Abstract
Flooding is constantly threatening the growth and yield of crops worldwide. When flooding kicks in, the soil becomes water-saturated and, therefore, the roots are the first organs to be exposed to excess water. Soon after flooding, the soil turns anoxic and the roots can no longer obtain molecular oxygen for respiration from the rhizosphere, rendering the roots dysfunctional. Rice, however, is a semi-aquatic plant and therefore relatively tolerant to flooding due to adaptive traits developed during evolution. In the present review, we have identified three key root traits, viz. cortical aerenchyma formation, a barrier to radial oxygen loss and adventitious root growth. The understanding of the physiological function, the molecular mechanisms, and the genetic regulation of these three traits has grown substantially and therefore forms the backbone of this review. Our synthesis of the recent literature shows each of the three key root traits contributes to flood tolerance in rice. One trait, however, is generally insufficient to enhance plant tolerance to flooding. Consequently, we suggest comprehensive use of all three adaptive traits in a pyramiding approach in order to improve tolerance to flooding in our major crops, in general, and in rice, in particular.
1. Introduction
The food demand is increasing annually owing to the world’s growing population [1]. In the last decades, the growth and yield of crops have been simultaneously threatened by various natural disasters, and among them are frequent flooding events [2]. The cultivation and breeding of flooding-tolerant plant varieties are thus becoming the prime task to alleviate the food crisis caused by soil flooding or even complete submergence. Wetland plants oftentimes exhibit higher waterlogging tolerance due to their evolutionary ability to thrive in such habitats, and various adaptive traits have been described in these plants, conferring tolerance to flash floods or prolonged flood events [3].
Rice is a semi-aquatic plant that survives soil flooding or even partial submergence. It possesses a range of adaptive traits to cope with floods, such as stem elongation [4], gas film retention by superhydrophobic leaves [5], aerenchyma formation in internodes [6] as well as in roots [7], and adventitious root growth [8,9] (Figure 1). Adaptive root traits are receiving considerable interest due to their high plasticity [10] and due to the fact that roots are more prone to soil flooding as compared to the shoot. In water-saturated (waterlogged) soils, O2 supply from the air to the soil is greatly restricted due to high resistance to molecular diffusion in water compared to in air. Moreover, the soil roots and microorganisms compete for the remaining O2, making the situation even more severe [9]. Lack of O2 inhibits root respiration, causing severe energy crisis and finally resulting in tissue death [11]. Consequently, the introgression of key root traits in the soil roots and the formation of a new root system are seen as two major strategies in rice to cope with soil flooding and partial submergence.
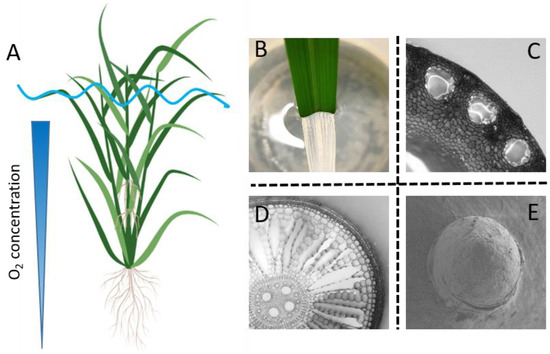
Figure 1.
Rice adaptive traits during waterlogging. Rice develops a range of adaptive traits upon soil flooding including stem elongation (A), gas film retention by the superhydrophobic leaves (B), aerenchyma formation in the internodes of the stem (C) and in the cortex of the roots (D), and floating adventitious roots (A,E). Panel (A) is created with BioRender.com, and photos and cross-sections are all original contributions by the authors.
Understanding the function of these adaptive traits is helpful to uncover the underlying mechanisms of plant adaptation to flooding, thus enabling the valuable quantitative trait loci (QTLs) or genes associated with these traits to be introgressed into flooding-susceptible genotypes through traditional hybridization or genetic engineering [12]. In this review, we summarize the progress in recent research of three important adaptive root traits expressed in rice and some other crops as a response to flooding: (i) cortical aerenchyma formation, (ii) the root barrier to radial oxygen loss, and (iii) aquatic adventitious root formation. The aim of this review is, therefore, to analyze recent research enabling breeding of climate-resilient rice based on pyramiding these three key root traits to optimize adaptation to flooding.
