Evaluation of Growth Characteristics and Biological Activities of ‘Dachul’, a Hybrid Medicinal Plant of Atractylodes macrocephala × Atractylodes japonica, under Different Artificial Light Sources
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristic Difference in Growth and Chlorophyll of Dachul Treated with Different Artificial Light Sources
2.2. Effects of Artificial Light Treatment on Antioxidants, Total Polyphenols, and Total Flavonoids in Dachul Plants
2.3. Antimicrobial Activity
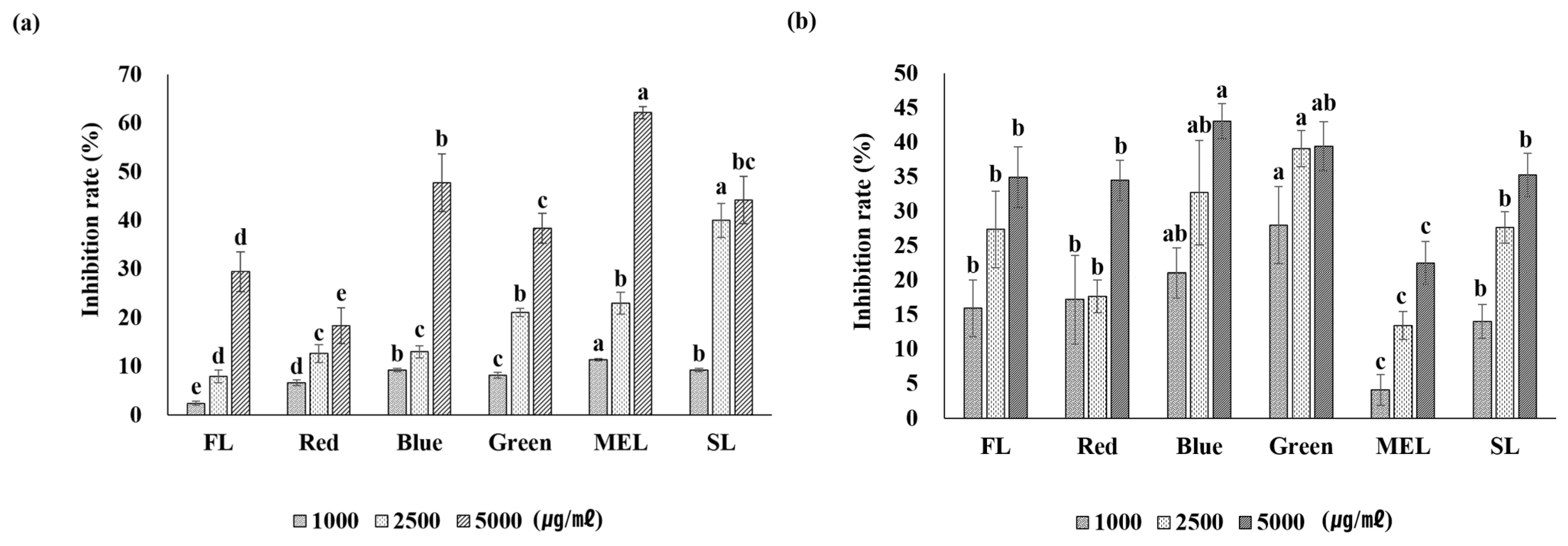
2.4. Whitening Activity
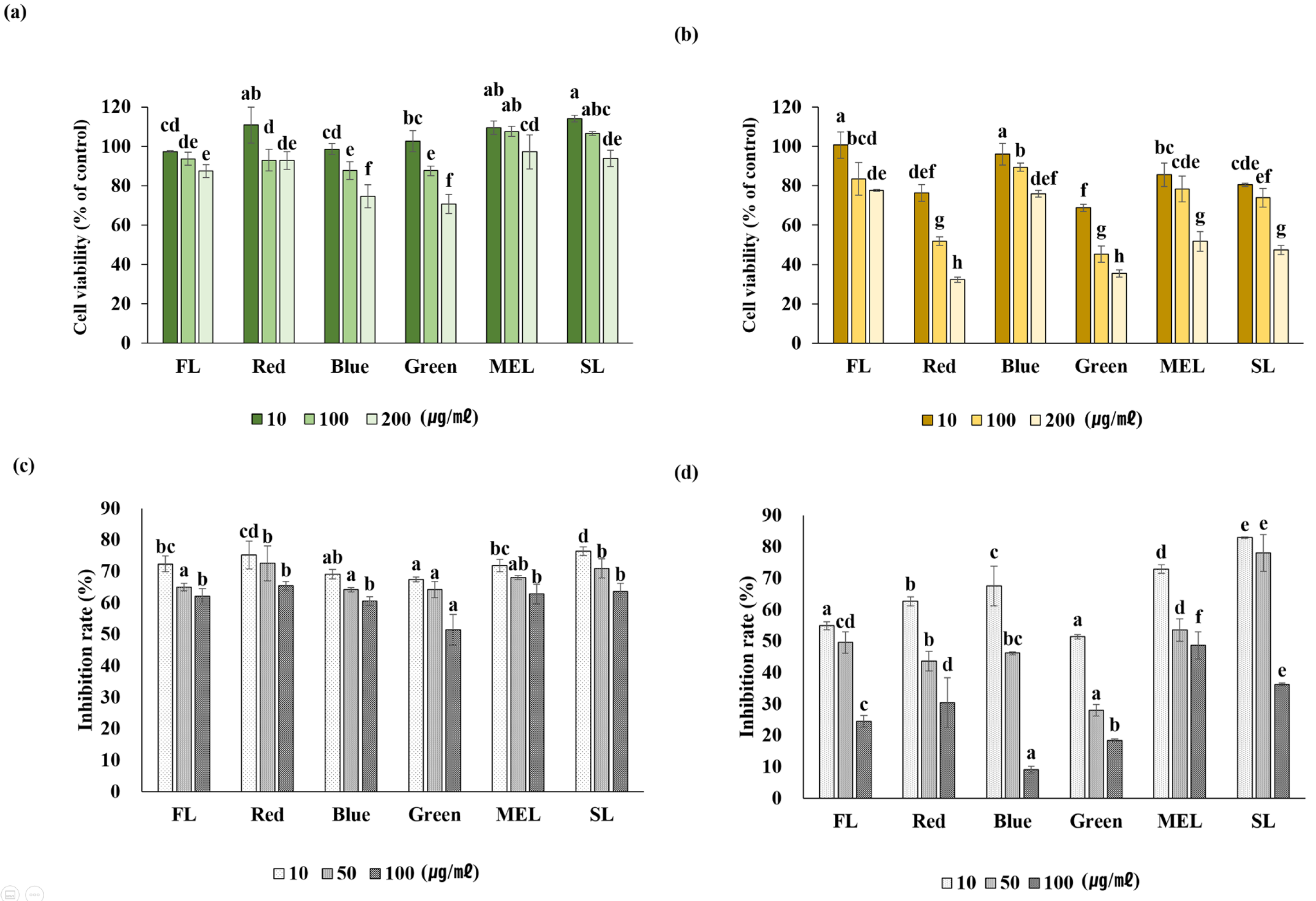
2.5. Inhibition of NO Production and Expression of Inflammation-Related Genes in LPS-Induced RAW 264.7 Cells
3. Materials and Methods
3.1. Plant Materials and Growth Environment Using Artificial Light Sources
3.2. Plant Gowth Characteristics
3.3. Chlorophyll Content
3.4. Extraction and Concentration of Plant Samples
3.5. Analysis of Antioxidant Activity
3.6. Analysis of Total Polyphenol Content
3.7. Analysis of Total Flavonoid Content
3.8. Analysis of Antimicrobial Activity
3.9. Analysis of Whitening Activity
3.10. Analysis of Cell Viability
3.11. Measurement of NO Production Rate in LPS-Induced Cell
3.12. Analysis of Gene Expression Using RT-PCR
