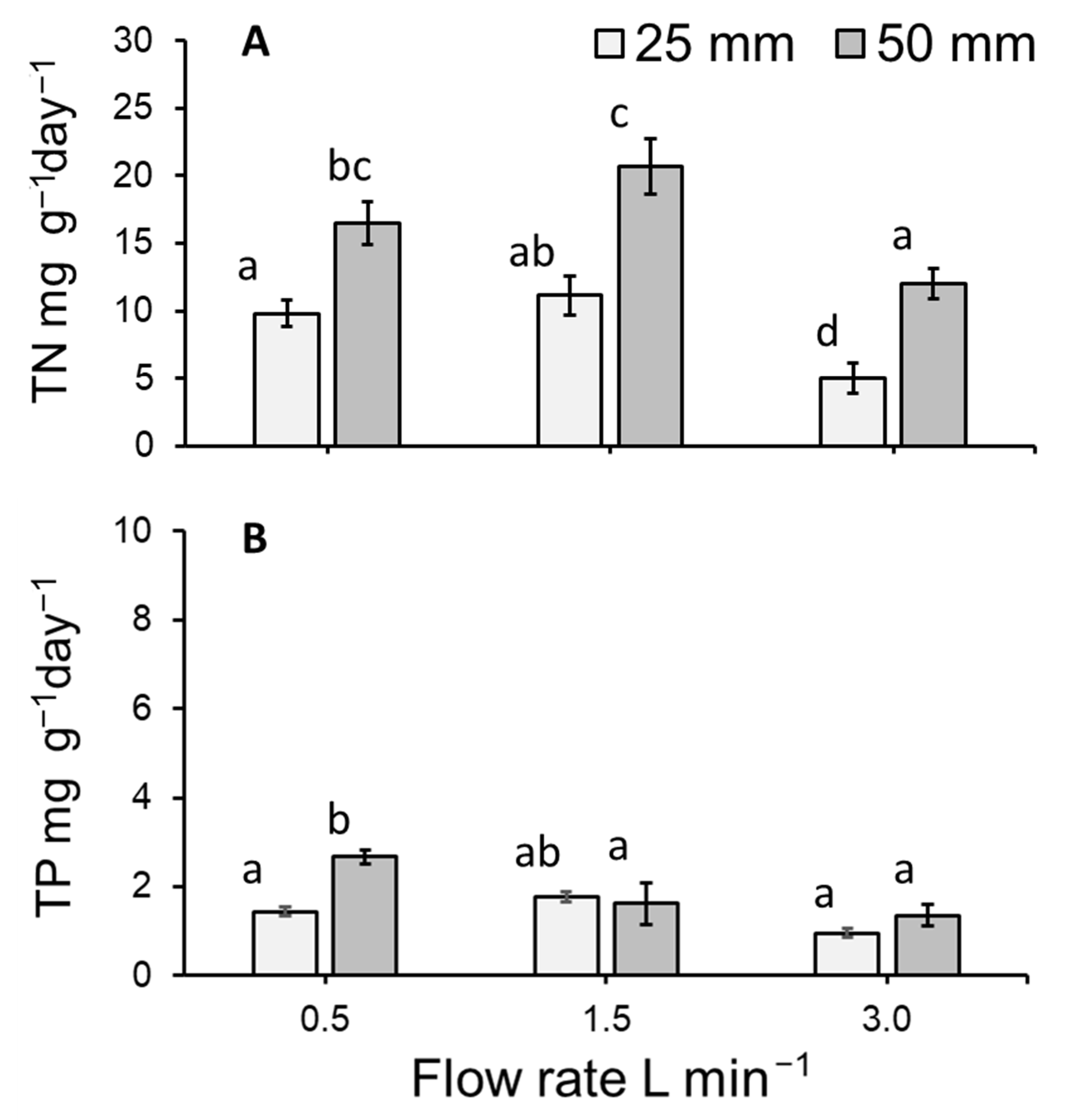
3.6. Total Nitrogen and Total Phosphorous Uptake by Lemna Minor
3.7. Fate of Total Nitrogen and Total Phosphorous within the System
4. Discussion
4.1. Duckweed Growth and Photosynthetic Health
4.2. Medium Concentration and Duckweed Removal (mg m−2 day−1) of Total Nitrogen and Total Phosphorous
4.3. Duckweed Uptake (mg g−1 day−1) of Total Nitrogen and Total Phosphorous
4.4. Fate of Total Nitrogen and Total Phosphorous within the System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050, Summary version; FAO: Rome, Italy, 2018; p. 60. [Google Scholar]
- Hamann, S. The global food system, agro-industrialization and governance: Alternative conceptions for sub-Saharan Africa. Globalizations 2020, 8, 1405–1420. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yu, C.; Yu, L.; Zhao, J.; Sun, C.; Ma, Y.; Zhou, G. The influence of light intensity and photoperiod on duckweed biomass and starch accumulation for bioethanol production. Bioresour. Technol. 2015, 187, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Rufí-Salís, M.; Petit-Boix, A.; Villalba, G.; Sanjuan-Delmás, D.; Parada, F.; Ercilla-Montserrat, M.; Arcas-Pilz, V.; Muñoz-Liesa, J.; Rieradevall, J.; Gabarrell, X. Recirculating water and nutrients in urban agriculture: An opportunity towards environmental sustainability and water use efficiency? J. Clean. Prod. 2020, 261, 121213. [Google Scholar] [CrossRef]
- UN. The Sustainable Development Goals Report 2021; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Morseletto, P. Targets for a circular economy. Resour. Conserv. Recycl. 2020, 153, 104553. [Google Scholar] [CrossRef]
- Walsh, É.; Coughlan, N.E.; O’Brien, S.; Jansen, M.A.K.; Kuehnhold, H. Density dependence influences the efficacy of wastewater remediation by Lemna minor. Plants 2021, 10, 1366. [Google Scholar] [CrossRef]
- Dinh, T.T.U.; Soda, S.; Nguyen, T.A.H.; Nakajima, J.; Cao, T.H. Nutrient removal by duckweed from anaerobically treated swine wastewater in lab-scale stabilization ponds in Vietnam. Sci. Total Environ. 2020, 722, 137854. [Google Scholar] [CrossRef]
- Cheng, J.J.; Stomp, A.-M. Growing duckweed to recover nutrients from wastewaters and for production of fuel ethanol and animal feed. Clean Soil Air Water 2009, 37, 17–26. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. Duckweeds for water remediation and toxicity testing. Toxicol. Environ. Chem. 2016, 98, 1127–1154. [Google Scholar] [CrossRef]