2. Aerenchyma Formation Facilitates Intra-Tissue O2 Diffusion in Flooded Soil Roots
Aerenchyma consist of gas-filled plant tissues, and the formation of cortical aerenchyma at the base of rice roots, for example, results from the lysis of cortex cells [13]. Root aerenchyma can either be constitutively formed or further induced as a response to the soil environment [14]. Aerenchyma is a key root trait enabling plants to survive flooded soils, because the gas-filled tissues provide a low-resistance pathway for O2 diffusion inside the roots, thus supplying molecular O2 to the growing root tip [15] (Figure 2A). Moreover, the programmed death of cortical cells reduces the root respiratory consumption of carbohydrates, thereby conserving organic carbon and nutrients, i.e., a longer root can be produced for the same investment in carbon and nutrients if a large proportion of the cortex is gas-filled [13,16]. In recent years, the molecular mechanism of aerenchyma formation in rice has gradually been uncovered. In rice, root cortical aerenchyma is constitutively formed [17], but great differences in constitutive aerenchyma have been found among genotypes and, interestingly, these differences have not been strongly correlated to their flooding tolerance. Non-wetland plants such as maize (Zea mays) and wheat (Triticum aestivum) barely form any constitutive aerenchyma and their roots easily suffer from tissue anoxia at the onset of waterlogging [17,18]. It is thus conceivable that constitutive aerenchyma formation in rice is closely associated with tolerance to waterlogging. Genetically, formation of constitutive aerenchyma in rice is regulated by auxin signaling. Auxin/indole-3-acetic acid protein IAA13 and the auxin response factor ARF19 together contribute to the development of constitutive aerenchyma by directly targeting the downstream transcription factor lateral organ boundaries domain (LBD)-containing protein 1–8 (LBD1–8), which is also required for constitutive aerenchyma formation [19].
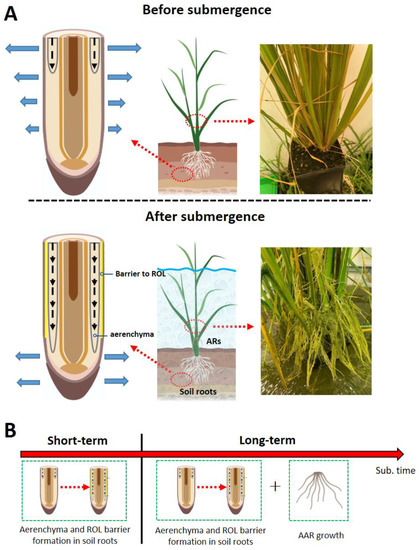
Figure 2.
Three adaptive root traits conferring tolerance to soil flooding in rice. (A) The inducible aerenchyma is developed inside the soil roots to enhance O2 diffusion from the shoot to the root. A barrier to radial oxygen loss (ROL) is formed at the outer part of the soil roots to restrict loss of molecular O2 to the anoxic rhizosphere. Furthermore, the shoot-borne aquatic adventitious roots (AARs) are formed aboveground. (B) Roots adaptive traits occur as the flooding progresses. Several components are created with BioRender.com.
In stark contrast, the inducible aerenchyma follows an ethylene-ROS regulatory manner upon soil flooding [7]. At the onset of flooding, the elevated ethylene in plant roots is a combination of slow gas diffusion (causing the constitutive production of ethylene to accumulate in the tissues) and upregulation of the ethylene biosynthesis genes, ACC synthase (ACS) and ACC oxidase (ACO) [20,21]. The expression of ACS1 and ACO5 is strongly induced in stagnant, deoxygenated hydroponics designed to mimic soil flooding [20], and the inhibition of ethylene biosynthesis and signaling significantly repressed the formation of inducible aerenchyma [20]. At later stages, ROS accumulation is also required for the formation of inducible aerenchyma [7]. Respiratory Burst Oxidase Homolog (RBOH) is the homolog of NADPH oxidase, playing a pivotal role in ROS generation in the apoplast; the accumulated ROS subsequently enters the cytosol. Expression of one of the RBOH isoforms (RBOHH) is induced in stagnant, deoxygenated conditions, and knockout of RBOHH significantly reduced the ROS production and aerenchyma formation [7]. Moreover, Ca2+-dependent signaling is needed for the induction of inducible aerenchyma [7]. Two calcium-dependent protein kinases (CPK/CDPK) CDPK5 and CDPK13 are crucial for the phosphorylation of RBOHH [7]. In addition, application of the RBOH inhibitor, diphenyleneiodonium (DPI), inhibited the formation of inducible aerenchyma as well. In conclusion, the formation of inducible aerenchyma is under strong control of ethylene, ROS, and Ca2+-dependent signaling.
