Abstract
Dianella ensifolia is a perennial herb with thickened rhizome and is widely distributed in tropical and subtropical regions of Asia, Australia, and the Pacific islands. This plant has the potential to be used as a source of herbal medicine. This study investigated further phytochemistry and tyrosinase inhibitory effect of some constituents isolated from D. ensifolia. Four new flavans, (2S)-4’-hydroxy-6,7-dimethoxyflavan (1), (2S)-3’,4’-dihydroxy-7-methoxy-8-methylflavan (2), (2S)-2’-hydroxy-7-methoxyflavan (3), and (2S,1′S)-4-hydroxy-4-(7-methoxy-8-methylchroman-2-yl)-cyclohex-2-enone (4), together with 67 known compounds, including 10 flavans (5–14), 5 flavanones (15–19), 3 flavone (20–22), 5 chalcones (23–27), 3 chromones (28–30), 15 aromatics (31–45), 7 phenylpropanoids (46–52), one lignan (53), 7 steroids (54–60), one monoterpene (61), one diterpene (62), 4 triterpenes (63–66), a carotenoid (67), 2 alkaloids (68 and 69), and 2 fatty acids (70 and 71) were isolated from D. ensifolia. Their structures were elucidated on the basis of physical and spectroscopic data analyses. Moreover, compounds 1–4, 8, 10–15, 20, 21, and 41 were evaluated for their mushroom tyrosinase inhibitory effect. Compounds 11 and 14 strongly inhibited mushroom tyrosinase activity with IC50 values of 8.6 and 14.5 μM, respectively.
1. Introduction
The Dianella Lam. ex Juss. genus includes perennial herbs (or subshrubs), glabrous, and rhizomatous with aerial stems. It comprises about 40 species with accepted names, mainly distributed in tropical Asia, Australia, and the southern Pacific islands [1]. The Dianella genus previously belongs to Liliaceae but has been revised to Asphodelaceae, recently [1,2]. Only one species, Dianella ensifolia (L.) DC, is native to Taiwan [3]. D. ensifolia is used as herbal medicine, such as topical medicines for ferunculosis, abscesses, lympharingitis, tuberculosis lymphadenitis, tinea, traumatic injuries, and wounds. The plant can also be taken internally for the treatment of dysentery, dysuria, leucorrhoea, blanorrhoea, and fatigue [4]. In Taiwan, the indigenous people use its roots for the treatment of abdominal pain; roots and leaves for treating poisonous snakebite [5]. Previous studies of D. ensifolia identified various phytochemical constituents, such as quinones [6,7], phenolics, steroids [8], flavonoids [8,9,10], glycosides [11], triterpenoids [12,13], and diarylpropanes [14,15,16]. Some of these constituents exhibited biological activities, including antioxidative [12,16], antibacterial [12], antivirus [12,17,18], anthelmintic [10], antitumor/anticancer [10,13], and anti-tyrosinase/melanogenesis activities [15,16,19]. Given the promise of a literature survey, an investigation was carried out to search for additional valuable bioactive constituents from the whole plant of D. ensifolia.
Melanin is one of the most widely distributed pigments and is found in fungi, bacteria, plants, and animals. It is the main protective pigment found in the hair, skin, and eyes of a human. The role of melanin is to protect the skin against ultraviolet (UV) damage by absorbing UV sunlight and removing reactive oxygen species (ROS) [20]. Melanin is formed through a series of oxidative reactions involving the amino acid tyrosine in the presence of tyrosinase. Tyrosinase (EC 1.14.18.1) is a membrane-bound, multifunctional copper-containing polyphenol oxidase (PPO) enzyme, involved in the initial steps of melanin biosynthesis in living organisms [21,22]. The enzyme catalyzes the hydroxylation of l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA) and l-DOPA to dopaquinone, which is the rate-limiting step of melanin synthesis and can cause unusual melanin pigments accumulation in the outermost layer of the skin [23,24]. Excessive production of melanin resulted in different dermatological disorders such as aging spots, wrinkles, melasma, freckles, lentigo, ephelides, post-inflammatory melanoderma, nevus, and melanoma [25]. In addition, a recent investigation suggests that abnormal melanogenesis disorders are related to some neurodegenerative diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s diseases [26]. Therefore, there is a great need for melanogenesis inhibitors to develop treatment or prevention of hyperpigmentation disorders. Tyrosinase inhibitors typically work by chelating with copper within the active site of tyrosinase, obviating the substrate-enzyme interaction, or disrupting the ensuing electrochemical oxidation process [23]. Since it has been observed that flavonoids, stilbenes, and phenolics manifest tyrosinase inhibitory activities, it encourages us the continuing search for more bioactive constituents from Formosan medicinal plants. Accordingly, this investigation of D. ensifolia products focused on identifying new tyrosinase inhibitors that could be whitening candidates. Here in this article, the structure elucidation of four new flavans and the results of the tyrosinase inhibitory effect are reported.
2. Results and Discussion
2.1. Isolation and Structure Elucidation of Compounds 1–4
The methanolic extract of roots of D. ensifolia was partitioned into ethyl acetate (EtOAc)- and water-soluble layers. Four new flavans (1–4) (Figure 1) and 41 known compounds including 8 flavans (5–12), 2 flavanones (15–16), a flavone (20), 4 chalcones (23–26), 2 chromones (28–29), 11 aromatics (31–41), 4 phenylpropanoids (46–49), one lignin (53), 7 steroids (54–60), and a monoterpene (61) (Figure S1) were isolated from the EtOAc-soluble layer of methanolic extract of roots.
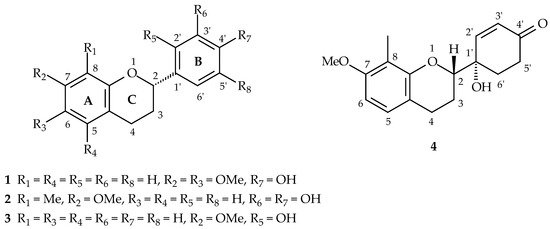
Figure 1.
The chemical structures of new flavans (1–4).
The methanolic extract of the aerial part of D. ensifolia was partitioned into EtOAc- and water-soluble layers. The new flavan (1) and 48 known compounds including 7 flavans (8–14), 5 flavanones (15–19), 2 flavones (21–22), 2 chalcones (26–27), 2 chromones (29–30), 10 aromatics (36–45), 4 phenylpropanoids (49–52), lignin (53), 4 steroids (54–57), a monoterpene (61), one diterpene (62), 4 triterpenes (63–66), a carotenoid (67), two alkaloids (68–69), and two fatty acids (70–71) (Figure S1) were isolated from the EtOAc-soluble layer of methanolic extract of the aerial part.