3.13. Statistical Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Kim, Y.K.; Hong, S.P.; Kim, C.S. Studies of taxonomic origins of Atractylodis rhizma alba and Atractylodis rhizma. Kor. J. Orient Med. 2002, 8, 55–63. [Google Scholar]
- Chung, H.G.; Bang, K.H.; Bang, J.K.; Lee, S.E.; Seong, N.S.; Cho, J.H.; Han, B.S.; Kim, S.M. Comparison of volatile components in essential oil from different origin of Atractylodes spp. Kor. J. Med. Crop Sci. 2004, 12, 149–153. [Google Scholar]
- Kim, J.K.; Park, K.L.; Rha, E.S. Variation analysis of Atractylodes japonica Koidzumi ex Kitamura based on quantitative characters. Kor. J. Plant Res. 2002, 15, 36–42. [Google Scholar]
- Hwang, M.H.; Seo, J.W.; Han, K.J.; Kim, M.J.; Seong, E.S. Effect of Artificial Light Treatment on the Physiological Property and Biological Activity of the Aerial and Underground Parts of Atractylodes macrocephala. Agronomy 2022, 12, 1485. [Google Scholar] [CrossRef]
- Rural Development Administration. List of Medicinal Crop Varieties; National Institute of Horticultural and Herbal Science: Eumseong-gun, Korea, 2008; pp. 106–109. [Google Scholar]
- Yoon, C.G. A Study on the LED Illumination Lamp Development and Application for Plant Factory. Ph.D. Thesis, Hongik University, Seoul, Korea, 2012; pp. 38–48.