3. Some Roots Develop a Barrier to Radial Oxygen Loss to Facilitate Internal Aeration
Due to the steep concentration gradient, oxygen entrapped in the root aerenchyma tends to diffuse radially to the surrounding anoxic soil rather than longitudinally to the root tip. In rice and other wetland plants, radial oxygen loss (ROL) from roots can be greatly restricted by the formation of a diffusive barrier at the exodermis (epidermal/hypodermal cell layers) when exposed to conditions of soil flooding or partial submergence [22,23]. The root barrier to ROL not only improves tissue oxygen status by restricting diffusive loss of oxygen, but also prevents soil phytotoxins from entering the root [24]. The ROL barrier is also capable of restricting the diffusion of gases other than O2, such as H2 or water vapour [25]. The root ROL barrier is presumably composed of enhanced cell wall depositions of suberin and/or lignin at the exodermis [26]. The location of the ROL barrier in the exodermis is supported by measurement using oxygen microsensors, indicating steep concentration gradients in the outer cell layers (epidermis, exodermis/hypodermis and sclerenchyma) [27]. Not only the exact components of the ROL barrier are still being debated, but it is also not yet clear which of the two known components (suberin and/or lignin) is of greater significance to reduce ROL in rice [27]. However, in four Amazonian tree species [28] and Hordeum marinum [29], it was found that suberization of the exodermis, more than lignification, was correlated with a reduction in ROL.
The formation of an ROL barrier is influenced by various environmental factors. Soil flooding leads to oxygen deprivation, accumulation of the phytohormone ethylene and also CO2, but none of these molecules act as an environmental signal(s) for ROL barrier formation [30]. In contrast, some soil phytotoxins such as Fe2+ [31], sulfides [32], and low molecular carboxylic acids [33] produced by anaerobic bacteria all induce a ROL barrier in rice. Interestingly, the molecular regulation of the ROL barrier formation in plants is still under investigation. By means of laser microdissection, the specific tissue where the ROL barrier is formed can be collected and used for transcriptome analysis [26]. Several genes involved in suberin biosynthesis, including CYTOCHROME P450 (OsCYP86B3) and ABC TRANSPORTER (OsABCG5), are strongly induced under soil flooding [26], suggesting that these are likely important molecular regulators required for ROL barrier formation.
In root tissues of the OsCYP86B3 rice mutant, the amount of C24 to C30 w/OH fatty acid was much lower compared to the wildtype, whereas the suberin lamellae were still detectable with histochemical staining [34]. In addition, oxygen leakage was observed at the root tip rather than at the basal part of the root, indicating that the lack of OsCYP86B3 did not influence the formation of the ROL barrier, and the knockout of OsCYP86B3 did also not alter root physiology. In contrast, OsABCG5 mutant plants also show a much lower C24 to C30 w/OH fatty acid, but unlike the OsCYP86B3 rice mutant, suberin lamellae was not histochemically detected in the OsACCG5 mutant and importantly, the apoplastic barrier was impaired [35]. To date, the ROL barrier in OsABCG5 mutant plants has not been systematically evaluated. In addition, the OsACCG5 mutant showed shorter roots as compared to the wildtype, indicating additional roles of OsACCG5 in root development.
Several transcription factors including WRKY, NAC, and MYB were upregulated under stagnant, deoxygenated conditions (simulating soil flooding), indicating that these transcription factors might be directly or indirectly involved in the ROL barrier formation [26]. Owning to the lack of relevant rice mutants, the functional analysis of these genes as related to the ROL barrier formation remains to be investigated. Recently, it was demonstrated that Abscisic Acid (ABA) signaling is required for ROL barrier formation, whereas the application of the ABA biosynthesis inhibitor FLU prevented the formation of ROL barrier. OsABA1 shows an impaired ROL barrier, but when complemented with ABA, it is perfectly able to form a ROL barrier under stagnant, deoxygenated conditions [36]. We therefore propose to investigate other ABA-related genes in order to further unravel the genes involved in the ROL barrier formation.
