Chemical Composition of Reichardia tingitana Methanolic Extract and Its Potential Antioxidant, Antimicrobial, Cytotoxic and Larvicidal Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic, Flavonoid, and Tannin Contents
2.2. Gas-Chromatography Mass Spectroscopy “GC-MS”
2.3. Biological Characteristics of the Plant Extracts
2.3.1. Antioxidant Activity—DPPH Assay
2.3.2. Antibacterial Activity
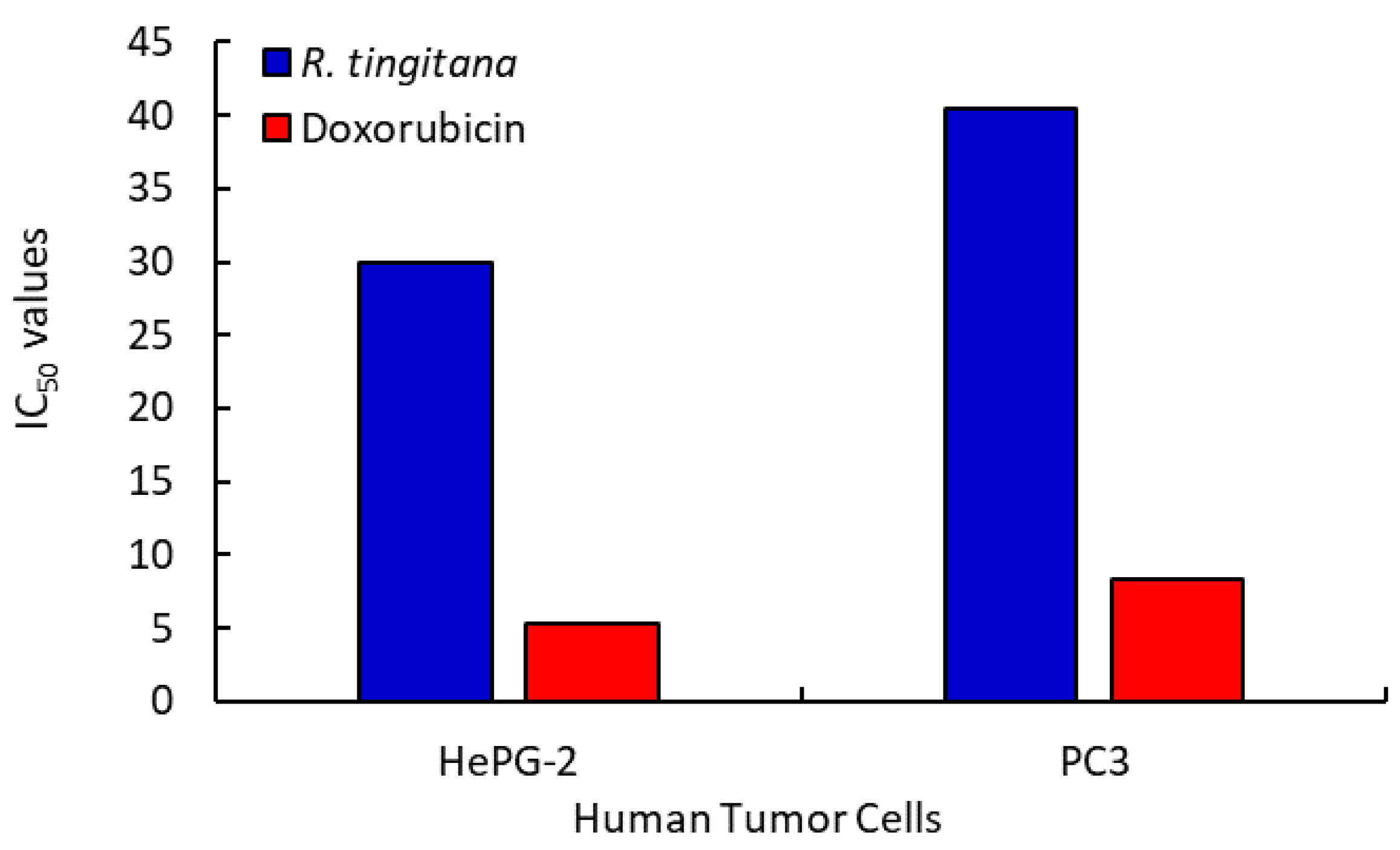
2.3.3. Anticancer Activity
2.3.4. Larvicidal Bioassay
3. Materials and Methods
3.1. Plant Material and Extraction Process
3.2. Phytochemical Analysis
3.2.1. Total Phenolic Contents
3.2.2. Total Flavonoid Contents
3.2.3. Total Tannin Contents
3.3. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
3.4. Antioxidant DPPH Assay
3.5. Antimicrobial Activity Procedure
3.6. Anticancer Activity Procedure
3.7. Mosquitocidal Assay
3.7.1. Rearing of Aedes aegypti
3.7.2. Larvicidal Activity Procedure
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memariani, Z.; Farzaei, M.H.; Ali, A.; Momtaz, S. Nutritional and Bioactive Characterization of Unexplored Food Rich in Phytonutrients. In Phytonutrients in Food; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–175. [Google Scholar]
- Gusain, P.; Uniyal, D.; Joga, R. Conservation and Sustainable Use of Medicinal Plants. In Preparation of Phytopharmaceuticals for the Management of Disorders; Apple Academic Press, Elsevier: Amsterdam, The Netherlands, 2021; pp. 409–427. [Google Scholar]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Tackholm, V. Students’ Flora of Egypt; Cairo University Publishing: Cairo, Egypt, 1974. [Google Scholar]
- Boulos, L. Flora of Egypt. Checklist; Al-Hadara Publishing: Cairo, Egypt, 1995. [Google Scholar]
- Boulos, L. Flora of Egypt; Al-Hadara Publishing: Cairo, Egypt, 2002; Volume 3. [Google Scholar]
- Sharma, P.; Prasad, G.; Archana, S. A comprehensive review: Medicinal plants with potential antidiabetic activity. Res. Rev. J. Herb. Sci. 2018, 4, 29–57. [Google Scholar]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Gawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.; Al-Rowaily, S.L. Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef]
