Biological Pests Management for Sustainable Agriculture: Understanding the Influence of Cladosporium-Bioformulated Endophytic Fungi Application to Control Myzus persicae (Sulzer, 1776) in Potato (Solanum tuberosum L.)
Abstract
1. Introduction
2. Results
2.1. The Aphicidal Activity of Formulations
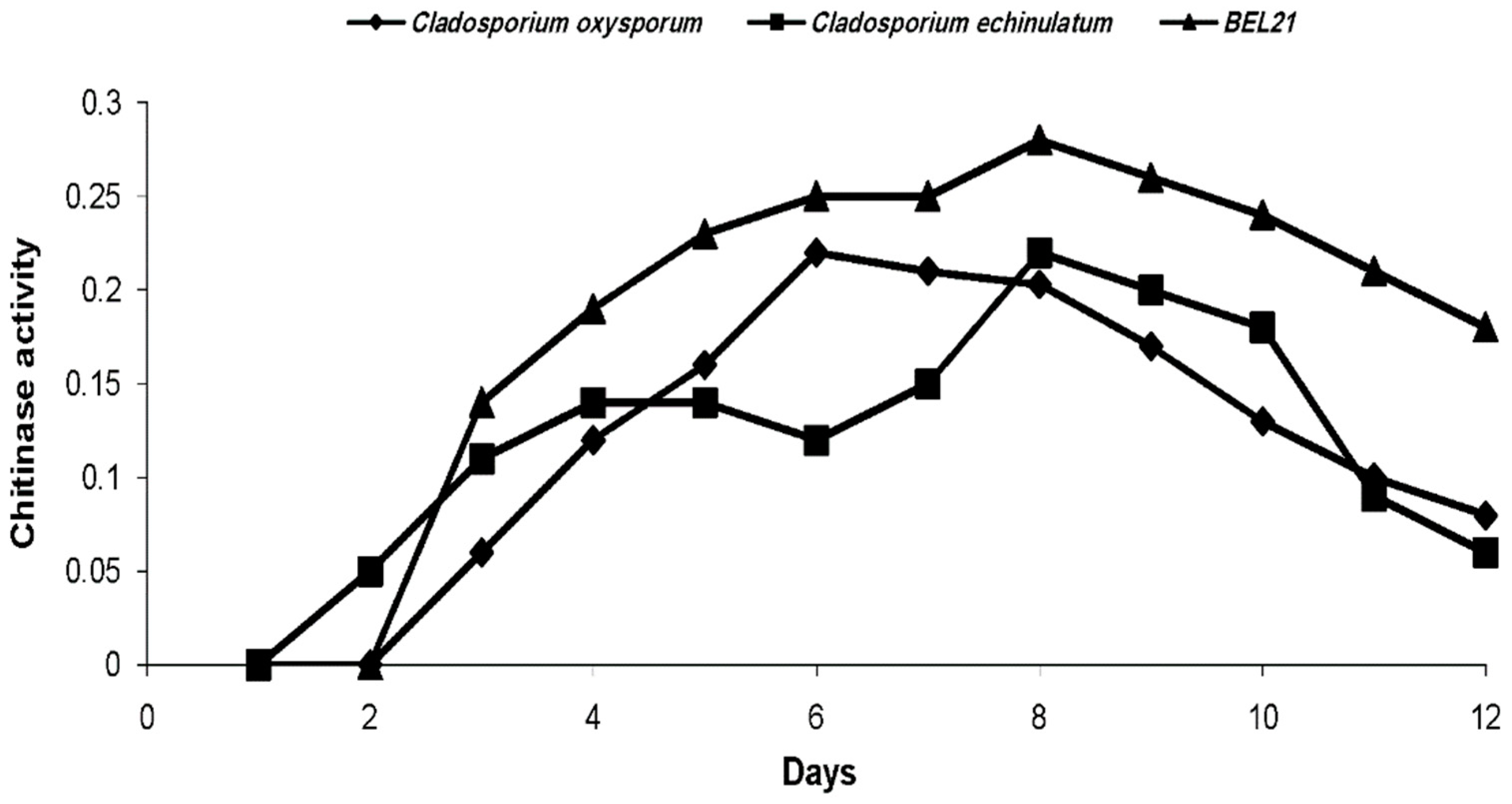
2.2. Evolution of Chitinolytic Activity of Endophytic Fungi
2.3. Effects of Plant Pre-Treatment on Aphids’ Demographic and Embryonic Parameters
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Aphids
4.3. Isolation and Preservation of Endophytic Fungi
4.4. Culture Filtrates Preparation
4.5. Formulations Conception
4.6. The Aphicidal Activity of Formulations
4.7. Evolution of Chitinolytic Activity of Endophytic Fungi
4.8. Effects of Plant Pre-Treatment on Aphid’s Demographic and Embryonic Parameters
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 14 October 2020).
