The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.)
Abstract
1. Introduction
2. The Interconnected Sub-Compartments of the Pea Seed
3. Sensing and Transmission through the Seed Coat
4. Maternal Inputs to the Developing Pea Seed
5. The Multi-Layered Legume Seed Coat
6. Seed Dormancy, Imbibition, and Permeability
7. Contributions of the Seed Coat to Diet and Nutrition
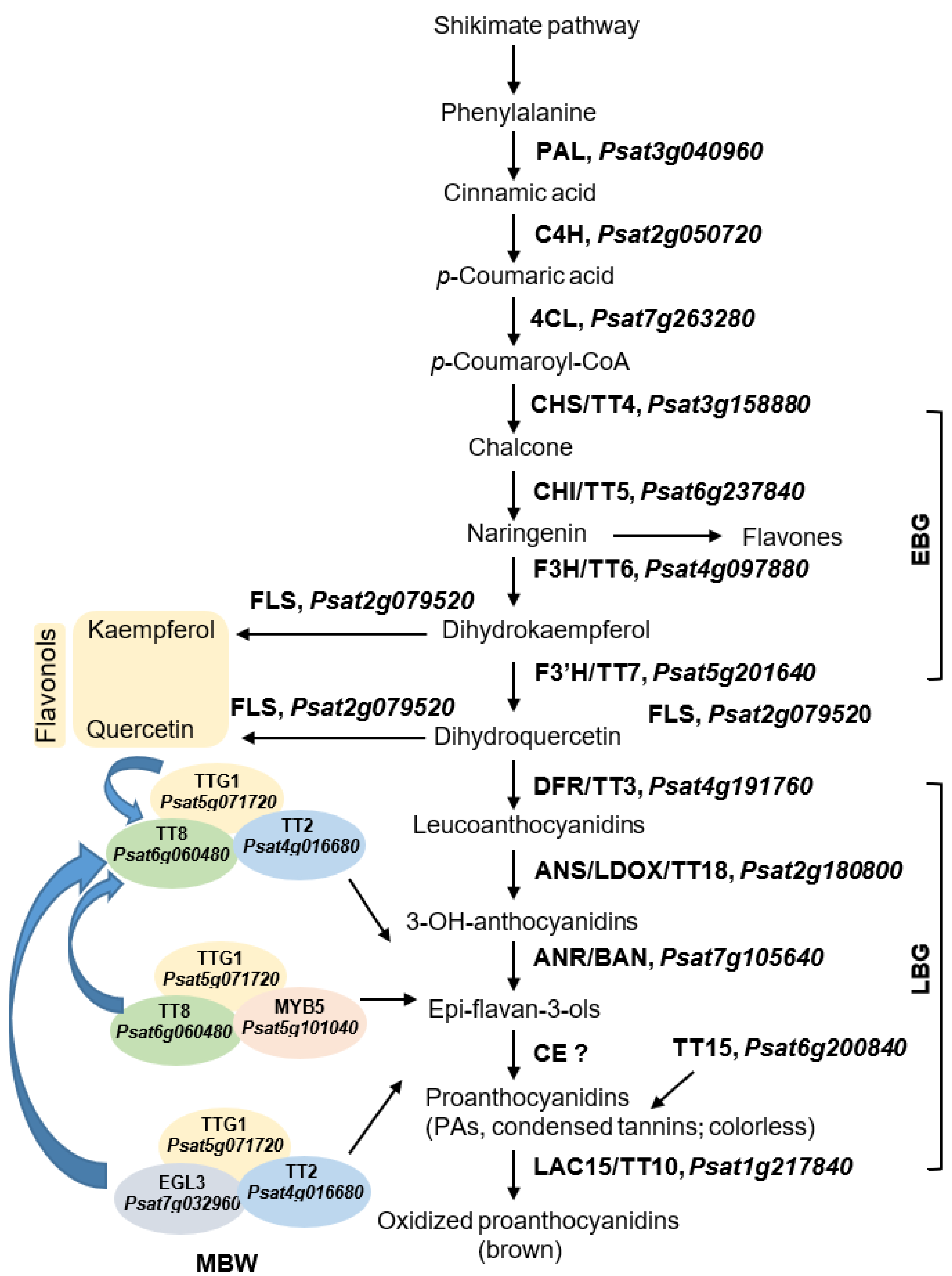
8. Seed Coat Chemistry
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzaki, T.; Nishida, H. Autoregulation of legume nodulation by sophisticated transcriptional regulatory networks. Mol. Plant. 2019, 12, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plant. 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Sun, B.; Hernández, T.; Estrella, I.; Spranger, M.I. Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.). J. Agric. Food Chem. 2003, 51, 7999–8004. [Google Scholar] [CrossRef]
- Smýkal, P.; Aubert, G.; Burstin, J.; Coyne, C.J.; Ellis, N.T.H.; Flavell, A.J.; Ford, R.; Hýbl, M.; Macas, J.; Neumann, P.; et al. Pea (Pisum sativum L.) in the genomic era. Agronomy 2012, 2, 74–115. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108 Suppl. S1, S3–S10. [Google Scholar] [CrossRef]
- Macas, J.; Neumann, P.; Navrátilová, A. Repetitive DNA in the pea (Pisum sativum L.) genome: Comprehensive characterization using 454 sequencing and comparison to soybean and Medicago truncatula. BMC Genom. 2007, 8, 427. [Google Scholar] [CrossRef]
- Tayeh, N.; Klein, A.; Le Paslier, M.-C.; Jacquin, F.; Houtin, H.; Rond, C.; Chabert-Martinello, M.; Magnin-Robert, J.-B.; Marget, P.; Aubert, G.; et al. Genomic prediction in pea: Effect of marker density and training population size and composition on prediction accuracy. Front. Plant. Sci. 2015, 6, 941. [Google Scholar] [CrossRef]
- Smýkal, P.; Hradilová, I.; Trněný, O.; Brus, J.; Rathore, A.; Bariotakis, M.; Das, R.R.; Bhattacharyya, D.; Richards, C.; Coyne, C.J.; et al. Genomic diversity and macroecology of the crop wild relatives of domesticated pea. Sci. Rep. 2017, 7, 17384. [Google Scholar] [CrossRef]
- Kreplak, J.; Madoui, M.-A.; Cápal, P.; Novák, P.; Labadie, K.; Aubert, G.; Bayer, P.E.; Gali, K.K.; Syme, R.A.; Main, D.; et al. A reference genome for pea provides insight into legume genome evolution. Nat. Genet. 2019, 51, 1411–1422. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Paiva, G.d.; Yadegari, R. Plant embryogenesis: Zygote to seed. Science 1994, 266, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Gehring, M.; Choi, Y.; Fischer, R.L. Imprinting and seed development. Plant. Cell 2004, 16, S203–S213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laux, T.; Würschum, T.; Breuninger, H. Genetic regulation of embryonic pattern formation. Plant. Cell 2004, 16 (Suppl. S1), S190–S202. [Google Scholar] [CrossRef] [PubMed]
- Moïse, J.A.; Han, S.; Gudynaitę-Savitch, L.; Johnson, D.A.; Miki, B.L.A. Seed coats: Structure, development, composition, and biotechnology. In Vitro Cell. Dev. Biol. Plant. 2005, 41, 620–644. [Google Scholar] [CrossRef]