- Recio, M.C.; Giner, R.M.; Hermenegildo, M.; Peris, J.B.; Mañez, S.; Rios, J.-L. Phenolics of Reichardia and their taxonomic implications. Biochem. Syst. Ecol. 1992, 20, 449–452. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. Anticancer, antimicrobial and antioxidant compounds of quinoa inflorescence. Adv. Life Sci. 2020, 8, 68–72. [Google Scholar]
- Javed, S.; Mahmood, Z.; Khan, K.M.; Sarker, S.D.; Javaid, A.; Khan, I.H.; Shoaib, A. Lupeol acetate as a potent antifungal compound against opportunistic human and phytopathogenic mold Macrophomina phaseolina. Sci. Rep. 2021, 11, 11. [Google Scholar]
- Gopal, R.; Vijayakumaran, M.; Venkatesan, R.; Kathiroli, S. Marine organisms in Indian medicine and their future prospects. Indian J. Nat. Prod. Resourc. 2008, 7, 139–145. [Google Scholar]
- Mostafa, H.; Elbakry, A.; Eman, A.A. Evaluation of antibacterial and antioxidant activities of different plant parts of Rumex vesicarius L. (Polygonaceae). Int. J. Pharm. Pharm. Sci. 2011, 3, 109–118. [Google Scholar]
- Weeratunga, P.; Rodrigo, C.; Fernando, S.D.; Rajapakse, S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017, 8, CD012759. [Google Scholar]
- Carvajal-Lago, L.; Ruiz-López, M.J.; Figuerola, J.; Martínez-de la Puente, J. Implications of diet on mosquito life history traits and pathogen transmission. Environ. Res. 2021, 195, 110893. [Google Scholar] [CrossRef] [PubMed]
- El Alfy, T.; El Tantawy, M.E.; Motaal, A.A.; Gamal, F.E.Z. Pharmacological, biological study and GC/MS analysis of the essential oil of the aerial parts and the alcohol soluble fraction of the n. Hexane extract of the flowers of Reichardia tingitana L. Can. J. Pure Appl. Sci. 2015, 9, 3167–3175. [Google Scholar]
- Daniewski, W.M.; Skibicki, P.; Gumułka, M.; Drożdż, B.; Grabarczyk, H.; Błoszczyk, E. Sesquiterpene lactones. XXXV. Constituents of Reichardia tingitana L. Roth. and their antifeedant activity. Acta Soc. Bot. Pol. 1988, 57, 539–545. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Abou-Elzahab, M.; Dawidar, A.; Ayyad, S. A sesquiterpene glucoside from Reichardia tingitana. Phytochemistry 1993, 34, 1434–1435. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Soufan, W.; Almutairi, K.F.; Zaghloul, N.S.; Abd-ElGawad, A.M. Proximate composition, bioactive compounds, and antioxidant potential of wild halophytes grown in coastal salt marsh habitats. Molecules 2021, 27, 28. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Zhang, Y.-T.; Guo, Y.-R.; Huang, Q.-L.; Peng, T.; Xu, Y.; Tang, L.; Chen, F. Antioxidant and anti-inflammatory activities of the phenolic extracts of Sapium sebiferum (L.) Roxb. leaves. J. Ethnopharmacol. 2013, 147, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Manickam, M.; Ramanathan, M.; Farboodniay Jahromi, M.; Chansouria, J.; Ray, A. Antihyperglycemic activity of phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, M.; Wu, K.; Chai, X.; Yu, H.; Tao, Z.; Wang, J. Immunomodulatory and anticancer activities of phenolics from emblica fruit (Phyllanthus emblica L.). Food Chem. 2012, 131, 685–690. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; la Rocca, A.; Marsili, S.; Mariotti, M. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M. Local Mediterranean food as a source of novel nutraceuticals. In Pharmaceutical Soc Great Britain; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Nengroo, Z.R.; Rauf, A. Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind. Crops Prod. 2019, 140, 111596. [Google Scholar] [CrossRef]
- Okwu, D.E. Citrus fruits: A rich source of phytochemicals and their roles in human health. Int. J. Chem. Sci. 2008, 6, 451–471. [Google Scholar]
- Al-Snafi, A.E. The medical benefit of Gnaphalium luteoalbum—A review. IOSR J. Pharm. 2019, 9, 40–44. [Google Scholar]
- Lu-Martínez, A.A.; Báez-González, J.G.; Castillo-Hernández, S.; Amaya-Guerra, C.; Rodríguez-Rodríguez, J.; García-Márquez, E. Studied of Prunus serotine oil extracted by cold pressing and antioxidant effect of P. longiflora essential oil. J. Food Sci. Technol. 2021, 58, 1420–1429. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.; Mashaly, I.A.; Al-Nafie, R.I. Antioxidant activity and allelopathic potential of five wild plants on germination and growth Bidens pilosa L. Int. J. Curr. Res. 2015, 7, 21019–21024. [Google Scholar]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophaë rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef] [PubMed]