4. Aquatic Adventitious Roots Confer Tolerance to Partial or Complete Submergence
Primordia of adventitious roots (AR) are present in the nodes of the aboveground parts of the stem in rice but do not emerge unless triggered by environmental factors such as soil flooding or partial submergence [8,37]. Some emerging roots penetrate into the soil, whereas others float in the water, and these are therefore referred to as aquatic ARs (AAR) [38]. Growth of ARs is coordinated with programmed cell death above the root primordia and induced by the gaseous phytohormone ethylene [39,40]. The new AR system alleviates the functional loss of soil roots, thus helping plants to endure flooding until the water recedes. AARs of deepwater rice have been shown to actively take up nutrients from the floodwater [38,41], and unlike the main roots exposed to anoxic soil, AAR can still obtain oxygen dissolved in the floodwater [42]. Interestingly, in several wild wetland plants, functional chloroplasts develop in AAR, indicating that oxygen and carbohydrates are produced by these roots [42,43]. During long-term and periodic flooding, the AARs are therefore of great importance to ensure plant survival and even continued growth.
The architecture of AR is not only determined by environmental signals but is also under strong genetic control. In the dark, ARs grow upwards presumably in a quest to obtain more oxygen [37]. Turbid floodwaters greatly restrict light penetration into the water, thereby minimizing the influence of light on directional root growth, and the light intensity decreases further with depth. The architecture of the AR system is comprehensively affected by altered gas diffusion, light intensity, and gravity. A number of genes have been characterized to be involved in crown root development and these genes participate in different molecular networks [44,45,46]. Crown roots and adventitious roots of rice have not been systematically distinguished because they both originate from the arial node. We thereby assumed that the regulation of crown roots and adventitious roots largely share overlapping gene networks, and we focus the remaining discussion on aboveground ARs.
It is believed that the aboveground AR root system is influenced by the following three parameters: (i) Position (number) of the aboveground node. AR primordia are present at the node and, therefore, more nodes result in the emergence of numerous ARs during flooding. The stem nodes in the majority of rice cultivars are buried in the soil or grow near the soil surface, whereas deepwater rice grows more aboveground nodes. The overexpression of several genes significantly increased the number of aboveground nodes such as WUSCHEL-related homeobox gene 11 (WOX11) [47], Mao Hu Zi 4 (MHZ4) [48], SNORKEL1 (SK1), and SNORKEL2 (SK2) [4]. (ii) The number of root primordia at each node. The number of root primordia at each node differs between rice genotypes. In addition, the number of AR is controlled by root-related genes such as CROWN ROOTLESS 1 (CRL1) [49], CROWN ROOTLESS 5 (CRL5) [50], WOX11 [47], TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1) [51] etc. (iii) The response to ethylene. Ethylene is required for adventitious root emergence, and the rapid induction of functional ARs is beneficial for plants to survive at the early flooding stage.
5. Discussion, Conclusions and Future Perspectives
One individual root trait is likely insufficient to substantially improve crop adaptation to flooding [52]. Thus, we propose that two or three adaptive traits should be simultaneously introduced into the target crops in order to provide sufficient flood tolerance. In traditional rice breeding, QTLs or valuable haplotypes of candidate genes can be introgressed into a modern high-yielding cultivar through cross breeding. However, such breeding approaches are usually time-consuming, laborious, and natural variations are unavailable for some traits. By contrast, modern genetic engineering holds the potential to greatly speed up the process.
Aerenchyma and ROL barrier induction occur at the onset of waterlogging and can therefore be seen as a flood response helping soil roots to adapt to short-term flooding soon after flooding kicks in. However, as the flooding progresses, a gradual functional loss of soil roots results from oxygen deficiency and accumulation of phytotoxins in the flooded soil [23]. The establishment of a new root system replacing the soil roots to maintain water and nutrient uptake is therefore an essential adaptive mechanism enabling survival and growth during continuous and periodic submergence (Figure 2B). Even if several environmental signaling molecules have been identified [53], the intra-tissue molecular mechanism of the ROL barrier induction in plants is still poorly understood. Nevertheless, we anticipate that rice varieties, which are tolerant to both short-term and long-term flooding, will be developed in the future.