- de Vries, S.C.; Weijers, D. Plant embryogenesis. Curr. Biol. 2017, 27, R870–R873. [Google Scholar] [CrossRef]
- Le, B.H.; Wagmaister, J.A.; Kawashima, T.; Bui, A.Q.; Harada, J.J.; Goldberg, R.B. Using genomics to study legume seed development. Plant. Physiol. 2007, 144, 562–574. [Google Scholar] [CrossRef]
- Roszak, P.; Köhler, C. Polycomb group proteins are required to couple seed coat initiation to fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 20826–20831. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Ammerlaan, A.M.; Wouterlood, M.; Van Aelst, A.C.; Borstlap, A.C. Structure of the developing pea seed coat and the post-phloem transport pathway of nutrients. Ann. Bot. 2003, 91, 729–737. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar]
- Weber, H.; Borisjuk, L.; Wobus, U. Molecular physiology of legume seed development. Annu. Rev. Plant. Biol. 2005, 56, 253–279. [Google Scholar] [CrossRef]
- Malovichko, Y.V.; Shtark, O.Y.; Vasileva, E.N.; Nizhnikov, A.A.; Antonets, K.S. Transcriptomic insights into mechanisms of early seed maturation in the garden pea (Pisum sativum L.). Cells 2020, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Obala, J.; Saxena, R.K.; Singh, V.K.; Kale, S.M.; Garg, V.; Kumar, C.V.S.; Saxena, K.B.; Tongoona, P.; Sibiya, J.; Varshney, R.K. Seed protein content and its relationships with agronomic traits in pigeonpea is controlled by both main and epistatic effects qtls. Sci. Rep. 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.G.; Hagedorn, D.J.; DeLoughery, R. Influence of herbicides on root rot in processing peas 1. Crop. Sci. 1975, 15, 67–71. [Google Scholar] [CrossRef]
- Grusak, M.A. Enhancing mineral content in plant food products. J. Am. Coll. Nutr. 2002, 21, 178S–183S. [Google Scholar] [CrossRef]
- Wang, Z.P.; Deloire, A.; Carbonneau, A.; Federspiel, B.; Lopez, F. An in vivo experimental system to study sugar phloem unloading in ripening grape berries during water deficiency stress. Ann. Bot. 2003, 92, 523–528. [Google Scholar] [CrossRef][Green Version]
- Haughn, G.; Chaudhury, A. Genetic analysis of seed coat development in arabidopsis. Trends Plant. Sci. 2005, 10, 472–477. [Google Scholar] [CrossRef]
- Hardham, A.R. Structural aspects of the pathways of nutrient flow to the developing embryo and cotyledons of Pisum sativum L. Aust. J. Bot. 1976, 24, 711–721. [Google Scholar] [CrossRef]
- Patrick, J.; Offler, C. Post-sieve element transport of sucrose in developing seeds. Funct. Plant. Biol. 1995, 22, 681–702. [Google Scholar] [CrossRef]
- Patrick, J.W.; Offler, C.E. Compartmentation of transport and transfer events in developing seeds. J. Exp. Bot. 2001, 52, 551–564. [Google Scholar] [CrossRef]
- Lanfermeijer, F.C.; Koerselman-Kooij, J.W.; Borstlap, A.C. Changing kinetics of l-valine uptake by immature pea cotyledons during development. Planta 1990, 181, 576–582. [Google Scholar] [CrossRef]