- Alasbahi, R.H. In vitro antibacterial activity of some Yemeni medicinal plants. Univ. Aden J. Nat. Appl. Sci. 2008, 12, 641–647. [Google Scholar]
- Sassi, A.B.; Harzallah-Skhiri, F.; Aouni, M. Investigation of some medicinal plants from Tunisia for antimicrobial activities. Pharm. Biol. 2007, 45, 421–428. [Google Scholar] [CrossRef]
- Fayed, E.M.; Abd-EIGawad, A.M.; Elshamy, A.I.; El-Halawany, E.S.F.; EI-Amier, Y.A. Essential oil of Deverra tortuosa aerial parts: Detailed chemical profile, allelopathic, antimicrobial, and antioxidant activities. Chem. Biodivers. 2021, 18, e2000914. [Google Scholar] [CrossRef]
- Salama, S.A.; Al-Faifi, Z.E.; Masood, M.F.; El-Amier, Y.A. Investigation and Biological Assessment of Rumex vesicarius L. Extract: Characterization of the Chemical Components and Antioxidant, Antimicrobial, Cytotoxic, and Anti-Dengue Vector Activity. Molecules 2022, 27, 3177. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E.; Gatea, F. Correlations between microbiota bioactivity and bioavailability of functional compounds: A mini-review. Biomedicines 2020, 8, 39. [Google Scholar] [CrossRef]
- Ullah, M.F.; Khan, M.W. Food as medicine: Potential therapeutic tendencies of plant derived polyphenolic compounds. Asian Pac. J. Cancer Prev. 2008, 9, 187–196. [Google Scholar] [PubMed]
- Shin, J.; Prabhakaran, V.-S.; Kim, K.-S. The multi-faceted potential of plant-derived metabolites as antimicrobial agents against multidrug-resistant pathogens. Microb. Pathog. 2018, 116, 209–214. [Google Scholar] [CrossRef] [PubMed]
- El-Amier, Y.A.; Abdelghany, A.M.; Abed Zaid, A. Green synthesis and antimicrobial activity of Senecio glaucus-Mediated silver nanoparticles. Res. J. Pharm. Biol. Chem. 2014, 5, 631–642. [Google Scholar]
- Maccelli, A.; Vitanza, L.; Imbriano, A.; Fraschetti, C.; Filippi, A.; Goldoni, P.; Maurizi, L.; Ammendolia, M.G.; Crestoni, M.E.; Fornarini, S. Satureja montana L. Essential oils: Chemical profiles/phytochemical screening, antimicrobial activity and o/w nanoemulsion formulations. Pharmaceutics 2019, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Krochmal-Marczak, B.; Skiba, D.; Patra, J.K.; Das, S.K.; Das, G.; Popović-Djordjević, J.B.; Kostić, A.