- Ziegler, P.; Sree, K.S.; Appenroth, K.J. The uses of duckweed in relation to water remediation. Desalination Water Treat. 2017, 63, 327–342. [Google Scholar] [CrossRef]
- Ceschin, S.; Crescenzi, M.; Iannelli, M.A. Phytoremediation potential of the duckweeds Lemna minuta and Lemna minor to remove nutrients from treated waters. Environ. Sci. Pollut. Res. 2020, 27, 15806–15814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Landesman, L.; Bergmann, B.A.; Classen, J.J.; Howard, J.W.; Yamamoto, Y.T. Nutrient removal from swine lagoon liquid by Lemna minor. Trans. Am. Soc. Agric. Eng. 2002, 45, 1003–1010. [Google Scholar] [CrossRef]
- Chikuvire, T.J.; Muchaonyerwa, P.; Zengeni, R. Biomass, nitrogen uptake and content of Wolffia arrhiza depends on strength of swine lagoon water. Water Environ. Res. 2018, 90, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Paolacci, S.; Stejskal, V.; Jansen, M.A.K. Aquaculture international, estimation of the potential of Lemna minor for effluent remediation in integrated multi-trophic aquaculture using newly developed synthetic aquaculture wastewater. Aquac. Int. 2021, 29, 2101–2118. [Google Scholar] [CrossRef]
- Walsh, É.; Kuehnhold, H.; O’Brien, S.; Coughlan, N.E.; Jansen, M.A.K. Light intensity alters the phytoremediation potential of Lemna minor. Environ. Sci. Pollut. Res. 2021, 28, 16394–16407. [Google Scholar] [CrossRef]
- Walsh, É.; Paolacci, S.; Burnell, G.; Jansen, M.A.K. The importance of the calcium-to-magnesium ratio for phytoremediation of dairy industry wastewater using the aquatic plant Lemna minor L. Int. J. Phytoremediation 2020, 22, 694–702. [Google Scholar] [CrossRef]
- Calicioglu, O.; Richard, T.L.; Brennan, R.A. Anaerobic bioprocessing of wastewater-derived duckweed: Maximizing product yields in a biorefinery value cascade. Bioresour. Technol. 2019, 289, 121716. [Google Scholar] [CrossRef]
- Yan, Y.; Candreva, J.; Shi, H.; Ernst, E.; Martienssen, R.; Schwender, J.; Shanklin, J. Survey of the total fatty acid and triacylglycerol composition and content of 30 duckweed species and cloning of a D6-desaturase responsible for the production of c-linolenic and stearidonic acids in Lemna gibba. BMC Plant Biol. 2013, 13, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appenroth, K.J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Fiordelmondo, E.; Ceschin, S.; Magi, G.E.; Mariotti, F.; Iaffaldano, N.; Galosi, L.; Roncarati, A. Effects of partial substitution of conventional protein sources with duckweed (Lemna minor) meal in the feeding of rainbow trout (Oncorhynchus mykiss) on growth performances and the quality product. Plants 2022, 11, 1220. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Bolger, P.; Burnell, G.; O’Leary, N.; O’Mahoney, M.; Paolacci, S.; Wall, D.; Jansen, M.A. Duckweed bioreactors: Challenges and opportunities for large-scale indoor cultivation of Lemnaceae. J. Clean. Prod. 2022, 336, 130285. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Stomp, A.M. Growing Spirodela polyrrhiza in Swine Wastewater for the Production of Animal Feed and Fuel Ethanol: A Pilot Study. Clean Soil Air Water 2012, 40, 760–765. [Google Scholar] [CrossRef]
- Goddek, S.; Joyce, A.; Kotzen, B.; Burnell, G.M. Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; Available online: https://link.springer.com/book/10.1007%2F978-3-030-15943-6 (accessed on 1 May 2022).
- Markou, G.; Wang, L.; Ye, J.; Unc, A. Using agro-industrial wastes for the cultivation of microalgae and duckweeds: Contamination risks and biomass safety concerns. Biotechnol. Adv. 2018, 36, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Bao, S.; He, Z.; Wang, F.; Zhao, H. Effects of operation parameters on nutrient removal from wastewater and high-protein biomass production in a duckweed-based (Lemma aequinoctialis) pilot-scale system. Water Sci. Technol. 2014, 70, 1195–1204. [Google Scholar] [CrossRef]
- Landolt, E. Biosystematic investigations in the family of duckweeds (Lemnaceae). In The Family of Lemnaceae—A Monographic Study; Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel: Zürich, Switzerland, 1986; Volume 1. [Google Scholar]
- Ceschin, S.; Abati, S.; Leacche, I.; Zuccarello, V. Ecological comparison between duckweeds in central Italy: The invasive Lemna minuta vs the native L. minor. Plant Biosyst. 2018, 152, 674–683. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 1993. [Google Scholar]
- Connolly, J.; Wayne, P. Asymmetric competition between plant species. Oecologia 1996, 108, 311–320. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA; American Water Works Association (AWWA): Denver, CO, USA; Water Environmental Federation (WEF): Alexandria, VI, USA, 2005. [Google Scholar]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis, 3rd ed.; John Wiley and Sons: Weinheim, Germany, 2009. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4.8. 2020. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 May 2022).