- Radchuk, V.; Borisjuk, L. Physical, metabolic and developmental functions of the seed coat. Front. Plant Sci. 2014, 5, 510. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Firnhaber, C.; Zuber, H.; Héricher, D.; Belghazi, M.; Henry, C.; Küster, H.; Thompson, R. A combined proteome and transcriptome analysis of developing Medicago truncatula seeds: Evidence for metabolic specialization of maternal and filial tissues *. Mol. Cell. Proteom. 2007, 6, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Weber, H. Controlling seed development and seed size in vicia faba: A role for seed coat-associated invertases and carbohydrate state. Plant. J. 1996, 10, 823–834. [Google Scholar] [CrossRef]
- Weber, H.; Borisjuk, L.; Heim, U.; Buchner, P.; Wobus, U. Seed coat: Associated invertases of fava bean control both unloading and storage functions: Cloning of cdnas and cell type: Specific expression. Plant. Cell 1995, 7, 1835–1846. [Google Scholar]
- Farley, S.; Patrick, J.; Offler, C. Functional transfer cells differentiate in cultured cotyledons of Vicia faba L. Seeds. Protoplasma 2000, 214, 102–117. [Google Scholar] [CrossRef]
- Offler, C.E.; Liet, E.; Sutton, E.G. Transfer cell induction in cotyledons of Vicia faba L. Protoplasma 1997, 200, 51–64. [Google Scholar] [CrossRef]
- Borisjuk, L.; Walenta, S.; Weber, H.; Mueller-Klieser, W.; Wobus, U. High-resolution histographical mapping of glucose concentrations in developing cotyledons of Vicia faba L. in relation to mitotic activity and storage processes: Glucose as a possible developmental trigger. Plant. J. 1998, 15, 583–591. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Taliercio, E.W.; Chourey, P.S. Sugars modulate an unusual mode of control of the cell-wall invertase gene (INCW1) through its 3′ untranslated region in a cell suspension culture of maize. Proc. Natl. Acad. Sci. USA 1999, 96, 10512–10517. [Google Scholar] [CrossRef]
- Sturm, A.; Tang, G.Q. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant. Sci. 1999, 4, 401–407. [Google Scholar] [CrossRef]
- Tegeder, M.; Wang, X.D.; Frommer, W.B.; Offler, C.E.; Patrick, J.W. Sucrose transport into developing seeds of Pisum sativum L. Plant. J. 1999, 18, 151–161. [Google Scholar] [CrossRef]
- Borisjuk, L.; Wang, T.L.; Rolletschek, H.; Wobus, U.; Weber, H. A pea seed mutant affected in the differentiation of the embryonic epidermis is impaired in embryo growth and seed maturation. Development 2002, 129, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Offler, C.E.; McCurdy, D.W.; Patrick, J.W.; Talbot, M.J. Transfer cells: Cells specialized for a special purpose. Annu. Rev. Plant. Biol. 2003, 54, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Zhou, Y.; Dibley, K.E.; Tyerman, S.D.; Furbank, R.T.; Patrick, J.W. Review: Nutrient loading of developing seeds. Funct. Plant. Biol. 2007, 34, 314–331. [Google Scholar] [CrossRef]
- Fernie, A.R. A push, and a pull, to enhance nitrogen use efficiency in rice. Plant. J. 2020, 103, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Doidy, J.; Vidal, U.; Lemoine, R. Sugar transporters in fabaceae, featuring sut mst and sweet families of the model plant medicago truncatula and the agricultural crop pisum sativum. PLoS ONE 2019, 14, e0223173. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant. J. 2020, 101, 217–236. [Google Scholar] [CrossRef]