Ž.; Anil Kumar, N.V.; Tripathi, A. Convolvulus plant-A comprehensive review from phytochemical composition to pharmacy. Phytother. Res. 2020, 34, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Khan, N.; Afaq, F.; Mukhtar, H. Chemoprevention of prostate cancer through dietary agents: Progress and promise. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.A.; Ali, Z.; Wang, Y.-H.; El-Amier, Y.A.; Khan, S.I.; Khan, I.A. Cytotoxic steroidal saponins from Panicum turgidum Forssk. Steroids 2017, 125, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Csupor-Löffler, B.; Hajdú, Z.; Réthy, B.; Zupkó, I.; Máthé, I.; Rédei, T.; Falkay, G.; Hohmann, J. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part II. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1109–1115. [Google Scholar] [CrossRef]
- Khacha-Ananda, S.; Tragoolpua, K.; Chantawannakul, P.; Tragoolpua, Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pac. J. Cancer Prev. 2013, 14, 6991–6995. [Google Scholar] [CrossRef]
- Bazou, D.; Coakley, W.T.; Hayes, A.J.; Jackson, S.K. Long-term viability and proliferation of alginate-encapsulated 3-D HepG2 aggregates formed in an ultrasound trap. Toxicol. Vitr. 2008, 22, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yang, J.; Wang, H.; Simons, F.E.R. Production and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem. Mol. Biol. 1999, 29, 909–914. [Google Scholar] [CrossRef]
- Mohankumar, T.K.; Shivanna, K.S.; Achuttan, V.V. Screening of methanolic plant extracts against larvae of Aedes aegypti and Anopheles stephensi in Mysore. J. Arthropod-Borne Dis. 2016, 10, 303. [Google Scholar] [PubMed]
- Nasir, S.; Nasir, I.; Asrar, M.; Debboun, M. Larvicidal and pupicidal action of medicinal plant extracts against dengue mosquito Aedes albopictus (Skuse)(Diptera: Culicidae). Indian J. Anim. Res. 2017, 51, 155–158. [Google Scholar] [CrossRef][Green Version]
- Elango, G.; Rahuman, A.A.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Venkatesan, C. Laboratory study on larvicidal activity of indigenous plant extracts against Anopheles subpictus and Culex tritaeniorhynchus. Parasitol. Res. 2009, 104, 1381–1388. [Google Scholar] [CrossRef]