- Zargar, M.; Rebouh, N.; Pakina, E.; Gadzhikurbanov, A.; Lyashko, M.; Ortskhanov, B. Impact of climate change on cereal production in the highlands of eastern Algeria. Res. Crop 2017, 18, 575–582. [Google Scholar] [CrossRef]
- Aissat, A. Etude du Secteur de la Pomme de Terre en Algérie; Agrico U.A.: Emmeloord, The Netherlands, 2013. [Google Scholar]
- Yi, X.; Gray, S.M. Aphids and their transmitted potato viruses: A continuous challenges in potato crops. J. Integr. Agric. 2020, 19, 367–375. [Google Scholar]
- Saguez, J.; Giordanengo, P.; Vincent, C. Aphids as major Potato pests. In Insect Pests of Potato: Global Perspectives on Biology and Management; Giordanengo, P., Vincent, C., Alyokhin, A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 31–63. [Google Scholar]
- Silva, A.X.; Jander, G.; Samaniego, H.; Ramsey, J.S.; Figueroa, C.C. Insecticide resistance mechanisms in the green peach aphid Myzus persicae (Hemiptera: Aphididae) I: A Transcriptomic Survey. PLoS ONE 2012, 7, e36366. [Google Scholar] [CrossRef] [PubMed]
- Laamari, M.; Coeur d’Acier, A.; Joussellin, E. Assesment of aphid diversity (Hemiptera: Aphididae) in Algeria: A fourteen-year investigation. Faun. Entomol. 2010, 62, 73–87. [Google Scholar]
- Kerlan, C. Potato viruses. In Desk Encyclopedia of Plant and Fungal Virology; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press: Barcelona, Spain, 2008; pp. 458–471. [Google Scholar]
- Gross, K.; Rosenheim, J.A. Quantifying secondary pest outbreaks in cotton and their monetary cost with causal-inference statistics. Ecol. Appl. 2011, 21, 2770–2780. [Google Scholar] [CrossRef]
- Foster, S.P.; Devonshire, A.L. Field-simulator study of insecticide resistance conferred by esterase-, MACE- and kdr-based mechanisms in the peach-potato aphid, Myzus persicae (Sulzer). Pest Manag. Sci. 1999, 55, 810–814. [Google Scholar] [CrossRef]
- Bueno, A.D.F.; Carvalho, G.A.; Santos, A.C.D.; Sosa-Gómez, D.R.; Silva, D.M.D. Pesticide selectivity to natural enemies: Challenges and constraints. Ciênc. Rural 2017, 47, e20160829. [Google Scholar]
- Gomes, H.D.O.; Menezes, J.M.C.; da Costa, J.G.M.; Coutinho, H.D.M.; Teixeira, R.N.P.; do Nascimento, R.F. A socio-environmental perspective on pesticideuse and food production. Ecotoxicol. Environ. Saf. 2020, 197, 110627. [Google Scholar] [CrossRef]
- Zemmouri, B.; Lammoglia, S.K.; Bouras, F.Z.; Seghouani, M.; Rebouh, N.Y.; Latati, M. Modelling human health risks from pesticide use in innovative legume-cereal intercropping systems in Mediterranean conditions. Ecotoxicol. Environ. Saf. 2022, 238, 113590. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Aliat, T.; Polityko, P.M.; Kherchouche, D.; Boulelouah, N.; Temirbekova, S.K.; Afanasyeva, Y.V.; Kucher, D.E.; Plushikov, V.G.; Parakhina, E.A.; et al. Environmentally Friendly Wheat Farming: Biological and Economic Efficiency of Three Treatments to Control Fungal Diseases in Winter Wheat (Triticum aestivum L.) under Field Conditions. Plants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Rebouh, N.Y.; Latati, M.; Polityko, P.; Kucher, D.; Hezla, L.; Norezzine, A.; Kalisa, L.; Utkina, A.; Vvedenskiy, V.; Gadzhikurbanov, A.; et al. Influence of three cultivation technologies to control Fusarium spp. In winter wheat (Triticum aestivum L.) production under Moscow conditions. Res. Crops 2020, 21, 17–25. [Google Scholar]