Compound 1 was isolated as yellowish oil. Its molecular formula, C17H18O4, was determined by HREIMS ([M]+, 286.1209). The 1H- and 13C-NMR spectral data (Table 1 and Table 2) of 1 were similar to that of 6,4’-dihydroxy-7-methoxyflavan [27], except that one hydroxy group at C-6 in 6,4’-dihydroxy-7-methoxyflavan was replaced by a methoxy group in 1. The 1H-NMR spectrum (Table 1 and Figure S4) of 1 showed five aliphatic protons at δ 2.05, 2.14, 2.70, 2.92, and 4.92, typical of pyran nucleus protons of a flavan, one set of A2B2 system phenyl protons [δ 6.83 (2H, d, J = 8.4 Hz, H-3’ and H-5’), 7.28 (2H, d, J = 8.4 Hz, H-2’ and H-6’)], two singlet aromatic protons [δ 6.46 (1H, s, H-8), 6.57 (1H, s, H-5)], two methoxy singlet at δ 3.80 and 3.82, and one hydroxy group at δ 5.05. The NOESY spectrum of 1 (Figure 2 and Figure S7) showed protons at δ 3.80 and 3.82 owing correlations with 6.46 (H-8) and 6.57 (H-5) respectively suggesting a methoxy group (δ 3.80) at C-7 and a methoxy group (δ 3.82) at C-6. Because 1 showed negative optical rotation { −17.1° (c 0.01, MeOH)}, C-2 possesses as S-configuration [28]. According to the above evidence, the structure of 1 was elucidated as (2S)-4’-hydroxy-6,7-dimethoxyflavan, which was further confirmed by COSY (Figure S6), NOESY (Figure 2 and Figure S7), 13C-NMR (Figure S5), DEPT, HMQC (Figure S8), and HMBC (Figure 2 and Figure S9) experiments.

Table 1.
1H-NMR data (CDCl3) of compounds 1–4. Chemical shifts δ in ppm, J in Hz.

Table 2.
13C-NMR data (CDCl3) of compounds 1–4. Chemical shifts δ in ppm.
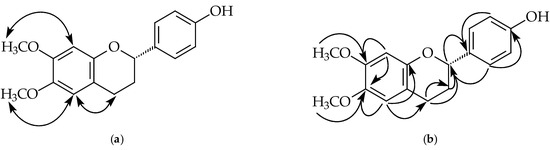
Figure 2.
Key NOESY contacts (a) and HMBC connectivities (b) of compound 1.
Compound 2 was isolated as a brown oil. Its molecular formula, C17H18O4, was determined by HREIMS ([M]+, 286.1207). The 1H-NMR spectrum (Table 1 and Figure S12) of 2 showed five aliphatic protons at δ 1.90, 2.16, 2.72, 2.92, and 4.96, typical of pyran nucleus protons of a flavan, one 3,4-dihydroxyphenyl group [δ 6.84 (2H, s, H-5’ and H-6’), 6.95 (1H, s, H-2’), 5.23 (br s, OH), 5.25 (br s, OH)], two ortho coupled doublets (J = 8.0 Hz) at δ 6.44 and 6.85, a methoxy singlet at δ 3.80, and one methyl singlet at δ 2.20. The NOESY experiment of 2 (Figure 3 and Figure S15) showed that protons at δ 6.85 correlated with δ 6.44 and 2.72 (H-4), δ 6.85 and 6.44 were assigned to H-5 and H-6, respectively. The methoxy singlet (δ 3.80) owning correlation with H-6 suggested the methoxy group at C-7. Compound 2 showed negative optical rotation { −15.8° (c 0.01, MeOH)}, which means C-2 has the S-configuration [28]. According to the above evidence, the structure of 2 was elucidated as (2S)-3’,4’-dihydroxy-7-methoxy-8-methylflavan, which was further confirmed by COSY (Figure S14), NOESY (Figure 3 and Figure S15), 13C-NMR (Figure S13), DEPT, HMQC (Figure S16,) and HMBC (Figure 3 and Figure S17) experiments.
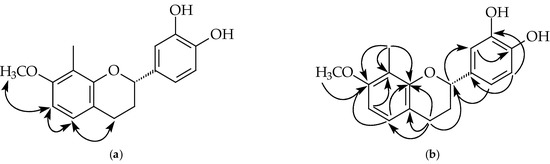
Figure 3.
Key NOESY contacts (a) and HMBC connectivities (b) of compound 2.
Compound 3 was isolated as a brown oil. Its molecular formula, C16H16O3, was determined by HREIMS ([M]+, 256.1104). The 1H-NMR spectrum (Table 1 and Figure S23) of 3 showed five aliphatic protons at δ 2.26, 2.80, 2.96, and 5.18, typical of pyran nucleus protons of a flavan, one set of ABX system phenyl protons [δ 6.46 (d, J = 2.4 Hz), 6.52 (dd, J = 8.4, 2.4 Hz), 7.00 (d, J = 8.4 Hz)], four aromatic protons at δ 6.90, 6.91, 7.14, and 7.20, a methoxy singlet at δ 3.75. Since the NOESY experiment of 3 (Figure 4) showed that protons at δ 7.00 correlated with δ 6.52 and H-4, δ 7.00 and 6.52 were assigned to H-5 and H-6, respectively. The methoxy singlet owning correlation with H-6 and δ 6.46 suggested the methoxy group at C-7 and δ 6.46 was assigned to H-8. 13C-NMR spectrum (Table 2 and Figure S21) of 3 showed three oxygenated quaternary C-atoms (δ 154.4, 154.7, 159.1) suggesting a hydroxyl group on B-ring. The NOESY correlations (Figure 4 and Figure S23) of four aromatic protons (δ 6.90, 6.91, 7.14, and 7.20) suggested the hydroxy group at C-2′. An S-configuration at C-2 of 3 was due to the negative optical rotation { −26.0° (c 0.01, MeOH)} [28]. According to the above evidence, the structure of 3 was elucidated as (2S)-2’-hydroxy-7-methoxyflavan, which was further confirmed by COSY (Figure S22), NOESY (Figure 4 and Figure S23), 13C-NMR (Figure S21), DEPT, HMQC (Figure S24), and HMBC (Figure 4 and Figure S25) experiments.

Figure 4.
Key NOESY contacts (a) and HMBC connectivities (b) of compound 3.