- Zhang, L.; Garneau, M.G.; Majumdar, R.; Grant, J.; Tegeder, M. Improvement of pea biomass and seed productivity by simultaneous increase of phloem and embryo loading with amino acids. Plant. J. 2015, 81, 134–146. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Improving plant nitrogen use efficiency through alteration of amino acid transport processes. Plant. Physiol. 2017, 175, 235–247. [Google Scholar] [CrossRef]
- Burstin, J.; Marget, P.; Huart, M.; Moessner, A.; Mangin, B.; Duchene, C.; Desprez, B.; Munier-Jolain, N.; Duc, G. Developmental genes have pleiotropic effects on plant morphology and source capacity, eventually impacting on seed protein content and productivity in pea. Plant. Physiol. 2007, 144, 768–781. [Google Scholar] [CrossRef]
- Dean, G.; Cao, Y.; Xiang, D.; Provart, N.J.; Ramsay, L.; Ahad, A.; White, R.; Selvaraj, G.; Datla, R.; Haughn, G. Analysis of gene expression patterns during seed coat development in arabidopsis. Mol. Plant. 2011, 4, 1074–1091. [Google Scholar] [CrossRef]
- Ranathunge, K.; Shao, S.; Qutob, D.; Gijzen, M.; Peterson, C.A.; Bernards, M.A. Properties of the soybean seed coat cuticle change during development. Planta 2010, 231, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A regulatory network-based approach dissects late maturation processes related to the acquisition of desiccation tolerance and longevity of medicago truncatula seeds. Plant. Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chan, K.; Wang, T.L.; Hedley, C.L.; Offler, C.E.; Patrick, J.W. Intracellular sucrose communicates metabolic demand to sucrose transporters in developing pea cotyledons. J. Exp. Bot. 2009, 60, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Kladnik, A.; Chamusco, K.; Dermastia, M.; Chourey, P. Evidence of programmed cell death in post-phloem transport cells of the maternal pedicel tissue in developing caryopsis of maize. Plant. Physiol. 2004, 136, 3572–3581. [Google Scholar] [CrossRef]
- Young, N.D.; Debellé, F.; Oldroyd, G.E.D.; Geurts, R.; Cannon, S.B.; Udvardi, M.K.; Benedito, V.A.; Mayer, K.F.X.; Gouzy, J.; Schoof, H.; et al. The medicago genome provides insight into the evolution of rhizobial symbioses. Nature 2011, 480, 520–524. [Google Scholar] [CrossRef]
- Debeaujon, I.; Lepiniec, L.; Pourcel, L.; Routaboul, J.-M. Seed Coat Development and Dormancy; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 25–49. [Google Scholar]
- Weber, H.; Borisjuk, L.; Wobus, U. Sugar import and metabolism during seed development. Trends Plant. Sci. 1997, 2, 169–174. [Google Scholar] [CrossRef]
- Rochat, C.; Boutin, J.P. Temporary storage compounds and sucrose-starch metabolism in seed coats during pea seed development (pisum sativum). Physiol. Plant. 1992, 85, 567–572. [Google Scholar] [CrossRef]
- Vigeolas, H.; Möhlmann, T.; Martini, N.; Neuhaus, H.E.; Geigenberger, P. Embryo-specific reduction of adp-glc pyrophosphorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant. Physiol. 2004, 136, 2676–2686. [Google Scholar] [CrossRef]