In stark contrast, the molecular mechanisms of aerenchyma development and AR growth have been comprehensively studied and are therefore much better understood. Development of both inducible aerenchyma and AR growth are triggered by ethylene and mediated via ROS accumulation, suggesting a shared overlapping gene network. Uncovering the functional genes regulating both traits will be crucial for breeding of flood-tolerant rice.
The gene network involved in AR development and growth has been systematically investigated [45,46], and the marker genes can be applied as major indicators for molecular breeding in agriculture. To date, the most extensively reported genes related to AR development are regulated by the phytohormone auxin and cytokinin [46]. To fully understand the role or potential application of these genes, it is commonly tested by overexpression in rice. Plants constitutively expressing these genes oftentimes cause phytohormone disorder, thus leading to plant growth defects. Constitutive overexpression of OsYUC in plants exhibits more ectopic ARs but at the expense of a dwarf phenotype [51]. Root growth is regulated genetically and by environmental factors. Roots with the potential to emerge from aboveground primordia are supposed to grow only when triggered by soil flooding, because roots lacking a cuticle are prone to desiccation in low humidity. Overexpression of WOX11 in rice shows more nodes and emerged ARs even in emergent conditions, which is seemingly not appropriate for the plant [47]; a similar observation has been made in the ABA4 rice mutant [48]. It is thus believed that driving these genes with hypoxia-inducible promoters may be preferable for the breeding of flood-tolerant rice. Submergence 1A (Sub1A) [54], SNORKEL1 (SK1), and SNORKEL2 (SK2) [4] are flooding tolerance-related genes detected in several wildtypes of rice. Their expressions are exclusively induced during soil flooding and the corresponding promoters can be cloned and further exploited. In addition, the expression of many other genes is also highly and specifically upregulated during soil flooding, such as Pyruvate Decarboxylase (PDC) [55] and Alcohol Dehydrogenase (ADH) [55], and their promoters are highly feasible as well [56].
The majority of the molecular elements characterizing adaptive root traits are positive regulators. Unfortunately, there are relatively few reports with respect to negative regulators and it is clear that biased research methods have resulted in ignoring the functions of these negative regulators. We therefore propose to pay more attention in the future to those negative regulatory factors related to the three traits. Owing to the development of cluttered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein (Cas) system and the gradual liberalization of genome editing crops [57], knocking out these important negative factors is not only conducive to basic scientific research but could have a large potential in crop application. Scientists from China have modified six important agronomic traits of wild allotetraploid rice through genome editing, making it possible to de novo domesticate wildtype plants [58]. It has been revealed that the rice gene Os8N3 (OsSWEET11) negatively regulates plant resistance to Xanthomonas and the knockout of Os8N3 significantly enhanced plant tolerance to Xanthomonas [59]. CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene has also been shown to significantly improve salinity tolerance [60]. These cases clearly indicate that the technology is feasible and reliable when used to modify adaptive traits under biotic and abiotic stress. An alternative strategy is to induce the specific nucleotide substitution of C to T or G to A in the regulatory elements at the promoter region; thereby, the expression of these identified positive regulators can be manipulated. However, the gain-of-function results are relatively hard to achieve, even though the predictable nucleotide-specific mutations can be realized through base editing using CRISPR/Cas technology. Moreover, the efficiency of such approaches remains low [61].
In addition to the three key root traits covered in the present review, several other traits have been described and will likely be involved in future crop improvement, including root porosity [18] and internode aerenchyma formation [6]. Similar to root aerenchyma, the root porosity enhances the internal aeration and facilitates oxygen diffusion to the root tip where it sustains respiration. Aerenchyma also develops in the internode of rice, where constitutive aerenchyma is primarily formed in the older internodes and further induced at the upper internode indicative of a developmental manner. Based on the research on 18 wild Poaceae species, three important indicators were developed to reflect plant root adaptation to soil flooding, including cortex to stele ratio (CSR), xylem to stele ratio (XSR), and aerenchyma to cortex ratio (ACR) [62]. These three key root parameters can be used for breeding flood-tolerant crops in the future. Because it is time-consuming to breed flooding-tolerant rice varieties through traditional hybridity, accurate targeted gene or QTLs might significantly shorten the breeding time. The development of these adaptive traits aims at either enhancing O2 uptake or improving O2 use efficiency. In the present review, we propose a breeding strategy involving the introgression of two or even three different flooding adaptive traits into high-yielding modern rice cultivars. Such an approach would lead to the improvement of plant survival and therefore reduce grain loss during soil flooding.