Compound 4 was isolated as a colorless oil. Its molecular formula, C17H20O4, was determined by HREIMS ([M]+, 288.1363). The 1H-NMR spectrum (Table 1 and Figure S28) of 4 was similar to that of 2, except the typical aromatic proton signals of the B-ring were absent. Instead, four aliphatic protons at δ 2.16, 2.49, 2.81, and two vinylic hydrogens [6.06 (d, J = 10.4 Hz, H-3’), 7.09 (dd, J = 10.4, 1.2 Hz, H-2’)] provided evidence of a cyclohexenone non-aromatic B-ring. Moreover, an additional carbonyl group at δ 198.2 (C-4’) and conjugated vinylic carbons at δ 129.5 (C-3’) and 148.7 (C-2’) in the 13C-NMR spectrum (Table 2 and Figure S29) of 4 provided strong support of this cyclohexenone moiety. HMBC correlations (Figure 5 and Figure S33) between H-2’ and the carbon at δ 80.5 (C-2), 198.2 (C-4’), 70.5 (C-1’), and 30.9 (C-6’) and between H-3’ and carbons at δ 70.5 (C-1’) and 33.9 (C-5’) supposed the connectivity between the non-aromatic B-ring and C-ring. According to the above evidence, the structure of 4 was elucidated as 4-hydroxy-4-(7-methoxy-8-methylchroman-2-yl)-cyclohex-2-enone, which was further confirmed by COSY (Figure S30), NOESY (Figure 5 and Figure S31), 13C-NMR (Figure S29), DEPT, HMQC (Figure S32), and HMBC (Figure 5 and Figure S33) experiments. The CD spectrum (Figure 6: orange curve) of 4 showed two positive Cotton effects around 392 and 334 nm, similar to the calculated ECD spectrum for (2S,1′S)-4 (Figure 6: purple curve). Therefore, the absolute configuration of 4 was decided as (2S,1′S)-4-hydroxy-4-(7-methoxy-8-methylchroman-2-yl)-cyclohex-2-enone.
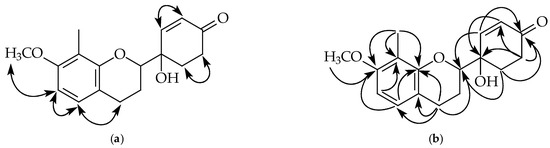
Figure 5.
Key NOESY contacts (a) and HMBC connectivities (b) of compound 4.
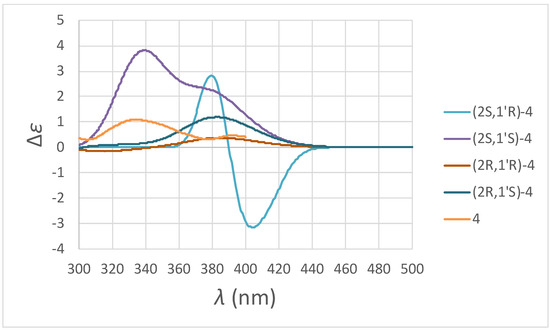
Figure 6.
Experimental and calculated ECD spectra of compound 4.
The known compounds including 10 flavans, (2R,3R)-3,4’-dihydroxy-7-methoxy-8-methylflavan (tupichinol A) (5) [29], (2S)-7,3’-dihydroxy-4’-methoxyflavan (6) [30], (2S)-4’-hydroxy-5,7-dimethoxy-8-methylflavan (7) [31], (2S)-7,4’-dihydroxyflavan (8) [28], (2S)-4’-hydroxy-7-methoxyflavan (9) [31], (2S)-7,4’-dihydroxy-8-methylflavan (10) [32], (2S)-2’,4’-dihydroxy-7-methoxy-8-methylflavan (11) [9], (2S)-3’,5’-dihydroxy-7,4’-dimethoxy-8-methylflavan (12) [33], (2S)-7,3′-dihydroxy-6,4’-dimethoxyflavan (13) [34], (2S)-2’,4’-dihydroxy-7-methoxyflavan (14) [8]; 5 flavanones, (2S)-liquiritigenin (15) [35], (2S)-farrerol (16) [36], (2S)-liquiritigenin 4’-methyl ether (17) [28], (2S)-5,7-dihydroxy-4’-methoxy-6,8-dimethylflavanone (18) [37], cyrtominetin (19) [38]; 3 flavones, luteolin (20) [39], apigenin (21) [39], syzalterin (22) [36]; 5 chalcones, broussonin A (23) [40], 1-(2,4-dimethoxyphenyl)-3-(4-hydroxyphenyl)propane (24) [41], 4,2’-dihydroxy-4’-methoxychalcone (25) [42], 4,2’,4’-trihydroxychalcone (26) [42], kukulkanin B (27) [42]; 3 chromones, noreugenin (28) [43] and 5,7-dihydroxy-2,8-dimethylchromen-4-one (29) [44], 5,7-dihydroxy-2-tricosylchromone (30) [45]; 15 aromatics, 1H,3H-5,7-dimethoxyisobenzofuran-l-one (31) [46], syringic acid (32) [47], 2,4-dimethoxy-6-methylbenzoic acid (33) [48], methyl 2,6-dihydroxy-3,4-dimethylbenzoate (34) [49], methyl 3,4-dihydroxybenzoate (35) [50], 3,5-dimethoxy-4-hydroxybenzaldehyde (36) [51], vanillic acid (37) [52], methyl 2,4-dimethoxy-6-methylbenzoate (38) [48], methyl 4-hydroxy-2-methoxy-6-methylbenzoate (39) [53], methyl 4-hydroxybenzoate (40) [54], 7-acetyl-4,8-dihydroxy-6-methyl-1-tetralone (41) [8], benzoic acid (42) [55], 4-hydroxybenzaldehyde (43) [52], 4-hydroxybenzoic acid (44) [54], phenylacetic acid (45) [56]; 7 phenylpropanoids, 4-(3-hydroxypropyl)-2-methoxyphenol (46) [57], ferulic acid (47) [58], tetracosanyl ferulate (48) [59], 4-hydroxycinnamic acid (49) [52], methyl 4-hydroxycinnamate (50) [47], feruloyloxytetracosanoic acid (51) [60], phloiorubein (52) [61]; one lignan, (+)-pinoresinol (53) [62]; 7 steroids, β-siosterol (54) [63], stigmasterol (55) [63], β-sitostenone (56) [64], stigmasta-4,22-dien-3-one (57) [65], 6β-hydroxystigmast-4-en-3-one (58) [66], 3β-hydroxystigmast-5-en-7-one (59) [52], (22E)-3β-hydroxystigmasta-5,22-dien-7-one (60) [67]; one monoterpene, loliolide (61) [68]; one diterpene, phytol (62) [54]; four triterpenes, 3β,24ξ-dihydroxy-25-cycloartene (63) [69], 3β-hydroxy-11-oxo-12-ursene (64) [70], 9 (11),12-oleanadien-3-one (65) [71], 3β-hydroxy-20-oxo-30-norlupane (66) [70]; a carotenoid, β,ε-carotene-3,3′-diol (67) [72]; two alkaloids, indole-3-aldehyde (68) [73], indole-3-carboxylic acid (69) [74]; two fatty acids, linoleic acid (70) [75] and palmitic acid (71) [76] were readily identified by comparison of physical and spectroscopic data (UV, IR, 1H-NMR, [α]D, and mass spectrometry data) with values found in the literature.