- Castillo Lopez, D.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The Entomopathogenic Fungal Endophytes Purpureocillium lilacinum (Formerly Paecilomyces lilacinus) and Beauveria bassiana Negatively Affect Cotton Aphid Reproduction under Both Greenhouse and Field Conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [PubMed]
- Bensaci, O.A.; Daoud, H.; Lombarkia, N.; Rouabah, K. Formulation of the endophytic fungus Cladosporium oxysporum Berk. & M.A. Curtis isolated from Euphorbia bupleuroides subsp. luteola, as a new biocontrol tool against the black bean aphid (Aphis fabae Scop.). J. Plant Prot. Res. 2015, 55, 81–87. [Google Scholar]
- Rouabah, K.; Lombarkia, N.; Bensaci, O.A.; Berna, T. Mycoendophytes as pest control agents, using culture filtrates, against green apple aphid; Aphis pomi (Hemiptera, Aphididae). Int. J. Biosci. 2018, 13, 16–26. [Google Scholar]
- Sayed, S.; El-Shehawi, A.; Al-Otaibi, S.; El-Shazly, S.; Al-Otaibi, S.; Ibrahim, R.; Alorabi, M.; Baazeem, A.; Elseehy, M. Isolation and efficacy of the endophytic fungus, Beauveria bassiana (Bals.) Vuillemin on grapevine aphid, Aphis illinoisensis Shimer (Hemiptera: Aphididae) under laboratory conditions. Egypt. J. Biol. Pest Control 2020, 30, 38. [Google Scholar] [CrossRef]
- Fingu-Mabola, J.C.; Bawin, T.; Francis, F. Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco. Insects 2021, 12, 89. [Google Scholar] [CrossRef]
- Yuningsih, D.; Anwar, R.; Wiyono, S. Endophytic colonization of entomopathogenic Lecanicillium lecanii (Zimm) Zare & Gams PTN 10, and its effect on tobacco resistance against Myzus persicae Sulzer (Hemiptera: Aphididae). The 2nd International Conference on Sustainable Plantation. IOP Conf. Ser. Earth Environ. Sci. 2022, 974, 012089. [Google Scholar] [CrossRef]
- Paul, N.C.; Yu, S.H. Two Species of Endophytic Cladosporium in Pine Trees in Korea. Microbiology 2008, 36, 211–216. [Google Scholar]
- Uzma, F.; Konappa, N.M.; Chowdappa, S. Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats, Karnataka. Egypt. J. Basic Appl. Sci. 2016, 3, 335–342. [Google Scholar] [CrossRef]
- Lasota, J.A.; Waldvogel, M.G.; Shetlar, D.J. Fungus found in galls of Adelges abietis (L.) (Homoptera: Adelgidae): Identification, within-tree distribution, and possible impact on insect survival. Environ. Entomol. 1983, 12, 245–246. [Google Scholar] [CrossRef]
- Rojas, T.; Pons, N.; Arnal, E. Cladosporium herbarum on whiteflies (Homoptera: Aleyrodidae) in Venezuela. Bol. De Entomol. Venez. Ser. Monogr. 1998, 13, 57–65. [Google Scholar]
- Rezende, S.R.F.; Curvello, F.A.; Fraga, M.E.; Reis, R.C.S.; Castilho, A.M.C.; Agostinho, T.S.P. Control of the Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae) with entomopathogenic fungi. Rev. Bras. De Ciênc. Avícola 2009, 11, 121–127. [Google Scholar] [CrossRef]
- Bensaci, O.A.; Lombarkia, N.; Laib, D.E. Initial evaluation of endophytic fungi, isolated from Nerium oleander L. for their biocontrol action against the bruchid Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) in Algeria. In Endophytes for Plant Protection: The State of the Art; Schneider, C., Leifert, C., Feldmann, F., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2013; pp. 162–167. [Google Scholar]