Author Contributions
C.L., O.P. and M.S. conceived the outline of the review. C.L. and T.Z. created the illustrations. C.L. and O.P. wrote the manuscript with the contribution from T.Z., L.L.P.O., Y.W. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research Fund Denmark grant number 8021-00120B; Danida Fellowship Centre grant number 19-03-KU; EU Horizon 2020 (Talent) Marie Skłodowska-Curie grant number 801199; Deutsche Forschungsgemeinschaft grant number SA 495/16-1; China Scholarship Council; Kiel Life Science ZMB Young Scientists Grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are thankful to Takaki Yamauchi (Bioscience Center, Nagoya Bioscience and Biotechnology Center, Nagoya University, Japan) for the discussion with respect to the gene regulation on the formation of root aerenchyma, as well as Shiono Katsuhiro (Fukui Prefectural University, Japan) for providing additional information about OsACCG5 and ABA1 mutants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Yeung, E.; Bailey-Serres, J.; Sasidharan, R. After the deluge: Plant revival post-flooding. Trends Plant Sci. 2019, 24, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Voesenek, L.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Hattori, Y.; Nagai, K.; Furukawa, S.; Song, X.J.; Kawano, R.; Sakakibara, H.; Wu, J.; Matsumoto, T.; Yoshimura, A.; Kitano, H.; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Winkel, A.; Pedersen, O.; Ella, E.; Ismail, A.M.; Colmer, T.D. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. J. Exp. Bot. 2014, 65, 3225–3233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffens, B.; Geske, T.; Sauter, M. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol. 2011, 190, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yoshioka, M.; Fukazawa, A.; Mori, H.; Nishizawa, N.K.; Tsutsumi, N.; Yoshioka, H.; Nakazono, M. An NADPH Oxidase RBOH Functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell 2017, 29, 775–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorbiecke, R.; Sauter, M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999, 119, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Sauter, M. Root responses to flooding. Curr. Opin. Plant Biol. 2013, 16, 282–286. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root plasticity under abiotic stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [Green Version]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Drew, M.C.; He, C.J.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef]
- Takahashi, H.; Yamauchi, T.; Colmer, T.D.; Nakazono, M. Aerenchyma Formation in Plants. In Low-Oxygen Stress in Plants; Springer: Vienna, Austria, 2014; pp. 247–265. [Google Scholar]
- Yamauchi, T.; Shimamura, S.; Nakazono, M.; Mochizuki, T. Aerenchyma formation in crop species: A review. Field Crops Res. 2013, 152, 8–16. [Google Scholar] [CrossRef]
- Colombi, T.; Chakrawal, A.; Herrmann, A.M. Carbon supply-consumption balance in plant roots: Effects of carbon use efficiency and root anatomical plasticity. New Phytol. 2022, 233, 1542–1547. [Google Scholar] [CrossRef]
- Colmer, T.D.; Voesenek, L. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Shiono, K.; Nagano, M.; Fukazawa, A.; Ando, M.; Takamure, I.; Mori, H.; Nishizawa, N.K.; Kawai-Yamada, M.; Tsutsumi, N.; et al. Ethylene biosynthesis is promoted by very-long-chain fatty acids during lysigenous aerenchyma formation in rice roots. Plant Physiol. 2015, 169, 180–193. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Mori, H.; Takamure, I.; Kato, K.; Nakazono, M. Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant Cell Environ. 2016, 39, 2145–2157. [Google Scholar] [CrossRef]
- Colmer, T.D.; Gibberd, M.R.; Wiengweera, A.; Tinh, T.K. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J. Exp. Bot. 1998, 49, 1431–1436. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef] [Green Version]
- Abiko, T.; Kotula, L.; Shiono, K.; Malik, A.I.; Colmer, T.D.; Nakazono, M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012, 35, 1618–1630. [Google Scholar] [CrossRef]