- Ullah, Z.; Ijaz, A.; Mughal, T.K.; Zia, K. Larvicidal activity of medicinal plant extracts against Culex quinquefasciatus Say. (Culicidae, Diptera). Int. J. Mosq. Res 2018, 5, 47–51. [Google Scholar]
- Shehata, A.Z. Biological activity of Prunus domestica (Rosaceae) and Rhamnus cathartica (Rhamnaceae) leaves extracts against the mosquito vector, Culex pipiens L.(Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. F Toxicol. Pest Control. 2019, 11, 65–73. [Google Scholar] [CrossRef]
- Dey, P.; Goyary, D.; Chattopadhyay, P.; Kishor, S.; Karmakar, S.; Verma, A. Evaluation of larvicidal activity of Piper longum leaf against the dengue vector, Aedes aegypti, malarial vector, Anopheles stephensi and filariasis vector, Culex quinquefasciatus. S. Afr. J. Bot. 2020, 132, 482–490. [Google Scholar] [CrossRef]
- Souza, M.M.; Silva, B.D.; Costa, C.S.; Badiale-Furlong, E. Free phenolic compounds extraction from Brazilian halophytes, soybean and rice bran by ultrasound-assisted and orbital shaker methods. An. Acad. Bras. Ciências 2018, 90, 3363–3372. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Issa, N.K.; Abdul Jabar, R.; Hammo, Y.; Kamal, I. Antioxidant activity of apple peels bioactive molecules extractives. Sci. Technol. 2016, 6, 76–88. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Burlingame, B. Wild nutrition. J. Food Compos. Anal. 2000, 13, 99–100. [Google Scholar] [CrossRef]
- Aberoumand, A. Nutritional evaluation of edible Portulaca oleracia as plant food. Food Anal. Methods 2009, 2, 204–207. [Google Scholar] [CrossRef]
- De Dobbeleer, I.; Gummersbach, J.; Huebschmann, H.-J.; Mayer, A.; Silcock, P. Analyzing PBDEs in House Dust Samples with the Thermo Scientific TSQ Quantum XLS Ultra GC-MS/MS in EI-SRM Mode; Thermo Fisher Scientific: Dreieich, Germany, 2012; pp. 1–6. [Google Scholar]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Fragr. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Parejo, I.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co (II)/EDTA-induced luminol chemiluminescence and DPPH·(2, 2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; de Capriles, C.H.; Pérez, C.; Colella, M.; Olaizola, C.; Ontiveros, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; Souza, S.M.D.