- Collemare, J.; Griffiths, S.; Iida, Y.; Karimi Jashni, M.; Battaglia, E.; Cox, R.J.; de Wit, P.J. Secondary metabolism and biotrophic lifestyle in the tomato pathogen Cladosporium fulvum. PLoS ONE 2014, 9, e85877. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.S. Fungal chitinases. In Advances in Agricultural and Food Biotechnology; Guevara-González, R.G., Torres-Pacheco, I., Eds.; Research Signpost: Thiruananthapuram, India, 2006; pp. 289–304. [Google Scholar]
- Arnold, A.E.; Lewis, L.C. Ecology and evolution of fungal endophytes and their roles against insects. In Insect–Fungal Associations: Ecology And Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: New York, NY, USA, 2006; pp. 74–96. [Google Scholar]
- Samways, M.J.; Grech, N.M. Assessment of the fungus Cladosporium oxysporum (Berk. and Curt.) as a potential biocontrol agent against certain Homoptera. Agric. Ecosyst. Environ. 1986, 15, 231–239. [Google Scholar] [CrossRef]
- Fenice, M.; Selbmann, L.; Di Giambattista, R.; Federici, F. Chitinolytic activity at low temperature of an Antarctic strain (A3) of Verticillium lecanii. Res. Microbiol. 1998, 149, 289–300. [Google Scholar] [CrossRef]
- Aldamen, H.; Gerowitt, B. Influence of selected potato cultivars on the reproduction rate of the aphid species Myzus persicae (Sulzer) and Macrosiphum euphorbiae (Thomas). J. Plant Dis. Prot. 2009, 116, 278–282. [Google Scholar] [CrossRef]
- Wratten, S.D. Reproductive strategy of winged and wingless morphs of the aphids Sitobion avenae and Metopolophium dirhodum. Ann. Appl. Biol. 1977, 85, 319–331. [Google Scholar] [CrossRef]
- Brisson, J.A.; Stern, D.L. The pea aphid, Acyrthosiphon pisum: An emerging genomic model system for ecological, developmental and evolutionary studies. BioEssays 2006, 28, 747–755. [Google Scholar] [CrossRef]
- Takada, H. Ovariole number and fecundity in fundatrices of Myzus persicae (Sulzer) (Homoptera: Aphididae). Jpn. J. Appl. Entomol. Zool. 1984, 28, 250–253. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Dharma, T.D. Number of ovarioles and fecundity in the black bean aphid, Aphis fabae. Entomol. Exp. Et Appl. 1980, 28, 1–14. [Google Scholar] [CrossRef]
- Heuchert, B.; Braun, U.; Schubert, K. Morphotaxonomic revision of fungicolous Cladosporium species (hyphomycetes). Schlechtendalia 2005, 13, 1–78. [Google Scholar]
- Bensch, K.; Braun, U.; Groenewald, J.Z.; Crous, P.W. The genus Cladosporium. Stud. Mycol. 2012, 72, 1–401. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Taylor, T.W. Isolation of DNA From fungal Mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Ennis, M., Gefland, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 282–287. [Google Scholar]
- White, J.; Bruns, T.; Lee, S.; Taylor, T.W. Isolation Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Ennis, M., Gefland, D., Sninsky, J., White, T., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Smith, D.; Onions, A.H.S. The preservation and maintenance of living fungi. In IMI Technical Handbooks, No.2. International Mycological Institute, 2nd ed.; CABI Publishing: Wallingford, UK, 1994; p. 122. Available online: https://www.researchgate.net/publication/285682868_Fungi_Preservation_of_cultures (accessed on 6 July 2022).