- Ogorek, L.L.P.; Pellegrini, E.; Pedersen, O. Novel functions of the root barrier to radial oxygen loss—Radial diffusion resistance to H2 and water vapour. New Phytol. 2022, 231, 1365–1376. [Google Scholar] [CrossRef]
- Shiono, K.; Yamauchi, T.; Yamazaki, S.; Mohanty, B.; Malik, A.I.; Nagamura, Y.; Nishizawa, N.K.; Tsutsumi, N.; Colmer, T.D.; Nakazono, M. Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J. Exp. Bot. 2014, 65, 4795–4806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotula, L.; Ranathunge, K.; Schreiber, L.; Steudle, E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J. Exp. Bot. 2009, 60, 2155–2167. [Google Scholar] [CrossRef]
- De Simone, O.; Haase, K.; Muller, E.; Junk, W.J.; Hartmann, K.; Schreiber, L.; Schmidt, W. Apoplasmic barriers and oxygen transport properties of hypodermal cell walls in roots from four amazonian tree species. Plant Physiol. 2003, 132, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Kotula, L.; Schreiber, L.; Colmer, T.D.; Nakazono, M. Anatomical and biochemical characterisation of a barrier to radial O2 loss in adventitious roots of two contrasting Hordeum marinum accessions. Funct. Plant Biol. 2017, 44, 845–857. [Google Scholar] [CrossRef]
- Colmer, T.D.; Cox, M.C.; Voesenek, L.A. Root aeration in rice (Oryza sativa): Evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytol. 2006, 170, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mongon, J.; Konnerup, D.; Colmer, T.D.; Rerkasem, B. Responses of rice to Fe2+ in aerated and stagnant conditions: Growth, root porosity and radial oxygen loss barrier. Funct. Plant Biol. 2014, 41, 922–929. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Rice and Phragmites: Effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. Am. J. Bot. 2001, 88, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Kotula, L.; Colmer, T.D.; Nakazono, M. Effects of organic acids on the formation of the barrier to radial oxygen loss in roots of Hordeum marinum. Funct. Plant Biol. 2014, 41, 187–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waßmann, F.F.M. Suberin Biosynthesis in O. Sativa: Characterization of a Cytochrome P450 Monooxygenase. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2014; p. 124. Available online: https://d-nb.info/1077289758/34 (accessed on 1 August 2022).
- Shiono, K.; Ando, M.; Nishiuchi, S.; Takahashi, H.; Watanabe, K.; Nakamura, M.; Matsuo, Y.; Yasuno, N.; Yamanouchi, U.; Fujimoto, M.; et al. RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J. 2014, 80, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Yoshikawa, M.; Kreszies, T.; Yamada, S.; Hojo, Y.; Matsuura, T.; Mori, I.C.; Schreiber, L.; Yoshioka, T. Abscisic acid is required for exodermal suberization to form a barrier to radial oxygen loss in the adventitious roots of rice (Oryza sativa). New Phytol. 2022, 233, 655–669. [Google Scholar] [CrossRef]
- Lin, C.; Sauter, M. Control of adventitious root architecture in rice by darkness, light, and gravity. Plant Physiol. 2018, 176, 1352–1364. [Google Scholar] [CrossRef] [Green Version]
- Rich, S.M.; Ludwig, M.; Pedersen, O.; Colmer, T.D. Aquatic adventitious roots of the wetland plant Meionectes brownii can photosynthesize: Implications for root function during flooding. New Phytol. 2011, 190, 311–319. [Google Scholar] [CrossRef]
- Mergemann, H.; Sauter, M. Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol. 2000, 124, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Sauter, M. Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiol. 2005, 139, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Rich, S.M.; Pedersen, O.; Ludwig, M.; Colmer, T.D. Shoot atmospheric contact is of little importance to aeration of deeper portions of the wetland plant Meionectes brownii; submerged organs mainly acquire O2 from the water column or produce it endogenously in underwater photosynthesis. Plant Cell Environ. 2013, 36, 213–223. [Google Scholar] [CrossRef]
- Lin, C.; Ogorek, L.L.P.; Pedersen, O.; Sauter, M. Oxygen in the air and oxygen dissolved in the floodwater both sustain growth of aquatic adventitious roots in rice. J. Exp. Bot. 2021, 72, 1879–1890. [Google Scholar] [CrossRef]