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.J. Relative in vitro growth rates of duckweeds (Lemnaceae)—The most rapidly growing higher plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef]
- Derksen, H.; Zwart, L. Eendenkroos Als Nieuw Eiwit-En Zetmeelgewas: Haalbaarheidsstudie November 2010, Eindrapport; Stichting Sustenable Forum: Singapore, 2010. [Google Scholar]
- Giblin, S.M.; Houser, J.N.; Sullivan, J.F.; Langrehr, H.A.; Rogala, J.T.; Campbell, B.D. Thresholds in the response of free-floating plant abundance to variation in hydraulic connectivity, nutrients, and macrophyte abundance in a large floodplain river. Wetlands 2014, 34, 413–425. [Google Scholar] [CrossRef]
- Janauer, G.A.; Schmidt-Mumm, U.; Schmidt, B. Aquatic macrophytes and water current velocity in the Danube River. Ecol. Eng. 2010, 36, 1138–1145. [Google Scholar] [CrossRef]
- Xu, J.; Cui, W.; Cheng, J.J.; Stomp, A.M. Production of high-starch duckweed and its conversion to bioethanol. Biosyst. Eng. 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Ran, N.; Agami, M.; Oron, G. A pilot study of constructed wetlands using duckweed (Lemna gibba L.) for treatment of domestic primary effluent in Israel. Water Res. 2004, 38, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Gatidou, G.; Oursouzidou, M.; Stefanatou, A.; Stasinakis, A.S. Removal mechanisms of benzotriazoles in duckweed Lemna minor wastewater treatment systems. Sci. Total Environ. 2017, 596, 12–17. [Google Scholar] [CrossRef]
- Adhikari, U.; Harrigan, T.; Reinhold, D.M. Use of duckweed-based constructed wetlands for nutrient recovery and pollutant reduction from dairy wastewater. Ecol. Eng. 2015, 78, 6–14. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. Pulse Amplitude Modul. Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Schoelynck, J.; Puijalon, S.; Meire, P.; Struyf, E. Thigmomorphogenetic responses of an aquatic macrophyte to hydrodynamic stress. Front. Plant Sci. 2015, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Körner, S.; Vermaat, J.E. The relative importance of Lemna gibba L., bacteria and algae for the nitrogen and phosphorus removal in duckweed-covered domestic wastewater. Water Res. 1998, 32, 3651–3661. [Google Scholar] [CrossRef]
- Zimmo, O.R.; van der Steen, N.P.; Gijzen, H.J. Nitrogen mass balance across pilot-scale algae and duckweed-based wastewater stabilisation ponds. Water Res. 2004, 38, 913–920. [Google Scholar] [CrossRef]