- Hwang, M.K.; Huh, C.S.; Seo, Y.J. Optic characteristics comparison and analysis of SMD type Y/G/W HB LED. J. Kor. Ins. Illum. Electric. Install. Eng. 2004, 18, 15–21. [Google Scholar]
- Kim, T.S.; Lee, S.P.; Park, S.I.; Lee, J.Y.; Lee, S.Y.; Jun, H.Y. Physico-chemical properties of Broccoli sprouts cultivated in a plant factory system with different lighting conditions. J. Kor. Soc. Food Sci. Nut. 2011, 40, 1757–1763. [Google Scholar] [CrossRef]
- Lee, J.G.; Oh, S.S.; Cha, S.H.; Jang, Y.A.; Kim, S.Y.; Um, Y.C.; Cheong, S.R. Effects of red/blue light ratio and short-term light quality conversion on growth and anthocyanin contents of baby leaf lettuce. J. Bio-Environ. Control 2010, 19, 351–359. [Google Scholar]
- Li, Q.; Kubota, C. Effects of supplementary light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Hwang, M.H. Influences of Different Light Sources on Growth Characteristics and Biological Activities in Atractylodes japonica cv. ‘Dachul’ and A. macrocephala. Master’s Thesis, Kangwon National University, Chuncheon, Korea, 2021. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis, and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Bio. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Kim, H.J.; Chung, C.H.; Cho, Y.S. Antioxidative activities and contents of polyphenolic compound of Cudrania tricuspidata. J. Kor. Soc. Food Sci. Nutr. 1999, 28, 1310–1315. [Google Scholar]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Hetog, M.G.; Hollman, P.C.; Van de Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines and fruit juice. J. Agri. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Ryu, H.Y.; Jang, Y.J.; Jang, H.S.; Park, Y.M.; Kim, S.Y. Evaluation of antimicrobial, antithrombin, and antioxidant activity of aerial part of Saxifraga stolonifera. Kor. J. Micro Biotech. 