2.2. Mushroom Tyrosinase Inhibitory Effect of Compounds Isolated from D. ensifolia
Tyrosinase is a copper-containing enzyme that catalyzes the oxidation of both monophenols (monophenolase activity) and diphenols (diphenolase activity) to the corresponding quinones and is responsible for the formation of melanin, which protects skin from the damage caused by UV radiation [20,24]. Excessive tyrosinase activity may lead to the overproduction or abnormal distribution of melanin, known as irregular hyperpigmentation of the skin. The use of tyrosinase inhibitors to prevent pigmentation is becoming increasingly important in the cosmetic and medicinal industries. These phenomena have motivated us to continue our research on natural tyrosinase inhibitors.
Compounds 1–4, 8, 10–15, 20, 21, and 41 were evaluated for their mushroom tyrosinase inhibitory effect. As shown in Table 3, compounds 11 and 14 exhibited potent concentration-dependent mushroom tyrosinase inhibitory activity, with IC50 values of 8.6 ± 0.8 and 14.5 ± 0.3 μM, respectively. However, the IC50 value of arbutin (positive control), a well-known whitening agent used in cosmetics, was 112.2 ± 5.4 μM. (2S)-2’,4’-Dihydroxy-7-methoxy-8-methylflavan (11) and (2S)-2’,4’-dihydroxy-7-methoxyflavan (14) showed approximately 13-fold and 7.7-fold stronger tyrosinase inhibitory activity than arbutin, respectively. Compounds 2, 15, 20, and 21 also showed a more substantial inhibitory effect against mushroom tyrosinase than arbutin. Whereas, compounds 1, 3, 4, 8, 10, 12, 13, and 41 had moderate or weak activity.

Table 3.
Mushroom tyrosinase inhibitory effect of tested compounds.
The results also noted interesting structure-activity relationships among the tyrosinase inhibitory activity of these tested compounds. The anti-tyrosinase activity of them can be ranked as follows: 11, 14 >> 20, 2 > 21 > 15 ≅ 41 ≅ arbutin > 8, 1 ≅ 10 > 13, 4 > 3, 12. Among flavonoids, it was found that compounds with a resorcinol skeleton in the ring B (e.g., 11 and 14) showed the most potent anti-tyrosinase activity than that bearing a catechol moiety (e.g., 2 and 20). As compounds with catechol moiety, the activity of 20 (a flavone) is better than 2 (a flavan), it was suggested that para-substituted hydroxy group in the B ring attached to an α,β-unsaturated carbonyl group of flavone forms a skeleton similar to that of tyrosine, thus, it showed better anti-tyrosinase activity than flavans. These relative activities are consistent with previous reports [77,78,79]. In addition, the position of resorcinol is also important for anti-tyrosinase activity. In comparison with 11, compound 12 dramatically decreased the tyrosinase inhibitory effect; it can be conducted that compounds with 2′,4′-resorcinol moiety is essential for potent enzyme inhibitor than that of 3′,5′-resorcinol moiety. Introducing a non-hydroxy substituent at C-4′ (e.g., 3, 4, 12, and 13) notably reduced the tyrosinase inhibitory activity. Further experiments are needed to pinpoint the mechanism of this activity.
3. Materials and Methods
3.1. General
Silica gel 60 F254 precoated plates (Merck). Column chromatography (CC): silica gel (Merk 70–230 mesh). High-performance liquid chromatography (HPLC): LDC Analytical-III system; column: Keystone spherisorb silica, 5 μm, 250 × 10 mm. M.p.: Yanaco-MP-S3 micro-melting-point apparatus; uncorrected. Optical rotation: Jasco-DIP-1000 polarimeter; in methanol. UV Spectra: Helios Beta UV-Visible spectrophotometer; λmax (logε) in nm. IR Spectra: Perkin-Elmer-983G FT-IR spectrophotometer; ν in cm−1. 1H-, 13C-, and 2D-NMR Spectra: Varian-Unity-Plus-400 and Bruker-DMX-500 spectrometers; δ in ppm, J in Hz. EIMS and HREIMS: Jeol-JMS-HX300 mass spectrometer; m/z (rel. %).
3.2. Plant Material
Dianella ensifolia were collected from Taipei, Taiwan, in July 2006 and positively identified by Y.-H. K. A voucher specimen (YHK 0671) has been deposited in the Herbarium of the College of Pharmacy, China Medical University, Taichung, Taiwan, R.O.C.
3.3. Extraction and Isolation
Dried roots (12.8 kg) of Dianella ensifolia were sliced and extracted with cold MeOH twice. After removal of the solvent under vacuum, the extract (376 g) was partitioned with EtOAc and water (1:1 v/v) three times and obtained EtOAc- and water-soluble fractions.
The EtOAc-soluble fraction (90 g) was chromatographed using silica gel (2.0 kg), using n-hexane, EtOAc, and MeOH of increasing polarity as eluent to give 10 fractions. Fraction 2 (7.6 g, n-hexane/EtOAc 95:5) was subjected to HPLC (n-hexane/EtOAc 9:1) to yield 3 (19.3 mg), 34 (583.2 mg), 38, (23.8 mg), 48 (62.5 mg), and the mixture (25.2 mg) of 56 and 57. Fraction 3 (10.1 g, n-hexane/EtOAc 90:10) was subjected to HPLC (CH2Cl2/EtOAc 97:3) to yield 7 (30.9 mg), 9 (85.5 mg), 24 (35.7 mg), the mixture (159.7 mg) of 54 and 55, and 69 (5.32 g). Fraction 4 (4.6 g, n-hexane/EtOAc 80:20) was subjected to HPLC (CH2Cl2/EtOAc 85:15) to yield 1 (31.9 mg), 2 (26.7 mg), 4 (33.2 mg), 6 (26.6 mg), 10 (37.0 mg), 11 (28.1 mg), 12 (24.4 mg), 16 (25.5 mg), 23 (31.0 mg), 25 (25.2 mg), 28 (22.9 mg), 29 (38.2 mg), 39 (47.8 mg), 40 (46.7 mg), 58 (15.8 mg), and the mixture (18.3 mg) of 59 and 60. Fraction 5 (4.6 g, n-hexane/EtOAc 70:30) was subjected to HPLC (CH2Cl2/EtOAc 85:15) to yield 5 (34.4 mg), 8 (33.2 mg), 26 (31.4 mg), 31 (14.1 mg), 35 (27.8 mg), 36 (18.4 mg), 41 (63.2 mg), 46 (16.5 mg), and 61 (15.6 mg). Fraction 6 (6.6 g, n-hexane/EtOAc 50:50) was subjected to HPLC (CH2Cl2/EtOAc 80:20) to yield 15 (51.8 mg), 20 (31.0 mg), 32 (28.9 mg), 33 (232.9 mg), 37 (22.8 mg), 47 (40.0 mg), 49 (21.6 mg), 53 (30.7 mg).