- Kumar, S.; Kaushik, N. Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS ONE 2013, 8, e56202. [Google Scholar] [CrossRef] [PubMed]
- Babel, W.; Müller, R.H.; Markuske, K.D. Improvement of growth yield of yeast on glucose to the maximum by using an additional energy source. Arch. Microbiol. 1983, 136, 203–208. [Google Scholar] [CrossRef]
- Costa, B.O.; Nahas, E. Growth and enzymatic responses of phytopathogenic fungi to glucose in culture media and soil. Braz. J. Microbiol. 2012, 43, 332–340. [Google Scholar] [CrossRef][Green Version]
- Mesquita, A.L.M.; Lacey, L.A.; Mercadier, G.; LeClant, F. Entomopathogenic activity of a whitefly-derived isolate of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) against the Russian wheat aphid, Diuraphis noxia (Hemiptera: Sternorrhyncha Aphididae) with the description of an effective bioassay method. Eur. J. Entomol. 1996, 93, 69–75. [Google Scholar]
- McArthur, D.A.J.; Knowles, N.R. Resistance responses of potato to vesicular arbuscular mycorrhizal fungi under varying abiotic phosphorus levels. Plant Physiol. 1992, 100, 341–351. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis; Cambridge University Press: London, UK, 1971. [Google Scholar]
- Nguyen, V.N.; Oh, I.J.; Kim, Y.J.; Kim, K.Y.; Kim, Y.C.; Park, R.D. Purification and characterization of chitinases from Paecilomyces variotii DG-3 parasitizing on Meloidogyne incognita eggs. J. Ind. Microbiol. Biotechnol. 2009, 36, 195–203. [Google Scholar] [CrossRef]
- Liu, D.; Wei, Y.; Yao, P.; Jiang, L. Determination of the degree of acetylation of chitosane by UV spectrophotometry using dual standards. Carbohydr. Res. 2006, 341, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Ledger, T.N.; Engler, G.; Robichon, A. Using the pea aphid Acyrthosiphon pisum as a tool for screening biological responses to chemicals and drugs. BMC Res. Notes 2009, 2, 185. [Google Scholar] [CrossRef] [PubMed]
| Filtrate Concentration (%) | Cladosporium echinulatum | Cladosporium oxysporum | Cladosporium BEL 21 | |||
|---|---|---|---|---|---|---|
| CM (%) 1 | LT50 (h) | CM (%) 1 | LT50 (h) | CM (%) 1 | LT50 (h) | |
| 20 | 44.96 ± 5.63 c | 15.71 | 45.79 ± 5.58 c | 16.64 | 61.63 ± 5.26 c | 10.03 |
| 40 | 53.29 ± 4.94 bc | 15.65 | 64.13 ± 6.72 b | 9.52 | 74.96 ± 4.90 bc | 6.93 |
| 60 | 72.46 ± 6.04 ab | 7.11 | 79.96 ± 4.74 ab | 7.01 | 84.96 ± 4.94 ab | 5.15 |
| 80 | 92.46 ± 22.14 a | 4.14 | 93.29 ± 3.01 a | 4.44 | 94.96 ± 2.48 a | 2.78 |
| Control | 3.34 ± 0.72 | / | 2.50 ± 0.88 | / | 1.67 ± 0.34 | / |
| Filtrate Concentration (%) | Cladosporium echinulatum | |||