- Abirached-Darmency, M.; Abdel-gawwad, M.R.; Conejero, G.; Verdeil, J.L.; Thompson, R. In situ expression of two storage protein genes in relation to histo-differentiation at mid-embryogenesis in Medicago truncatula and Pisum sativum seeds. J. Exp. Bot. 2005, 56, 2019–2028. [Google Scholar] [CrossRef]
- Harris, W.M. On the development of macrosclereids in seed coats of Pisum sativum L. Am. J. Bot. 1983, 70, 1528–1535. [Google Scholar] [CrossRef]
- Shao, S.; Meyer, C.J.; Ma, F.; Peterson, C.A.; Bernards, M.A. The outermost cuticle of soybean seeds: Chemical composition and function during imbibition. J. Exp. Bot. 2007, 58, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.J.; Steudle, E.; Peterson, C.A. Patterns and kinetics of water uptake by soybean seeds. J. Exp. Bot. 2007, 58, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant. Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Ozga, J.E.; Catherine, C.; Alena, J.; Han, Y.; Zohre, H.S.; Kaiyuan, Y. Pea (Pisum sativum L.) Seed Coats and Seed Coat Fractions. U.S. Patent US-2016058813-A1, 3 March 2016. [Google Scholar]
- Hradilová, I.; Trněný, O.; Válková, M.; Cechová, M.; Janská, A.; Prokešová, L.; Aamir, K.; Krezdorn, N.; Rotter, B.; Winter, P.; et al. A combined comparative transcriptomic, metabolomic, and anatomical analyses of two key domestication traits: Pod dehiscence and seed dormancy in pea (Pisum sp.). Front. Plant. Sci. 2017, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.H.; Fortune, J.A.; Gallagher, J. Anatomical structure and nutritive value of lupin seed coats. Aust. J. Agric. Res. 2001, 52, 985–993. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J.M. Seed Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic press: Cambridge, MA, USA, 2014; pp. 1–1586. [Google Scholar]
- Janská, A.; Pecková, E.; Sczepaniak, B.; Smýkal, P.; Soukup, A. The role of the testa during the establishment of physical dormancy in the pea seed. Ann. Bot. 2019, 123, 815–829. [Google Scholar] [CrossRef]
- Niemann, S.; Riederer, M. Seed Coat Permeability of Active Ingredients: Permeabilität Von Samenschalen Für Aktivsubstanzen; Universitätsbibliothek der Universität Würzburg: Würzburg, Germany, 2013. [Google Scholar]
- Ramakrishna, G.; Kaur, P.; Nigam, D.; Chaduvula, P.K.; Yadav, S.; Talukdar, A.; Singh, N.K.; Gaikwad, K. Genome-wide identification and characterization of indels and snps in Glycine max and Glycine soja for contrasting seed permeability traits. BMC Biol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Chai, M.; Zhou, C.; Molina, I.; Fu, C.; Nakashima, J.; Li, G.; Zhang, W.; Park, J.; Tang, Y.; Jiang, Q.; et al. A class ii knox gene, knox4, controls seed physical dormancy. Proc. Natl. Acad. Sci. USA 2016, 113, 6997–7002. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant. Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by myb-bhlh-wdr complexes. Trends Plant. Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Routaboul, J.M.; Kerhoas, L.; Debeaujon, I.; Pourcel, L.; Caboche, M.; Einhorn, J.; Lepiniec, L. Flavonoid diversity and biosynthesis in seed of arabidopsis thaliana. Planta 2006, 224, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Hatcher, D.W.; Warkentin, T.; Toews, R. Effect of cultivar and environment on physicochemical and cooking characteristics of field pea (pisum sativum). Food Chem. 2010, 118, 109–115. [Google Scholar] [CrossRef]