The dried aerial part of D. ensifolia (4.9 kg) was chopped and extracted with cold MeOH twice. The mixtures were filtered and concentrated to dryness under reduced pressure, producing a methanolic extract (331.0 g). The methanolic extract was partitioned with EtOAc and water (1:1, v/v) to obtain EtOAc- and water-soluble layers.
The EtOAc-soluble layer (185.0 g) was subjected to silica gel (5.0 kg) column chromatography with a gradient of n-hexane/ EtOAc /MeOH to obtain 11 fractions. Fraction 4 (35.3 g, n-hexane/EtOAc 90:10) was subjected to preparative HPLC, eluting with n-hexane/EtOAc (8:2) and n-hexane/CH2Cl2/EtOAc (5.5:4:0.5) to yield four steroids: 54 (1442 mg), 55 (961 mg), 56 (45.4 mg), 57 (31.5 mg). Fraction 5 (9.6 g, n-hexane/EtOAc 80:20) was subjected to preparative HPLC, eluting with n-hexane/EtOAc (6:4), CH2Cl2/EtOAc (8.5/1.5), and n-hexane/CH2Cl2/EtOAc (5.5:4:0.5) to yield three flavonoids: 8 (10.8 mg), 9 (21.6 mg), and 18 (6.7 mg); six aromatics: 38 (1.3 mg), 39 (2.4 mg), 40 (22.2 mg), 43 (2.2 mg), 50 (2.9 mg), and 52 (6.0 mg); four triterpenoids: 63 (7.0 mg), 64 (6.6 mg), 65 (4.8 mg), and 66 (11.5 mg); one chromone: 29 (7.7 mg), one diterpene: 62 (21.2 mg) and a fatty acid: 71 (18.5 mg). Fraction 6 (8.9 g, n-hexane/EtOAc 70:30) was purified by HPLC, eluting with n-hexane/EtOAc (7:3) to obtain 17 (4.8 mg), follow by CH2Cl2/EtOAc (9.5:0.5 to 8:2) to yield 1 (3.1 mg), 10 (5.2 mg), 11 (12.1 mg), 12 (27.5 mg), 13 (29.7 mg), 14 (33.3 mg), and 16 (54.2 mg) was purified by n-hexane/EtOAc (6:4). Total are eight flavonoids isolated from fraction 6. In addition, four aromatics, 36 (11.8 mg), 41 (9.4 mg), 42 (5.3 mg), 51 (2.5 mg), were obtained by eluting with n-hexane/EtOAc (6.5:3.5 to 6:4) and CH2Cl2/EtOAc (8.5:1.5 to 8:2). One chromone, 44 (25.3 mg), was obtained by eluting with n-hexane/CH2Cl2/EtOAc (5.5:4:0.5). One chalcone, 27 (3.1 mg) was obtained by eluting with n-hexane/EtOAc (6:4). A carotenoid, 67 (1.7 mg), was obtained by eluting with n-hexane/EtOAc (7:3) and CH2Cl2/EtOAc (9.5:0.5 to 8:2). Fraction 7 (12.4 g, n-hexane/EtOAc 50:50) was purified by HPLC, eluting with n-hexane/EtOAc (6:4 to 1:1) and CH2Cl2/EtOAc (9.5:0.5 to 1:1) to yield four flavonoids [15 (10.1 mg), 21 (9.4 mg), 22 (4.7 mg), 19 (29.6 mg)], seven aromatics [29 (7.7 mg), 30 (25.3 mg), 49 (63.8 mg), 37 (13.0 mg), one chalcone [26 (23.1 mg)], two alkaloids [68 (9.0 mg), 69 (11.6 mg)], one lignan [53 (17.8 mg)], and one monoterpene [61 (79.8 mg)].
3.4. (2S)-4’-Hydroxy-6,7-Dimethoxyflavan (1)
Yellow oil; −17.1° (c 0.01, MeOH); UV (MeOH) λmax (log ε): 286 (4.12), 225 (4.66) nm; IR (neat) νmax: 3424, 3010, 2935, 2859, 1605, 1512, 1214, 1116 cm−1; EIMS: m/z (rel. int.): 286 (M+, 100), 180 (8), 167 (41), 166 (13), 120 (7); HREIMS: 286.1209 (C17H18O4+, calc. 286.1205); 1H-NMR: see Table 1; 13C-NMR: see Table 2.
3.5. (2S)-3’,4’-Dihydroxy-7-Methoxy-8-Methylflavan (2)
Brown oil; −15.8° (c 0.01, MeOH); UV (MeOH) λmax (log ε): 282 (3.66), 227 (4.08) nm; IR (neat) νmax: 3403, 3090, 2952, 2853, 1619, 1510, 1454, 1275, 1162 cm−1; EIMS: m/z (rel. int.): 286 (M+, 77), 269 (13), 164 (29), 151 (100), 136 (34); HREIMS: 286.1207 (C17H18O4+, calc. 286.1205); 1H-NMR: see Table 1; 13C-NMR: see Table 2.
3.6. (2S)-2’-Hydroxy-7-Methoxyflavan (3)
Brown oil; −26.0° (c 0.01, MeOH); UV (MeOH) λmax (log ε): 280 (3.84), 221 (4.25) nm; IR (neat) νmax: 3368, 3012, 2927, 2853, 1607, 1513 cm−1; EIMS: m/z (rel. int.): 256 (M+, 34), 239 (50), 150 (28), 137 (58), 73 (26), 59 (100); HREIMS: 256.1104 (C16H16O3+, calc. 256.1099); 1H-NMR: see Table 1; 13C-NMR: see Table 2.
3.7. 4-Hydroxy-4-(7-Methoxy-8-Methylchroman-2-yl)-Cyclohex-2-Enone (4)
Colorless oil; +30.8° (c 0.01, MeOH); UV (MeOH) λmax (log ε): 281 (3.51), 227 (4.48) nm; IR (neat) νmax: 3435, 3044, 2935, 1676, 1601, 1493, 1434, 1113 cm−1; EIMS: m/z (rel. int.): 288 (M+, 29), 270 (5), 178 (13), 177 (100), 112 (33); HREIMS: 288.1363 (C17H20O4+, calc. 288.1362); CD (MeOH): Δε392 0.47, Δε334 1.10 (dm3 mol−1 cm−1); 1H-NMR: see Table 1; 13C-NMR: see Table 2.
3.8. In Vitro Mushroom Tyrosinase Inhibition Assay
The mushroom tyrosinase inhibitory assay has been described in detail previously [79]. Briefly, various concentrations of test compounds (100 μL) and 2 mM l-tyrosine (80 μL, Sigma-Aldrich, St. Louis, MO, USA) were mixed in 96-well plates. 20 μL of mushroom tyrosinase (1000 U/mL, Sigma-Aldrich, USA) was added to initiate the assay. The absorbance of the mixture was measured at 490 nm using a microplate reader (μQuant™, BioTek Winooski, VT, USA). The percentage inhibition of mushroom tyrosinase was calculated according to the following equation: Inhibition (%) = [1 − (S − SB)/(C − CB)] × 100%, where S, SB, C, and CB are the absorbance of the sample, the blank sample, the control, and the blank control, respectively.