| 1st Instar Number | 3rd Instar Number | Apterous Adult Number | Winged Adult Number | |
| 20 | 5.37 ± 1.86 bc | 6.38 ± 0.85 bc | 3.25 ± 0.66 b | 0.25 ± 0.43 bc |
| 40 | 5.12 ± 2.14 bc | 5.00 ± 1.41 de | 2.75 ± 1.47 bc | 0.25 ± 0.43 bc |
| 60 | 5.25 ± 2.10 bc | 4.87 ± 1.16 de | 2.88 ± 0.92 bc | 0.37 ± 0.48 bc |
| 80 | 4.50 ± 1.80 cd | 4.62 ± 1.49 de | 3.38 ± 0.99 b | 0.62 ± 0.48 ab |
| Control | 9.00 ± 1.63 a | 8.00 ± 3.74 b | 6.33 ± 1.69 a | 0.00 c |
| Filtrate Concentration (%) | Cladosporium oxysporum | |||
| 1st Instar Number | 3rd instar Number | Apterous Adult Number | Winged Adult Number | |
| 20 | 5.63 ± 0.85 bc | 3.88 ± 0.7 efg | 2.63 ± 0.69 bc | 0.13 ± 0.33 c |
| 40 | 4.88 ± 1.26 bcd | 5.25 ± 1.56 cd | 2.88 ± 0.78 bc | 0.13 ± 0.33 c |
| 60 | 5.38 ± 1.72 bc | 4.25 ± 1.29 def | 2.75 ± 0.66 bc | 0.25 ± 0.43 bc |
| 80 | 5.75 ± 1.85 bc | 4.63 ± 0.99 de | 3.50 ± 0.86 b | 0.25 ± 0.43 bc |
| Control | 7.00 ± 0.81 ab | 7.33 ± 1.24 b | 5.00 ± 0.81 a | 0.00 c |
| Filtrate Concentration (%) | Cladosporium BEL21 | |||
| 1st Instar Number | 3rd Instar Number | Apterous Adult Number | Winged Adult Number | |
| 20 | 3.25 ± 0.66 ef | 2.88 ± 0.59 gh | 2.88 ± 0.78 bc | 0.25 ± 0.43 bc |
| 40 | 2.75 ± 0.82 f | 3.12 ± 0.92 fgh | 3.13 ± 0.92 bc | 0.62 ± 0.48 ab |
| 60 | 3.50 ± 0.86 def | 2.25 ± 0.43 h | 2.75 ± 0.82 bc | 0.50 ± 0.50 abc |
| 80 | 3.13 ± 1.05 ef | 2.12 ± 0.59 h | 2.13 ± 0.92 c | 0.87 ± 0.59 a |
| Control | 9.00 ± 1.63 a | 11.00 ± 2.16 a | 2.67 ± 0.94 bc | 0.00 c |
| Filtrate Concentration (%) | C. echinulatum | ||||
| Ovariole Number | Embryo Number | Embryo Number/Ovariole | Mature Embryo Number | Mature Embryo Number/Ovariole | |
| 20 | 11.43 ± 1.17 ab | 26.43 ± 4.95 ab | 2.30 ± 0.24 abc | 5.71 ± 1.57 bcde | 0.51 ± 0.13 bcde |
| 40 | 11.71 ± 0.88 ab | 29.57 ± 1.76 a | 2.54 ± 0.21 a | 6.71 ± 1.48 abcd | 0.58 ± 0.15 abcd |
| 60 | 11.29 ± 0.88 ab | 21.43 ± 3.73 def | 1.90 ± 0.31 def | 7.14 ± 1.24 ab | 0.64 ± 0.13 abc |
| 80 | 11.71 ± 0.69 ab | 27.29 ± 5.00 ab | 2.35 ± 0.33 abc | 6.71 ± 1.27 abcd | 0.58 ± 0.11 abcd |
| Control | 11.43 ± 0.90 ab | 24.14 ± 2.23 bcd | 2.12 ± 0.19 cde | 7.71 ± 1.66 a | 0.66 ± 0.10 a |
| Filtrate Concentration (%) | C. oxysporum | ||||
| Ovariole Number | Embryo Number | Embryo Number/Ovariole | Mature Embryo Number | Mature Embryo Number/Ovariole | |
| 20 | 10.71 ± 0.88 b | 26.29 ± 2.71 ab | 2.45 ± 0.14 ab | 7.57 ± 0.72 a | 0.71 ± 0.07 a |
| 40 | 11.43 ± 0.49 ab | 26.57 ± 2.71 ab | 2.38 ± 0.23 abc | 6.86 ± 0.83 abc | 0.60 ± 0.08 abc |
| 60 | 11.71 ± 1.03 ab | 26.29 ± 3.14 ab | 2.24 ± 0.14 bc | 5.29 ± 1.57 de | 0.46 ± 0.12 de |