2008, 36, 195–200. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Baek, G.Y.; Kwon, S.J.; Yoon, Y.C.; Kim, H.T. Effect of LED light wavelength on lettuce growth, vitamin C and anthocyanin contents. Protect. Hort. Plant Fac. 2014, 23, 19–25. [Google Scholar] [CrossRef]
- Lee, G.J.; Heo, J.W.; Jung, C.R.; Kim, H.H.; Jo, J.S.; Lee, J.G.; Lee, G.J.; Nam, S.Y.; Hong, E.Y. Effects of artificial light sources on growth and glucosinolate contents of hydroponically grown kale in plant factory. Protect. Hort. Plant Fac. 2016, 25, 77–82. [Google Scholar] [CrossRef]
- Lee, G.J.; Heo, J.W.; Kim, H.H.; Jung, C.R.; Kim, D.E.; Nam, S.Y. Effects of artificial light sources on growth and yield of Peucedanum japonicum hydroponically grown in plant factory. Protect. Hort. Plant Fac. 2016, 25, 16–23. [Google Scholar] [CrossRef]
- Wongnok, A.; Piluek, C.; Techasilpitak, T.; Tantivivat, S. Effects of light emitting diodes on micropropagation of Phalaenopsis orchids. Acta Hort. 2008, 788, 149–156. [Google Scholar] [CrossRef]
- Choi, C.S.; Lee, J.G.; Jang, Y.A.; Lee, S.G.; Oh, S.S.; Lee, H.J.; Um, Y.C. Effect of artificial light sources on growth and quality characteristics of leaf lettuce in closed plant factory system. J. Agri. Life Sci. 2016, 47, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.S.; Jeong, J.T.; Lee, J.H.; Park, C.G.; Choi, J.H.; Jang, G.Y.; Kim, J.W.; Chang, J.K.; Kim, D.H.; Lee, S.E. Comparison of anti-oxidative and anti-inflammatory effect of Atractylodes interspecific hybrid cultivar roots. Kor. J. Med. Crop Sci. 2018, 26, 391–400. [Google Scholar] [CrossRef]
- Lee, S.O.; Lee, H.J.; Yu, M.H.; Im, H.G.; Lee, I.S. Total polyphenol contents and antioxidant activities of methanol extracts from vegetables produced in Ullung island. Kor. J. Food Sci. Tech. 2005, 37, 233–240. [Google Scholar]