4. Conclusions
Four new flavans, (2S)-4’-hydroxy-6,7-dimethoxyflavan (1), (2S)-3’,4’-dihydroxy-7-methoxy-8-methylflavan (2), (2S)-2’-hydroxy-7-methoxyflavan (3), and (2S,1’S)-4-hydroxy-4-(7-methoxy-8-methylchroman-2-yl)-cyclohex-2-enone (4), along with 67 known compounds were isolated from whole plants (roots and the aerial part) of D. ensifolia. Selected compounds of D. ensifolia display anti-tyrosinase properties. The presence of 2′,4′-resorcinol (11 and 14) or catechol (2 and 20) moiety in the ring B of flavonoids appears to be required for the anti-tyrosinase activity. Although the detailed mechanism of action of these compounds remains to be determined, the results confirmed that D. ensifolia is a valuable source of herbal medicine from which natural-based whitening agents can be derived.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11162142/s1, Figure S1: The chemical structures of compounds 1–71, and Figures S2–S33: the IR, EIMS, 1D- and 2D-NMR spectra of compounds 1–4.
Author Contributions
Conceptualization, Y.-H.K. and H.-H.K.; methodology, S.-H.S., J.-C.H., C.-Y.C. and H.-H.K.; validation, Y.-C.C., P.-J.S., Y.-H.K. and H.-H.K.; investigation, Y.-C.C., S.-H.S. and J.-C.H.; writing—original draft preparation, Y.-C.C.; writing—review and editing, H.-H.K., Y.-F.C. and Y.-H.K.; funding acquisition, Y.-H.K. and H.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Kaohsiung Medical University grant in Taiwan (KMU-M109024, M110027, and M111004), China Medical University grant in Taiwan (CMU110-Z-08 and CMU109-AWARD-02), and “Chinese Medicine Research Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CHM-2-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary material.
Acknowledgments
We are grateful to Chih-Chuang Liaw (Department of Marine Biotechnology and Resources, National Sun Yat-Sen University) for recording the CD spectrum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plants of the World Online, Royal Botanic Gardens, Kew. Dianella Lam. Ex Juss. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:24187-1 (accessed on 13 April 2022).
- Plants of the World Online, Royal Botanic Gardens, Kew. Asphodelaceae Juss. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:60006629-3 (accessed on 13 April 2022).
- Ying, S.S. Liliaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 2000; Volume 5, pp. 35–71. [Google Scholar]
- Widyaning, E.A.; Rahayu, I.; Timotius, K.H. Ethno medical uses, phytochemistry and pharmacology of Dianella ensifolia (Linnaeus) de Candolle: A systematic review. Int. J. Herb. Med. 2020, 8, 10–18. [Google Scholar]
- Ho, Y.L.; Chang, Y.S. Compendium of Medicinal Plants Used by the Indigenous People of Taiwan, 2nd ed.; Ministry of Health and Welfare: Taipei, Taiwan, 2018; pp. 65–66. [Google Scholar]
- Lojanapiwatna, V.; Chancharoen, K.; Sakarin, K.; Wiriyachitra, P. Chemical constituents of Dianella ensifolia redoute. J. Sci. Soc. Thail. 1982, 8, 95–102. [Google Scholar] [CrossRef]
- Randrianasolo, R.; Raharinirina, A.; Rasoanaivo, H.L.; Krebs, H.C.; Raharisolololao, A.; Razakarivony, A.A.; Rakotondramanga, M.F. A new dihydronaphtaquinone from Dianella ensifolia L. Redout. J. Pharmacogn. Phytochem. 2015, 3, 140–144. [Google Scholar]
- Nhung, L.T.H.; Linh, N.T.T.; Cham, B.T.; Thuy, T.T.; Tam, N.T.; Thien, D.D.; Huong, P.T.M.; Tan, V.M.; Tai, B.H.; Anh, N.T.H. New phenolics from Dianella ensifolia. Nat. Prod. Res. 2021, 35, 3063–3070. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Q.; Huang, S.S.; Liang, Y.E.; Sun, J.B.; Ma, Y.; Zeng, B.; Lee, S.M.Y.; Lu, J.L. Two new flavans from the roots of Dianella ensifolia (L.) DC. Nat. Prod. Res. 2017, 31, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Q.; Chen, Z.Y.; Sun, J.B.; Lee, S.M.Y.; Lu, J.L. Phytochemical and chemotaxonomic study on Dianella ensifolia (L.) DC. Biochem. Syst. Ecol. 2017, 72, 12–14. [Google Scholar] [CrossRef]
- Dias, D.A.; Silva, C.A.; Urban, S. Naphthalene aglycones and glycosides from the Australian medicinal plant, Dianella callicarpa. Planta Med. 2009, 75, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Shen, X.Y.; Cheng, Z.Y.; Wang, R.L.; Lai, P.X.; Xing, X. Chemical composition, antibacterial, antioxidant and cytotoxic activities of the essential oil of Dianella ensifolia. Rec. Nat. Prod. 2020, 14, 160–165. [Google Scholar] [CrossRef]
- Tang, B.Q.; Li, C.W.; Sun, J.B.; Chang, Y.; Chan, J.Y.W.; Lee, S.M.Y.; Zeng, B. A new cycloartane-type triterpenoid from the roots of Dianella ensifolia (L.) DC. Nat. Prod. Res. 2017, 31, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xu, B.T.; Xu, X.R.; Qin, X.S.; Gan, R.Y.; Li, H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules 2010, 15, 8602–8617. [Google Scholar] [CrossRef]
- Nesterov, A.; Zhao, J.; Minter, D.; Hertel, C.; Ma, W.; Abeysinghe, P.; Hong, M.; Jia, Q. 1-(2,4-dihydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl) propane, a novel tyrosinase inhibitor with strong depigmenting effects. Chem. Pharm. Bull. 2008, 56, 1292–1296. [Google Scholar] [CrossRef]
- Mammone, T.; Muizzuddin, N.; Declercq, L.; Clio, D.; Corstjens, H.; Sente, I.; Rillaer, K.V.; Matsui, M.; Niki, Y.; Ichihashi, M.; et al. Modification of skin discoloration by a topical treatment containing an extract of Dianella ensifolia: A potent antioxidant. J. Cosmet. Dermatol. 2010, 9, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.J.; Reynolds, G.D.; O’Leary, M.C.; Flower, R.L.P. Screening of Australian medicinal plants for antiviral activity. J. Ethnopharmacol. 1998, 60, 163–172. [Google Scholar] [CrossRef]