- Blair, M.W.; Izquierdo, P.; Astudillo, C.; Grusak, M.A. A legume biofortification quandary: Variability and genetic control of seed coat micronutrient accumulation in common beans. Front. Plant Sci. 2013, 4, 275. [Google Scholar] [CrossRef]
- Stanley, D.W.; Aguilera, J.M. A review of textural defects in cooked reconstituted legumes —the influence of structure and composition. J. Food Biochem. 1985, 9, 277–323. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Y.; Li, X.; Wang, L.; Xu, S.; Yang, J.; Weng, L.; Sato, S.; Tabata, S.; Ambrose, M.; et al. Genetic control of floral zygomorphy in pea (Pisum sativum L.). Proc. Natl. Acad. Sci. USA 2008, 105, 10414–10419. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S330–S375. [Google Scholar] [CrossRef]
- Li, X.; Buirchell, B.; Yan, G.; Yang, H. A molecular marker linked to the mollis gene conferring soft-seediness for marker-assisted selection applicable to a wide range of crosses in lupin (Lupinus angustifolius L.) breeding. Mol. Breed. 2012, 29, 361–370. [Google Scholar] [CrossRef]
- Whitlock, K.A.; Kozicky, L.; Jin, A.; Yee, H.; Ha, C.; Morris, J.; Field, C.J.; Bell, R.C.; Ozga, J.A.; Chan, C.B. Assessment of the mechanisms exerting glucose-lowering effects of dried peas in glucose-intolerant rats. Br. J. Nutr. 2012, 108 (Suppl. S1), S91–S102. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M.M. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88 (Suppl. S3), S293–S306. [Google Scholar] [CrossRef]
- Dueñas, M.; Estrella, I.; Hernández, T. Occurrence of phenolic compounds in the seed coat and the cotyledon of peas (Pisum sativum L.). Eur. Food Res. Technol. 2004, 219, 116–123. [Google Scholar] [CrossRef]
- Hoover, R.; Hughes, T.; Chung, H.J.; Liu, Q. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res. Int. 2010, 43, 399–413. [Google Scholar] [CrossRef]
- Tosh, S.M.; Yada, S. Dietary fibres in pulse seeds and fractions: Characterization, functional attributes, and applications. Food Res. Int. 2010, 43, 450–460. [Google Scholar] [CrossRef]
- Devi, J.; Sanwal, S.K.; Koley, T.K.; Mishra, G.P.; Karmakar, P.; Singh, P.M.; Singh, B. Variations in the total phenolics and antioxidant activities among garden pea (Pisum sativum L.) genotypes differing for maturity duration, seed and flower traits and their association with the yield. Sci. Hortic. 2019, 244, 141–150. [Google Scholar] [CrossRef]