- Yoo, J.H.; Choi, J.H.; Kang, B.J.; Jeon, M.R.; Lee, C.O.; Kim, C.H.; Seong, E.S.; Heo, K.; Yu, C.Y.; Choi, S.K. Antioxidant and tyrosinase inhibition activity promoting effects of perilla by the light emitting plasma. Kor. J. Med. Crop Sci. 2017, 25, 37–44. [Google Scholar] [CrossRef]
- Heo, J.W.; Lee, J.S.; Lee, G.I.; Kim, H.H. Growth of kale seedlings affected by the control of light quality and intensity under smart greenhouse conditions with artificial lights. Kor. J. Environ. Agri. 2017, 36, 193–200. [Google Scholar] [CrossRef]
- Choi, H.L.; Seo, J.W.; Hwang, M.H.; Lee, H.I.; Kim, M.J.; Yu, C.Y. Growth characteristics and functional analysis of Salvia miltiorrhiza Bunge by artificial light sources. Kor. J. Med. Crop Sci. 2020, 28, 200–208. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Pimentel, T.; Serôdio, J.; Rosa, R.; Calado, R. Comparative performance of light emitting plasma(LEP) and light emitting diode(LED) in ex situ aquaculture of scleractinian corals. Aquaculture 2013, 402, 38–45. [Google Scholar] [CrossRef]
- Lawrence, T.; Willoughby, D.A.; Gilroy, D.W. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat. Rev. Immunol. 2002, 2, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kean, W.F.; Buchanan, W.W. The use of NSAIDs in rheumatic disorders 2005: A global perspective. Inflammopharmacol. 2005, 13, 343–370. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. A correlation analysis on chlorophyll content and SPAD value in tomato leaves. Hort. Res. 2017, 71, 37–42. [Google Scholar]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Tech. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Ramarathnam, N.; Suzuki, Y.; Ohkubo, T.; Takeuchi, M.; Ochi, H. Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources. J. Agri. Food Chem. 1996, 44, 37–41. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical-scavenging activity of propolis from several regions of argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Kobayashi, A.; Koguchi, Y.; Takahashi, S.; Kanzaki, H.; Kawazu, K. ST-1, a novel radical scavenger from fungus F-124. Biosci. Biotech. Biochem. 1993, 57, 1034–1036. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Green, L.C.; Wagnerm, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Analytic. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
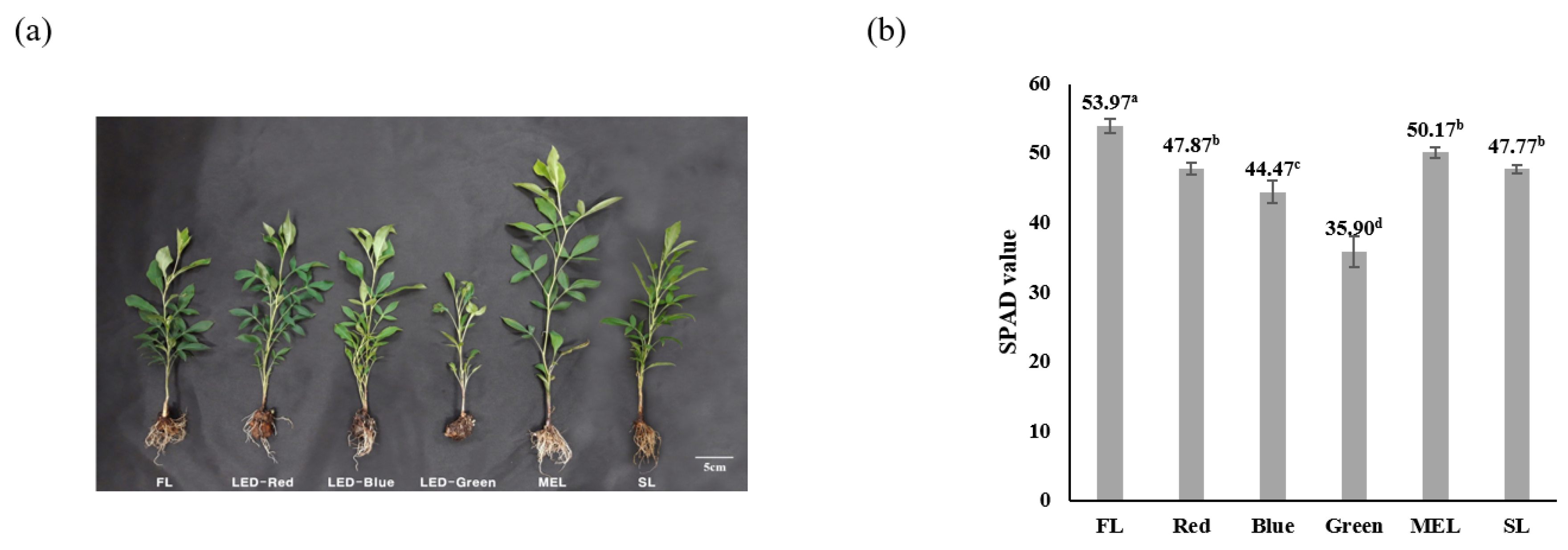
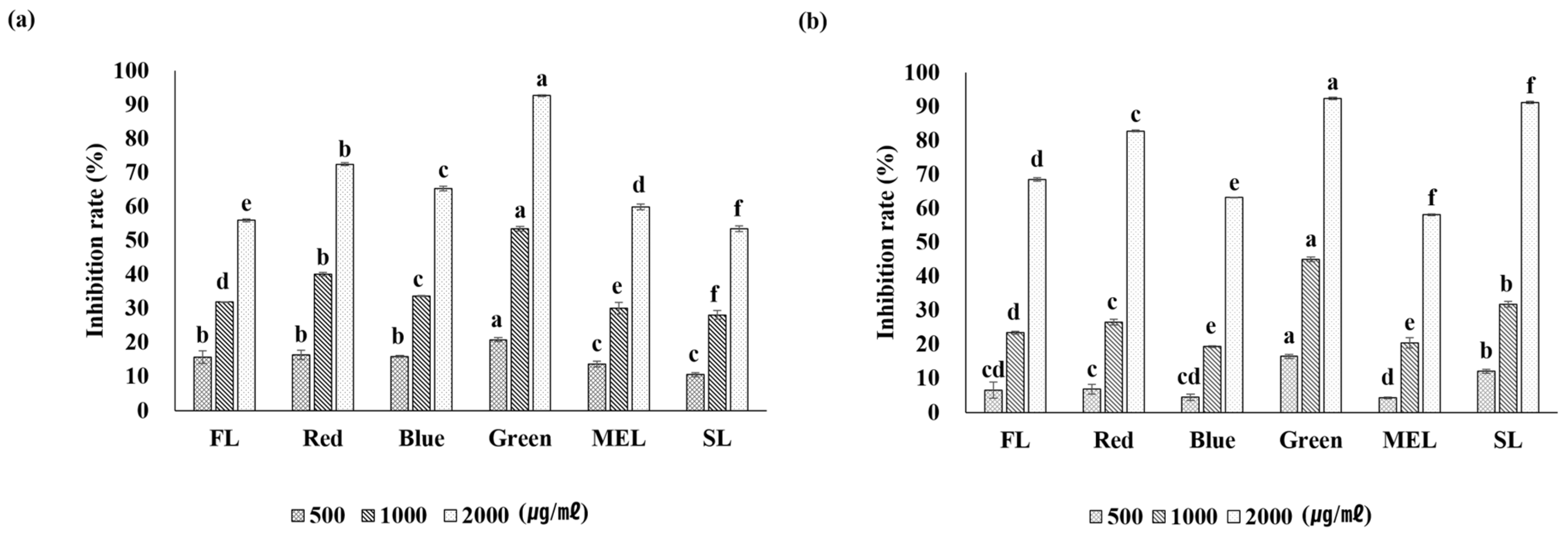
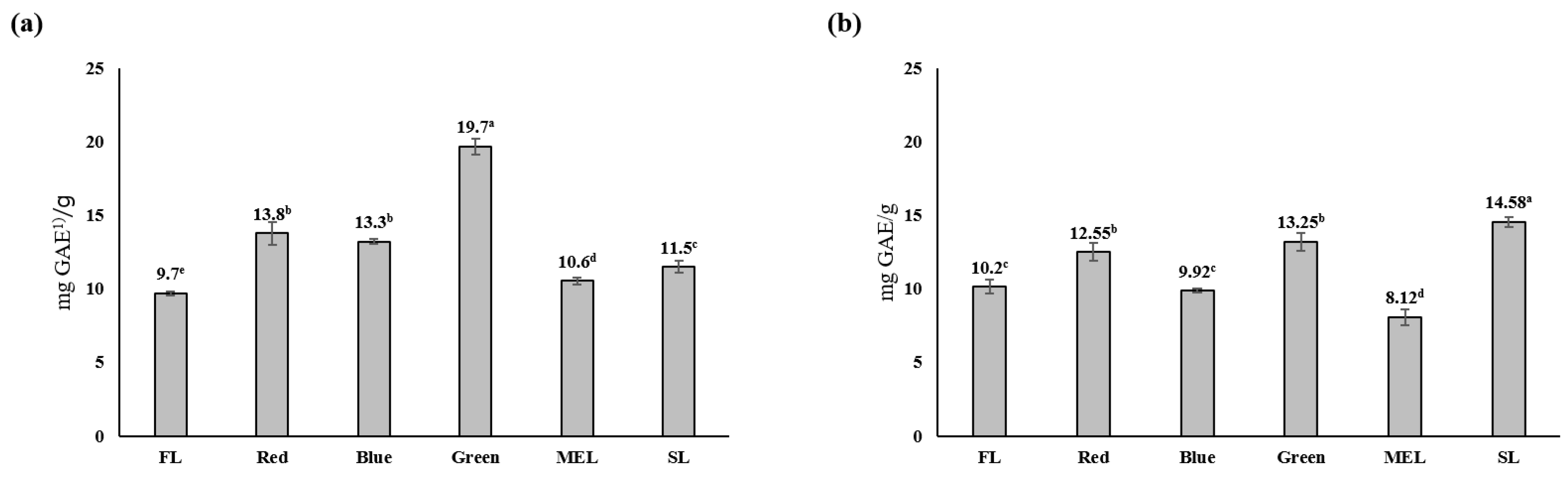
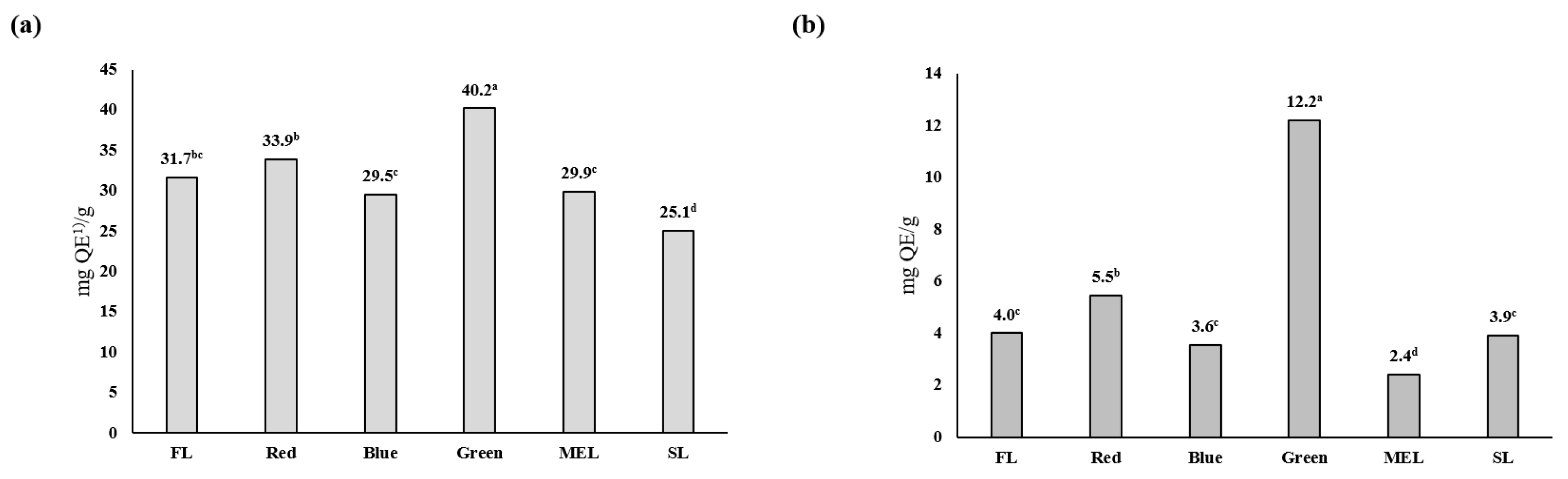

| Light Source | Aerial Part | Underground Part | |||||
|---|---|---|---|---|---|---|---|
| Plant Length (cm) | Leaf Length (cm) | Leaf Width (cm) | Number of Leaves (ea) | Dry Weight (g) | Root Length (cm) | Dry Weight (g) | |
| FL (1) | 24.47 ± 0.59 c | 6.03 ± 0.45 d | 5.20 ± 1.25 bc | 15.67 ± 0.58 a | 1.11 ± 0.06 ab | 12.13 ± 2.67 a | 0.19 ± 0.04 ab |
| LED-Red | 29.90 ± 3.06 b | 7.57 ± 0.64 ab | 6.23 ± 0.46 ab | 16.67 ± 1.53 a | 0.85 ± 0.06 b | 12.00 ± 1.20 a | 0.13 ± 0.01 bc |
| LED-Blue | 30.23 ± 1.94 b | 7.07 ± 0.51 bc | 5.57 ± 0.21 abc | 18.00 ± 1.63 a | 1.17 ± 0.24 ab | 13.66 ± 1.84 a | 0.18 ± 0.03 ab |
| LED-Green | 23.07 ± 1.56 c | 5.67 ± 0.06 d | 4.80 ± 0.00 c | 12.33 ± 0.58 b | 0.45 ± 0.18 c | 5.53 ± 0.57 b | 0.10 ± 0.01 c |
| MEL (2) | 38.20 ± 1.95 a | 8.27 ± 0.64 a | 6.87 ± 0.90 a | 17.67 ± 1.53 a | 1.14 ± 0.06 ab | 11.43 ± 1.10 a | 0.21 ± 0.06 a |