| 80 | 11.14 ± 0.98 ab | 23.71 ± 1.97 bcde | 2.14 ± 0.20 cd | 4.71 ± 0.69 e | 0.43 ± 0.08 e |
| Control | 10.71 ± 0.88 b | 25.43 ± 4.40 bc | 2.38 ± 0.40 abc | 6.86 ± 1.88 abc | 0.65 ± 0.18 ab |
| Filtrate Concentration (%) | Cladosporium BEL21 | ||||
| Ovariole Number | Embryo Number | Embryo Number/Ovariole | Mature Embryo Number | Mature Embryo Number/Ovariole | |
| 20 | 11.29 ± 0.69 ab | 20.71 ± 3.69 def | 1.83 ± 0.28 f | 5.57 ± 0.90 cde | 0.50 ± 0.09 cde |
| 40 | 11.57 ± 0.49 ab | 21.86 ± 2.23 def | 1.84 ± 0.16 ef | 5.29 ± 1.48 de | 0.45 ± 0.11 de |
| 60 | 11.43 ± 1.04 ab | 20.14 ± 3.18 ef | 1.76 ± 0.18 fg | 4.71 ± 1.27 e | 0.42 ± 0.13 e |
| 80 | 12.00 ± 0.75 a | 18.00 ± 2.82 f | 1.51 ± 0.27 g | 4.43 ± 0.90 e | 0.37 ± 0.09 e |
| Control | 11.43 ± 1.04 ab | 27.57 ± 4.30 ab | 2.37 ± 0.19 abc | 6.71 ± 1.66 abcd | 0.58 ± 0.15 abcd |
| p | 0.504 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensaci, O.A.; Rouabah, K.; Aliat, T.; Lombarkia, N.; Plushikov, V.G.; Kucher, D.E.; Dokukin, P.A.; Temirbekova, S.K.; Rebouh, N.Y. Biological Pests Management for Sustainable Agriculture: Understanding the Influence of Cladosporium-Bioformulated Endophytic Fungi Application to Control Myzus persicae (Sulzer, 1776) in Potato (Solanum tuberosum L.). Plants 2022, 11, 2055. https://doi.org/10.3390/plants11152055
Bensaci OA, Rouabah K, Aliat T, Lombarkia N, Plushikov VG, Kucher DE, Dokukin PA, Temirbekova SK, Rebouh NY. Biological Pests Management for Sustainable Agriculture: Understanding the Influence of Cladosporium-Bioformulated Endophytic Fungi Application to Control Myzus persicae (Sulzer, 1776) in Potato (Solanum tuberosum L.). Plants. 2022; 11(15):2055. https://doi.org/10.3390/plants11152055
Chicago/Turabian StyleBensaci, Oussama A., Khamsa Rouabah, Toufik Aliat, Nadia Lombarkia, Vadim G. Plushikov, Dmitry E. Kucher, Petr A. Dokukin, Sulukhan K. Temirbekova, and Nazih Y. Rebouh. 2022. "Biological Pests Management for Sustainable Agriculture: Understanding the Influence of Cladosporium-Bioformulated Endophytic Fungi Application to Control Myzus persicae (Sulzer, 1776) in Potato (Solanum tuberosum L.)" Plants 11, no. 15: 2055. https://doi.org/10.3390/plants11152055
APA StyleBensaci, O. A., Rouabah, K., Aliat, T., Lombarkia, N., Plushikov, V. G., Kucher, D. E., Dokukin, P. A., Temirbekova, S. K., & Rebouh, N. Y. (2022). Biological Pests Management for Sustainable Agriculture: Understanding the Influence of Cladosporium-Bioformulated Endophytic Fungi Application to Control Myzus persicae (Sulzer, 1776) in Potato (Solanum tuberosum L.). Plants, 11(15), 2055. https://doi.org/10.3390/plants11152055