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Bondock, S.; Adel, S.; Etman, H.A.; Badria, F.A. Synthesis and antitumor evaluation of some new 1, 3, 4-oxadiazole-based heterocycles. Eur. J. Med. Chem. 2012, 48, 192–199. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, T.M.; Hassan, M.I.; Moselhy, W.A.; Amer, M.S.; Shehata, A.Z. Evaluation of the biological activity of some Cupressus semprevirens (Cupressaceae) extracts against the mosquito vector Culex pipiens L.(Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 33–48. [Google Scholar] [CrossRef][Green Version]
- Briggs, J.D. Reduction of adult house-fly emergence by the effects of Bacillus spp. on the development of immature forms. J. Insect Pathol. 1960, 2, 418–432. [Google Scholar]
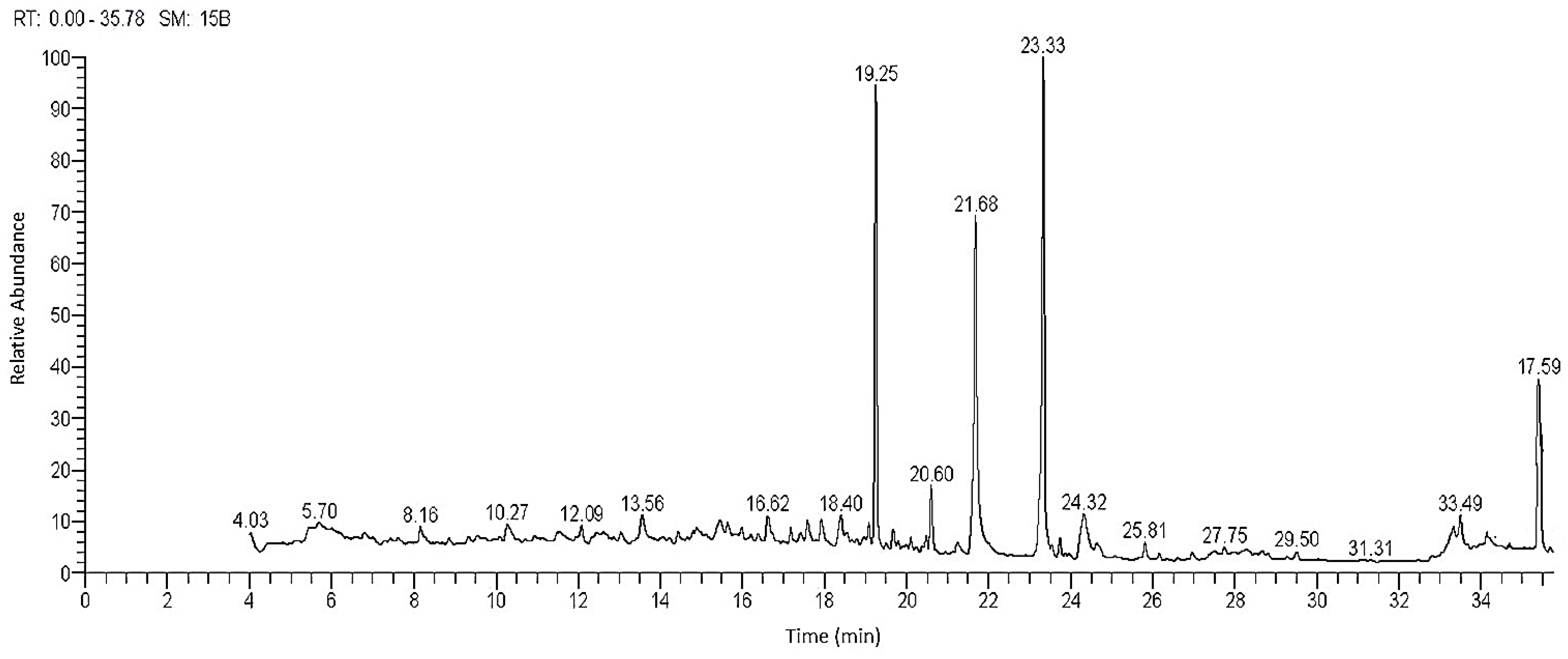
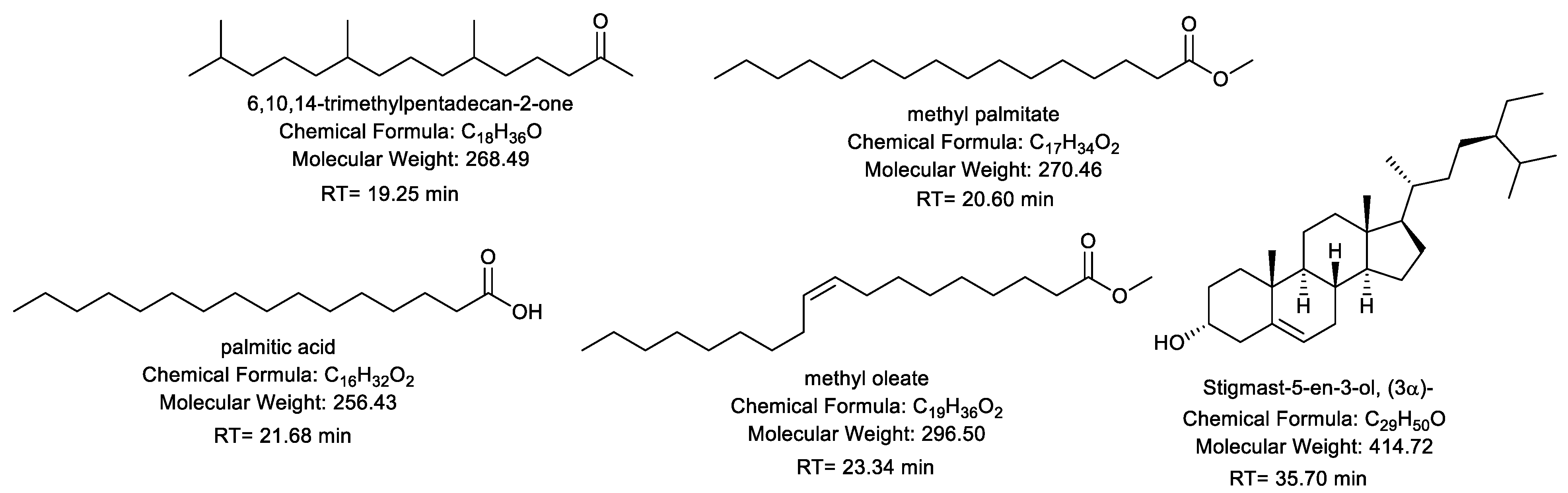
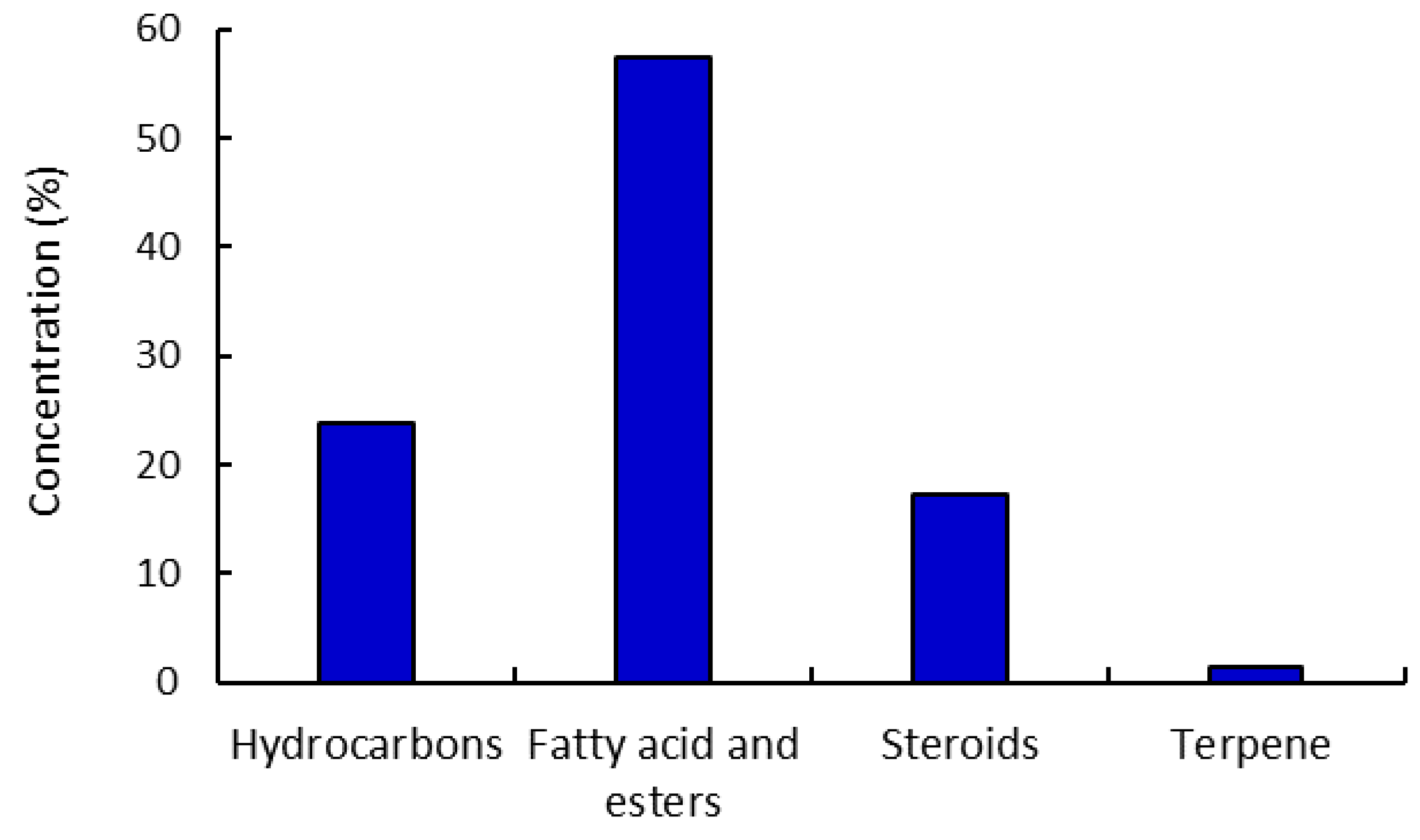

| No. | RT a | Conc. % b | Compound | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| Hydrocarbons | |||||
| 1 | 13.56 | 1.84 ± 0.04 | 5,5,8a-trimethylhexahydro-2H-chromen-4a(5H)-yl acetate | 240.34 | C14H24O3 |
| 2 | 19.25 | 21.98 ± 0.22 | 6,10,14-trimethylpentadecan-2-one | 268.49 | C18H36O |
| Fatty Acid and Esters | |||||
| 3 | 16.62 | 2.16 ± 0.05 | (E)-octadec-9-enoic acid | 282.47 | C18H34O2 |
| 4 | 17.92 | 1.44 ± 0.03 | Ethyl (9Z,12Z)-octadeca-9,12-dienoate | 308.51 | C20H36O2 |
| 5 | 19.08 | 1.13 ± 0.02 | Oleic acid | 282.47 | C18H34O2 |
| 6 | 19.67 | 1.16 ± 0.02 | Isobutyl octadecyl phthalate | 474.73 | C30H50O4 |
| 7 | 20.6 | 3.29 ± 0.07 | Methyl palmitate | 270.46 | C17H34O2 |
| 8 | 21.68 | 14.85 ± 0.18 | Palmitic acid | 256.43 | C16H32O2 |
| 9 | 23.34 | 27.26 ± 0.24 | Methyl oleate | 296.5 | C19H36O2 |
| 10 | 23.74 | 1.06 ± 0.02 | Methyl stearate | 298.51 | C19H38O2 |
| 11 | 24.31 | 3.56 ± 0.06 | (Z)-octadec-11-enoic acid | 282.47 | C18H34O2 |
| 12 | 33.5 | 1.55 ± 0.03 | 2-hydroxypropane-1,3-diyl (9E,9′E)-bis(octadec-9-enoate) | 621 | C39H72O5 |
| Steroids | |||||
| 13 | 18.4 | 1.75 ± 0.04 | Estra-1,3,5(10)-trien-17α-ol | 256.39 | C18H24O |