| SL (3) | 30.87 ± 3.06 b | 6.23 ± 0.50 cd | 4.97 ± 0.72 bc | 17.67 ± 2.31 a | 1.21 ± 0.01 a | 12.10 ± 2.00 a | 0.23 ± 0.06 a |
| Minimal Inhibitory Concentration (mg/mℓ) | |||||||
|---|---|---|---|---|---|---|---|
| Dachul | S. aureus | V. litoralis | B. subtilis | E. coli | S. typhimurium | P. aeruginosa | |
| Light Source | Plant Part | ||||||
| FL | Aerial | ND (1) | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥1 | ND | ≥1 | ND | ND | |
| LED-Red | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| LED-Blue | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥1 | ND | ≥0.5 | ND | ND | |
| LED-Green | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.25 | ND | ≥0.25 | ND | ND | |
| MEL | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| SL | Aerial | ND | ND | ND | ≥1 | ND | ≥1 |
| Underground | ND | ≥0.5 | ND | ≥0.5 | ND | ND | |
| Tetracycline | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | ≥0.007 | |
| Type | Wavelength | Light Intensity |
|---|---|---|
| Fluorescent light | continuous spectrum | 24 µmol/m2·s |
| LED-Red | 630 nm | 22 µmol/m2·s |
| LED-Blue | 450 nm | 14 µmol/m2·s |
| LED-Green | 520 nm | 12 µmol/m2·s |
| Microwave electrodeless light | continuous spectrum | 52 µmol/m2·s |
| Sun light | continuous spectrum | 2.8 µmol/m2·s |
| Microorganism | Media | Incubation Temperature | |
|---|---|---|---|
| Bacillus subtilis | KCTC 1021 | Nutrient | 30 °C |
| Staphylococcus aureus | KCTC 1916 | Nutrient | 37 °C |
| Escherichia coli | KCTC 1924 | Nutrient | 37 °C |
| Salmonella typhimurium | KCTC 1925 | Nutrient | 37 °C |
| Pseudomonas aeruginosa | KCTC 2742 | Nutrient | 37 °C |
| Vibrio litoralis | KCTC 13228 | Nutrient | 37 °C |
| Primer | Primer Sequence (5′ → 3′) | |
|---|---|---|
| β-actin | Forward Reverse | TGACGGGGTCACCCACACTGTGCCCATCTA CTAGAAGCATTTGCGGTGGACGATGGAGGG |