- Mohedano, R.A.; Costa, R.H.R.; Hofmann, S.M.; Belli Filho, P. Using full-scale duckweed ponds as the finish stage for swine waste treatment with a focus on organic matter degradation. Water Sci. Technol. 2014, 69, 2147–2154. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Y.; Jin, Y.; Huang, J.; Ma, X.; He, K.; He, Z.; Wang, F.; Zhao, H. Microbial community and removal of nitrogen via the addition of a carrier in a pilot-scale duckweed-based wastewater treatment system. Bioresour. Technol. 2015, 179, 549–558. [Google Scholar] [CrossRef]
- Paolacci, S.; Harrison, S.; Jansen, M.A.K. A comparative study of the nutrient responses of the invasive duckweed Lemna minuta, and the native, co-generic species Lemna minor. Aquat. Bot. 2016, 134, 47–53. [Google Scholar] [CrossRef]
- Landolt, E.; Kandeler, R. Biosystematic investigations in the family of duckweeds (Lemnaceae). In The Family of Lemnaceae—A Monographic Study; Veröffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rübel: Zürich, Switzerland, 1987; Volume 2. [Google Scholar]
- Lasfar, S.; Monette, F.; Millette, L.; Azzouz, A. Intrinsic growth rate: A new approach to evaluate the effects of temperature, photoperiod and phosphorus–nitrogen concentrations on duckweed growth under controlled eutrophication. Water Res. 2007, 41, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
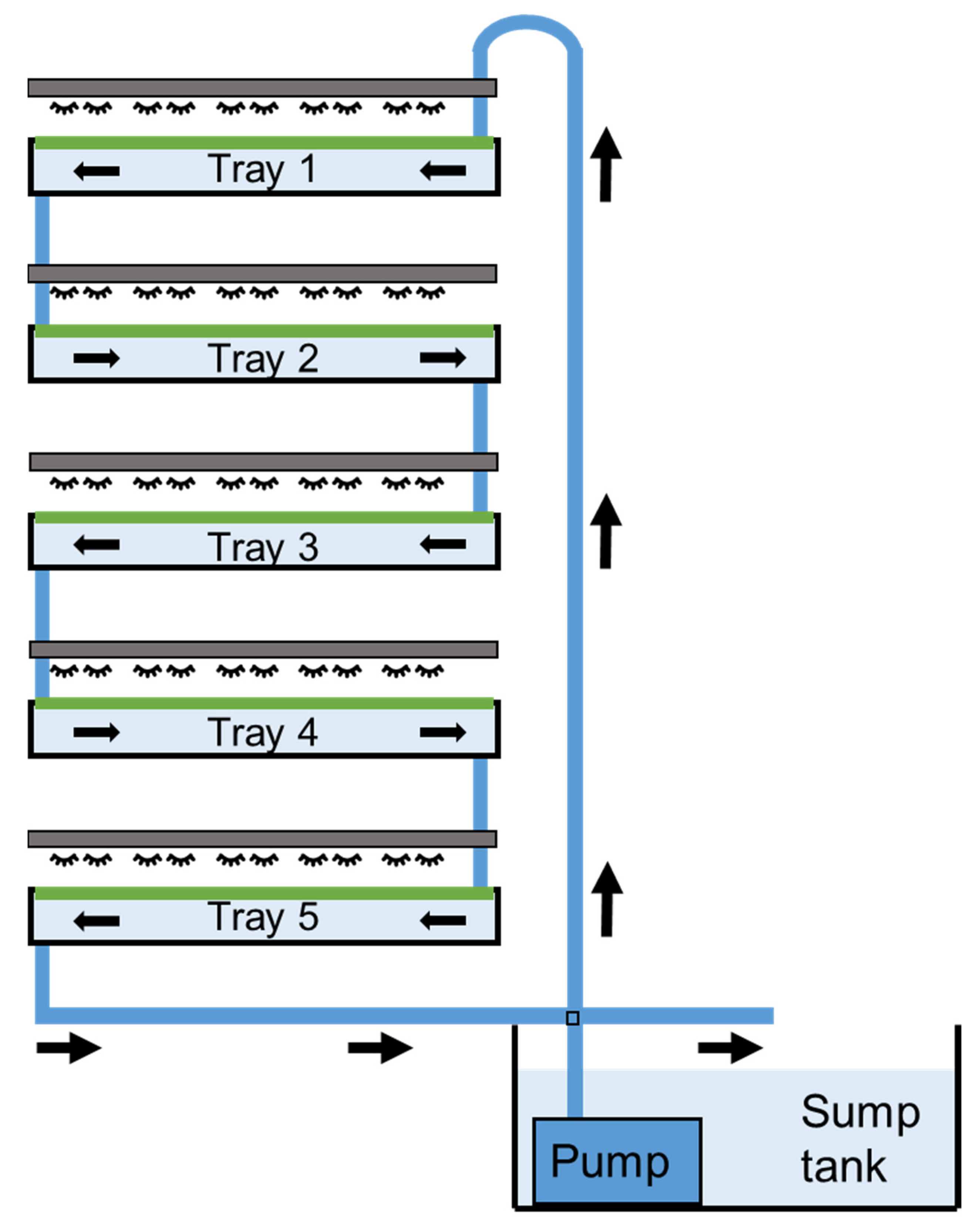
| Treatment | Flow Rates (L min−1) | Medium Depth (mm) | Velocity (m s−1) |
|---|---|---|---|
| 1 | 0.5 | 25 | 0.0008 |
| 2 | 1.5 | 25 | 0.0024 |
| 3 | 3.0 | 25 | 0.0048 |
| 4 | 0.5 | 50 | 0.0004 |
| 5 | 1.5 | 50 | 0.0012 |
| 6 | 3.0 | 50 | 0.0024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coughlan, N.E.; Walsh, É.; Ahern, R.; Burnell, G.; O’Mahoney, R.; Kuehnhold, H.; Jansen, M.A.K. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants 2022, 11, 2170. https://doi.org/10.3390/plants11162170
Coughlan NE, Walsh É, Ahern R, Burnell G, O’Mahoney R, Kuehnhold H, Jansen MAK. Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants. 2022; 11(16):2170. https://doi.org/10.3390/plants11162170
Chicago/Turabian StyleCoughlan, Neil E., Éamonn Walsh, Roger Ahern, Gavin Burnell, Rachel O’Mahoney, Holger Kuehnhold, and Marcel A. K. Jansen. 2022. "Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor" Plants 11, no. 16: 2170. https://doi.org/10.3390/plants11162170
APA StyleCoughlan, N. E., Walsh, É., Ahern, R., Burnell, G., O’Mahoney, R., Kuehnhold, H., & Jansen, M. A. K. (2022). Flow Rate and Water Depth Alters Biomass Production and Phytoremediation Capacity of Lemna minor. Plants, 11(16), 2170. https://doi.org/10.3390/plants11162170