- Troszynska, A.; Ciska, E. Phenolic compounds of seed coats of white and coloured varieties of pea (Pisum sativum L.) and their total antioxidant activity. Czech. J. Food Sci. 2002, 20, 15–22. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Ilic, M.; Jovanović, Ž.; Cupic, T.; Dabić, D.; Natić, M.; Tesic, Z.; Radovic, S. Identification of seed coat phenolic compounds from differently colored pea varieties and characterization of their antioxidant activity. Arch. Biol. Sci. 2015, 67, 829–840. [Google Scholar] [CrossRef]
- Dueñas, M.; Hernández, T.; Estrella, I. Assessment of in vitro antioxidant capacity of the seed coat and the cotyledon of legumes in relation to their phenolic contents. Food Chem. 2006, 98, 95–103. [Google Scholar] [CrossRef]
- Fahim, J.R.; Attia, E.Z.; Kamel, M.S. The phenolic profile of pea (pisum sativum): A phytochemical and pharmacological overview. Phytochem. Rev. 2019, 18, 173–198. [Google Scholar] [CrossRef]
- Han, H.; Baik, B.-K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Wang, X.; Warkentin, T.; Briggs, C.; Oomah, B.D.; Campbell, C.; Woods, S. Total phenolics and condensed tannins in field pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.). Euphytica 1998, 101, 97–102. [Google Scholar] [CrossRef]
- Griffiths, D.W. The polyphenolic content and enzyme inhibitory activity of testas from bean (Vicia faba) and pea (Pisum spp.) varieties. J. Sci. Food Agric. 1981, 32, 797–804. [Google Scholar] [CrossRef]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in arabidopsis1. Plant. Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Peel, G.J.; Wright, E.; Wang, Z.; Dixon, R.A. Early steps in proanthocyanidin biosynthesis in the model legume medicago truncatula. Plant. Physiol. 2007, 145, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dixon, R.A. Mate transporters facilitate vacuolar uptake of epicatechin 3’-o-glucoside for proanthocyanidin biosynthesis in medicago truncatula and arabidopsis. Plant. Cell 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wenger, J.P.; Saathoff, K.; Peel, G.J.; Wen, J.; Huhman, D.; Allen, S.N.; Tang, Y.; Cheng, X.; Tadege, M.; et al. A wd40 repeat protein from medicago truncatula is necessary for tissue-specific anthocyanin and proanthocyanidin biosynthesis but not for trichome development. Plant. Physiol. 2009, 151, 1114–1129. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilichini, T.D.; Gao, P.; Yu, B.; Bing, D.; Datla, R.; Fobert, P.; Xiang, D. The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.). Plants 2022, 11, 2056. https://doi.org/10.3390/plants11152056
Quilichini TD, Gao P, Yu B, Bing D, Datla R, Fobert P, Xiang D. The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.). Plants. 2022; 11(15):2056. https://doi.org/10.3390/plants11152056
Chicago/Turabian StyleQuilichini, Teagen D., Peng Gao, Bianyun Yu, Dengjin Bing, Raju Datla, Pierre Fobert, and Daoquan Xiang. 2022. "The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.)" Plants 11, no. 15: 2056. https://doi.org/10.3390/plants11152056
APA StyleQuilichini, T. D., Gao, P., Yu, B., Bing, D., Datla, R., Fobert, P., & Xiang, D. (2022). The Seed Coat’s Impact on Crop Performance in Pea (Pisum sativum L.). Plants, 11(15), 2056. https://doi.org/10.3390/plants11152056