| iNOS | Forward Reverse | CCCTTCCGAAGTTTCTGGCAGCAGC GGCTGTCAGAGCCTCGTGGCTTTGG |
| COX-2 | Forward Reverse | CACTACATCCTGACCCACTT ATGCTCCTGCTTGAGTATGT |
| TNF-α | Forward Reverse | TTGACCTCAGCGCTGAGTTG CCTGTAGCCCACGTCGTAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, M.H.; Seo, J.W.; Park, B.J.; Han, K.J.; Lee, J.G.; Kim, N.Y.; Kim, M.J.; Seong, E.S. Evaluation of Growth Characteristics and Biological Activities of ‘Dachul’, a Hybrid Medicinal Plant of Atractylodes macrocephala × Atractylodes japonica, under Different Artificial Light Sources. Plants 2022, 11, 2035. https://doi.org/10.3390/plants11152035
Hwang MH, Seo JW, Park BJ, Han KJ, Lee JG, Kim NY, Kim MJ, Seong ES. Evaluation of Growth Characteristics and Biological Activities of ‘Dachul’, a Hybrid Medicinal Plant of Atractylodes macrocephala × Atractylodes japonica, under Different Artificial Light Sources. Plants. 2022; 11(15):2035. https://doi.org/10.3390/plants11152035
Chicago/Turabian StyleHwang, Myeong Ha, Ji Won Seo, Byung Jun Park, Kyeong Jae Han, Jae Geun Lee, Na Young Kim, Myong Jo Kim, and Eun Soo Seong. 2022. "Evaluation of Growth Characteristics and Biological Activities of ‘Dachul’, a Hybrid Medicinal Plant of Atractylodes macrocephala × Atractylodes japonica, under Different Artificial Light Sources" Plants 11, no. 15: 2035. https://doi.org/10.3390/plants11152035
APA StyleHwang, M. H., Seo, J. W., Park, B. J., Han, K. J., Lee, J. G., Kim, N. Y., Kim, M. J., & Seong, E. S. (2022). Evaluation of Growth Characteristics and Biological Activities of ‘Dachul’, a Hybrid Medicinal Plant of Atractylodes macrocephala × Atractylodes japonica, under Different Artificial Light Sources. Plants, 11(15), 2035. https://doi.org/10.3390/plants11152035