- Ayi, Q.; Zeng, B.; Liu, J.; Li, S.; van Bodegom, P.M.; Cornelissen, J.H.C. Oxygen absorption by adventitious roots promotes the survival of completely submerged terrestrial plants. Ann. Bot. 2016, 118, 675–683. [Google Scholar] [CrossRef]
- Coudert, Y.; Perin, C.; Courtois, B.; Khong, N.G.; Gantet, P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010, 15, 219–226. [Google Scholar] [CrossRef]
- Meng, F.; Xiang, D.; Zhu, J.; Li, Y.; Mao, C. Molecular mechanisms of root development in rice. Rice 2019, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Sauter, M. Control of root system architecture by phytohormones and environmental signals in rice. Isr. J. Plant Sci. 2020, 67, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, Y.; Dai, M.; Huang, L.; Zhou, D.X. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell 2009, 21, 736–748. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Yin, C.C.; He, S.J.; Lu, X.; Zhang, W.K.; Lu, T.G.; Chen, S.Y.; Zhang, J.S. Ethylene-induced inhibition of root growth requires abscisic acid function in rice (Oryza sativa L.) seedlings. PLoS Genet. 2014, 10, e1004701. [Google Scholar] [CrossRef]
- Inukai, Y.; Sakamoto, T.; Ueguchi-Tanaka, M.; Shibata, Y.; Gomi, K.; Umemura, I.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 2005, 17, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Kitomi, Y.; Ito, H.; Hobo, T.; Aya, K.; Kitano, H.; Inukai, Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011, 67, 472–484. [Google Scholar] [CrossRef]
- Zhang, T.; Li, R.; Xing, J.; Yan, L.; Wang, R.; Zhao, Y. The YUCCA-Auxin-WOX11 module controls crown root development in rice. Front Plant Sci. 2018, 9, 523. [Google Scholar] [CrossRef]
- Kuroha, T.; Ashikari, M. Molecular mechanisms and future improvement of submergence tolerance in rice. Mol. Breed. 2020, 40, 41. [Google Scholar] [CrossRef]
- Ejiri, M.; Fukao, T.; Miyashita, T.; Shiono, K.A. barrier to radial oxygen loss helps the root system cope with waterlogging-induced hypoxia. Breed. Sci. 2021, 71, 40–50. [Google Scholar] [CrossRef]
- Xu, K.; Xu, X.; Fukao, T.; Canlas, P.; Maghirang-Rodriguez, R.; Heuer, S.; Ismail, A.M.; Bailey-Serres, J.; Ronald, P.C.; Mackill, D.J. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 2006, 442, 705–708. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Grover, A.; Peacock, W.J.; Dennis, E.S.; Ellis, M.H. Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Funct. Plant Biol. 2001, 12, 1231–1241. [Google Scholar] [CrossRef]
- Reynoso, M.A.; Kajala, K.; Bajic, M.; West, D.A.; Pauluzzi, G.; Yao, A.I.; Hatch, K.; Zumstein, K.; Woodhouse, M.; Rodriguez-Medina, J.; et al. Evolutionary flexibility in flooding response circuitry in angiosperms. Science 2019, 365, 1291–1295. [Google Scholar] [CrossRef]
- Gao, C. The future of CRISPR technologies in agriculture. Nat. Rev. Mol. Cell Biol. 2018, 19, 275–276. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Meng, X.; Du, H.; Zhang, J.; Liu, G.; Chen, M.; Jing, Y.; Kou, L.; Li, X.; et al. A route to de novo domestication of wild allotetraploid rice. Cell 2021, 184, 1156–1170. [Google Scholar] [CrossRef]
- Kim, Y.A.; Moon, H.; Park, C.J. CRISPR/Cas9-targeted mut A route to de novo domestication of wild allotetraploid rice agenesis of Os8N3 in rice to confer resistance to Xanthomonas oryzae pv. oryzae. Rice 2019, 12, 67. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Wang, F.; Li, T.; Chen, Z.; Kong, D.; Bi, J.; Zhang, F.; Luo, X.; Wang, J.; et al. Enhanced rice salinity tolerance via CRISPR/Cas9-targeted mutagenesis of the OsRR22 gene. Mol. Breed. 2019, 39, 47. [Google Scholar] [CrossRef] [Green Version]
- Scheben, A.; Edwards, D. Towards a more predictable plant breeding pipeline with CRISPR/Cas-induced allelic series to optimize quantitative and qualitative traits. Curr. Opin. Plant Biol. 2018, 45, 218–225. [Google Scholar] [CrossRef]
- Yamauchi, T.; Pedersen, O.; Nakazono, M.; Tsutsumi, N. Key root traits of Poaceae for adaptation to soil water gradients. New Phytol. 2021, 229, 3133–3140. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).