- Semple, S.J.; Pyke, S.M.; Reynolds, G.D.; Flower, R.L.P. In vitro antiviral activity of the anthraquinone chrysophanic acid against poliovirus. Antivir. Res. 2001, 49, 169–178. [Google Scholar] [CrossRef]
- Niki, Y.; Yoshida, M.; Ando, H.; Wakamatsu, K.; Ito, S.; Harada, N.; Matsui, M.S.; Yarosh, D.B.; Ichihashi, M. 1-(2,4-Dihydroxyphenyl)-3-(2,4-dimethoxy-3-methylpheny) propane inhibits melanin synthesis by dual mechanisms. J. Dermatol. Sci. 2011, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Garratt, P.J.; Land, E.J.; Pavel, S.; Ramsden, C.A.; Riley, P.A.; Smit, N.P.M. Evidence of the indirect formation of the catecholic intermediate substrate responsible for the autoactivation kinetics of tyrosinase. J. Biol. Chem. 1997, 272, 26226–26235. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Kothari, S.; Chavan, B.; Spencer, J.D. Regulation of melanogenesis-controversies and new concepts. Exp. Dermatol. 2008, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef]
- Chang, T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Hałdys, K.; Goldeman, W.; Jewgiński, M.; Wolińska, E.; Anger, N.; Rossowska, J.; Latajka, R. Inhibitory properties of aromatic thiosemicarbazones on mushroom tyrosinase: Synthesis, kinetic studies, molecular docking and effectiveness in melanogenesis inhibition. Bioorg. Chem. 2018, 81, 577–586. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickamb, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Shirota, O.; Sekita, S.; Hirayama, Y.; Hakamata, Y.; Hayashi, T.; Yanagawa, T.; Satake, M. Antiandrogenic phenolic constituents from Dalbergia cochinchinensis. Phytochemistry 1997, 46, 1219–1223. [Google Scholar] [CrossRef]
- Achenbach, H.; Stöcker, M.; Constenla, M.A. Flavonoid and other constituents of Bauhinia manca. Phytochemistry 1988, 27, 1835–1841. [Google Scholar] [CrossRef]
- Pan, W.B.; Chang, F.R.; Wei, L.M.; Wu, Y.C. New flavans, spirostanol sapogenins, and a pregnane genin from Tupistra chinensis and their cytotoxicity. J. Nat. Prod. 2003, 66, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Masaoud, M.; Ripperger, H.; Porzel, A.; Adam, G. Flavonoids of dragon’s blood from Dracaena cinnabari. Phytochemistry 1995, 38, 745–749. [Google Scholar] [CrossRef]
- Ali, A.A.; Makboul, M.A.; Attia, A.A.; Ali, D.T. Chromones and flavans from Pancratium maritimum. Phytochemistry 1990, 29, 625–627. [Google Scholar] [CrossRef]
- Ioset, J.R.; Marston, A.; Gupta, M.P.; Hostettmann, K. A methylflavan with free radical scavenging properties from Pancratium littorale. Fitoterapia 2001, 72, 35–39. [Google Scholar] [CrossRef]
- Tang, B.; Li, M.; Yu, G. Extraction of New Flavanes from Roots of Dianella ensifolia. Faming Zhuanli Shenqing. 2021. Available online: https://worldwide.espacenet.com/patent/search/family/075313538/publication/CN112625048A?q=CN112625048A:CN112625048A20210409 (accessed on 16 June 2022).
- Su, Y.S.; Chen, J.J.; Cheng, M.J.; Chai, C.Y.; Kwan, A.L.; Huang, J.C.; Kuo, Y.H. Saccharpiscinols A-C: Flavans with potential anti-inflammatory activities from one actinobacteria Saccharomonospora piscinae. Molecules 2021, 26, 4909. [Google Scholar] [CrossRef] [PubMed]
- Yahara, S.; Ogata, T.; Saijo, R.; Konishi, R.; Yamahara, J.; Miyahara, K.; Nohara, T. Isoflavan and related compounds from Dalbergia odorifera. I. Chem. Pharm. Bull. 1989, 37, 979–987. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Ramadan, M.A.; Khalifa, A.A. Acetophenones, a chalcone, a chromone and flavonoids from Pancratium maritimum. Phytochemistry 1998, 49, 2579–2583. [Google Scholar] [CrossRef]
- Takahashi, H.; Hirata, S.; Minami, H.; Fukuyama, Y. Triterpene and flavanone glycoside from Rhododendron simsii. Phytochemistry 2001, 56, 875–879. [Google Scholar] [CrossRef]
- Kishimoto, Y. Pharmaceutical studies on ferns. XI. Flavonoids of Cyrtomium species (3). Constitution of cyrtominetin and cyrtopterinetin. Chem. Pharm. Bull. 1956, 4, 24–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, Y.R.; Song, A.X.; Wang, H.Q. Chemical components of Seriphidium santolium Poljak. J. Chin. Chem. Soc. 2004, 51, 629–636. [Google Scholar] [CrossRef]
- Lee, D.; Bhat, K.P.L.; Fong, H.H.S.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod. 2001, 64, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.R.; Sujatha, K.; Kapil, R.S. Phenolic oxidative coupling with hypervalent organo iodine compound (diacetoxyiodo) benzene. Tetrahedron Lett. 1990, 31, 1351–1352. [Google Scholar] [CrossRef]
- Veitch, N.C.; Sutton, P.S.E.; Kite, G.C.; Ireland, H.E. Six new isoflavones and a 5-deoxyflavonol glycoside from the leaves of Ateleia herbert-smithii. J. Nat. Prod. 2003, 66, 210–216. [Google Scholar] [CrossRef]
- Tane, P.; Ayafor, J.F.; Sondengam, B.L.; Connolly, J.D. Chromone glycosides from Schumanniophyton magnificum. Phytochemistry 1990, 29, 1004–1007. [Google Scholar] [CrossRef]
- Schmid, H.; Bolleter, A. Über die inhaltstoffe von Eugenia caryophyllata (L.) Thunbg. IV. Isolierung des isoeugenitols. Helv. Chim. Acta 1949, 32, 1358–1360. [Google Scholar] [CrossRef]
- Buske, A.; Schmidt, J.; Porzei, A.; Adam, G. Benzopyranones and ferulic acid derivatives from Antidesma membranaceum. Phytochemistry 1997, 46, 1385–1388. [Google Scholar] [CrossRef]
- Saá, J.M.; Dopico, M.; Martorell, G.; Garcia-Raso, A. Deoxygenation of highly hindered phenols. J. Org. Chem. 1990, 55, 991–995. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Fürstner, A.; Castanet, A.S.; Radkowski, K.; Lehmann, C.W. Total synthesis of (S)-(+)-citreofuran by ring closing alkyne metathesis. J. Org. Chem. 2003, 68, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bakshi, K.; Zavalij, P.; Doyle, M.P. Pericyclic reaction of a zwitterionic salt of an enedione-diazoester. A novel strategy for the synthesis of highly functionalized resorcinols. Org. Lett. 2010, 12, 4304–4307. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Oshima, T.; Koshio, K.; Itsuzaki, Y.; Anzai, J. Tyrosinase inhibitor from black rice bran. J. Agric. Food Chem. 2003, 51, 6953–6956. [Google Scholar] [CrossRef] [PubMed]
- Baik, W.; Lee, H.J.; Jang, J.M.; Koo, S.; Kim, B.H. NBS-promoted reactions of symmetrically hindered methylphenols via p-benzoquinone methide. J. Org. Chem. 2000, 65, 108–115. [Google Scholar] [CrossRef]
- Lee, C.K.; Lu, C.K.; Kuo, Y.H.; Chen, J.Z.; Sun, G.Z. New prenylated flavones from the roots of Ficus beecheyana. J. Chin. Chem. Soc. 2004, 51, 437–441. [Google Scholar] [CrossRef]
- Scott, A.L.; Guilford, H.; Ryan, J.J.; Skingle, D. Biogenetic-type synthesis of polyketides part VIII: Experiments with the tetra- and hexa-acetate systems. Tetrahedron 1971, 27, 3025–3038. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The constituents of Lindera glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Webb, K.S.; Ruszkay, S.J. Oxidation of aldehydes with Oxone® in aqueous acetone. Tetrahedron 1998, 54, 401–410. [Google Scholar] [CrossRef]
- Zara, C.L.; Jin, T.; Giguere, R.J. Microwave heating in organic synthesis: Decarboxylation of malonic acid derivatives in water. Synth. Commun. 2000, 30, 2099–2104. [Google Scholar] [CrossRef]
- Suga, T.; Ohta, S.; Munesada, K.; Ide, N.; Kurokawa, M.; Shimizu, M.; Ohta, E. Endogeneous pine wood nematicidal substances in pines, Pinus massoniana, P. strobus and P. palustris. Phytochemistry 1993, 33, 1395–1401. [Google Scholar] [CrossRef]
- Masuda, T.; Hidaka, K.; Shinohara, A.; Maekawa, T.; Takeda, Y.; Yamaguchi, H. Chemical studies on antioxidant mechanism of curcuminoid: Analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999, 47, 71–77. [Google Scholar] [CrossRef]
- Addae-Mensah, I.; Achenbach, H.; Thoithi, G.N.; Waibel, R.; Mwangi, J.W. Epoxychiromodine and other constituents of Croton megalocarpus. Phytochemistry 1992, 31, 2055–2058. [Google Scholar] [CrossRef]
- Kawanushi, K.; Hashimoto, Y. Long chain esters of Virola species. Phytochemistry 1987, 26, 749–752. [Google Scholar] [CrossRef]
- Hylands, P.J.; Ingolfsdottir, K. The isolation of methyl β-orsellinate from Stereocaulon alpinum and comments on the isolation of 4,6-dihydroxy-2-methoxy-3-methylacetophenone from Stereocaulon species. Phytochemistry 1985, 24, 127–129. [Google Scholar] [CrossRef]
- Della Greca, M.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Jain, T.C.; Banks, C.M. 22-Dihydrostigmasterol from Saussurea lappa Clarke. Can. J. Chem. 1968, 46, 2325–2327. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Li, Y.C. Constituents of the bark of Ficus microcarpa L.f. J. Chin. Chem. Soc. 1997, 44, 321–325. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Wu, Y.C. The constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1997, 44, 313–319. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chu, P.H. Studies on the constituents from the bark of Bauhinia purpurea. J. Chin. Chem. Soc. 2002, 49, 269–274. [Google Scholar] [CrossRef]
- Jones, S.R.; Selinsky, B.S.; Rao, M.N.; Zhang, X.; Kinney, W.A.; Tham, F.S. Efficient route to 7α-(benzoyloxy)-3-dioxolane cholestan-24(R)-ol, a key intermediate in the synthesis of squalamine. J. Org. Chem. 1998, 63, 3786–3789. [Google Scholar] [CrossRef]
- Kimura, J.; Maki, N. New loliolide derivatives from the brown alga Undaria pinnatifida. J. Nat. Prod. 2002, 65, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Della Greca, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Cycloartane triterpenes from Juncus effuses. Phytochemistry 1994, 35, 1017–1022. [Google Scholar] [CrossRef]
- Matsunaga, S.; Tanaka, R.; Akagi, M. Triterpenoids from Euphorbia maculata. Phytochemistry 1988, 27, 535–537. [Google Scholar] [CrossRef]
- Tanaka, R.; Matsunaga, S. Triterpene dienols and other constituents from the bark of Phyllanthus flexuosus. Phytochemistry 1988, 27, 2273–2277. [Google Scholar] [CrossRef]
- Khachik, F.; Chang, A.N. Total synthesis of (3R,3′R,6′R)-lutein and its stereoisomers. J. Org. Chem. 2009, 74, 3875–3885. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.; Nakano, H.; Yamada, K.; Shigemori, H.; Hasegawa, K. Isolation and identification of lateral bud growth inhibitor, indole-3-aldehyde, involved in apical dominance of pea seedlings. Phytochemistry 2002, 61, 863–865. [Google Scholar] [CrossRef]
- Chiji, H.; Arakawa, Y.; Ueda, S.; Kuroda, M.; Izawa, M. 5,2′-Dihydroxy-6,7-methylenedioxyisoflavone from seed balls of sugar beet. Phytochemistry 1986, 25, 281–282. [Google Scholar]
- Radeglia, R.; Poleschner, H.; Heydenreich, M. The (Z)/(E)-configurational analysis of isolated double bonds in pheromones and unsaturated fatty acids. The use of 1D and 2D J.-resolved 1H CW off-resonance techniques in 13C NMR spectroscopy. Magn. Reson. Chem. 1991, 29, 1028–1035. [Google Scholar] [CrossRef]
- Casu, M.; Lai, A.; Erriu, G.; Onnis, S.; Zucca, N. Nuclear magnetic resonance evidence for radiation damage of saturated phosphatidylcholine in bilayers. Magn. Reson. Chem. 1992, 30, 408–412. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Yoshikawa, K.; Shimizu, K.; Konda, R. Isoprenoid-substituted flavonoids from wood of Artocarpus heterophyllus on B16 melanoma cells: Cytotoxicity and structural criteria. Fitoterapia 2010, 81, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.C.; Tzeng, C.W.; Lin, C.C.; Yen, F.L.; Ko, H.H. Prenylated flavonoids from Artocarpus altilis: Antioxidant activities and inhibitory effects on melanin production. Phytochemistry 2013, 89, 78–88. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).