| 14 | 34.45 | 1.31 ± 0.02 | Ethyl 3,7,12-trihydroxycholan-24-oate | 436.63 | C26H44O5 |
| 15 | 35.39 | 6.63 ± 0.05 | 3,7,12-trihydroxycholan-24-oic acid | 408.58 | C24H40O5 |
| 16 | 35.7 | 7.57 ± 0.08 | Stigmast-5-en-3-ol, (3α)- | 414.72 | C29H50O |
| Terpene | |||||
| 17 | 17.59 | 1.48 ± 0.02 | Corymbolone (4a-hydroxy-4,8a-dimethyl-6-(prop-1-en-2-yl) octahydronaphthalen-1(2H)-one) | 236.36 | C15H24O2 |
| Total | 100.0 | ||||
| Treatment | Conc. (mg L−1) | Radical Scavenging Activity (%) | IC50 (mg L−1) |
|---|---|---|---|
| R. tingitana | 5 | 8.32 ± 0.42 F | 30.77 |
| 10 | 23.84 ± 1.64 E | ||
| 20 | 42.27 ± 2.37 D | ||
| 30 | 51.61 ± 2.88 C | ||
| 40 | 62.33 ± 3.20 B | ||
| 50 | 71.91 ± 3.72 A | ||
| LSD0.05 | 1.62 *** | ||
| Ascorbic acid | 1 | 2.81 ± 0.01 F | 12.02 |
| 2.5 | 11.38 ± 0.03 E | ||
| 5 | 38.57 ± 0.19 D | ||
| 10 | 47.92 ± 0.51 C | ||
| 15 | 61.34 ± 1.42 B | ||
| 20 | 72.61 ± 1.55 A | ||
| LSD0.05 | 1.40 *** |
| Microbes | R. tingitana (10 mg mL−1) | Standard Antibiotic (10 mg L−1) | |||
|---|---|---|---|---|---|
| Cephradin | Tetracycline | Azithromycin | Ampicillin | ||
| Gram-Negative Bacteria | |||||
| Escherichia coli | 22.85 ± 0.87 B | 17.81 ± 0.82 D | 20.34 ± 0.71 BC | 20.45 ± 0.51 B | 20.51 ± 0.73 C |
| Enterobacter aerogenes | 11.36 ± 0.44 E | 0.00 F | 0.00 F | 14.56 ± 0.63 C | 0.00 F |
| Salmonella typhimurium | 25.71 ± 1.63 A | 0.00 F | 12.82 ± 0.54 D | 9.35 ± 0.07 D | 0.00 F |
| Klebsella pneumonius | 15.13 ± 0.51 D | 12.08 ± 0.42 E | 20.17 ± 0.68 C | 13.75 ± 0.49 C | 8.03 ± 0.09 E |
| Gram-Positive bacteria | |||||
| Bacillus cereus | 24.42 ± 0.81 AB | 20.33 ± 0.55 BC | 12.09 ± 0.50 D | 19.44 ± 0.42 B | 10.62 ± 0.42 D |
| Clostridium tetani | 9.25 ± 0.04 F | 0.00 F | 8.54 ± 0.05 E | 0.00 D | 10.51 ± 0.27 D |
| Staphylococcus aureus | 18.62 ± 0.64 C | 21.82 ± 0.57 B | 22.77 ± 0.61 AB | 20.08 ± 0.91 B | 30.67 ± 1.92 A |
| Streptococcus pyogenes | 13.66 ± 0.32 D | 25.60 ± 1.31 A | 23.63 ± 1.58 A | 23.74 ± 0.87 A | 22.07 ± 1.37 C |
| Staphylococcus xylosus | 10.54 ± 0.51 E | 19.73 ± 0.98 C | 21.15 ± 1.65 ABC | 22.81 ± 0.69 A | 25.32 ± 1.51 B |
| LSD0.05 | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** | 0.0001 *** |
| Samples | Conc. (µg mL−1) | In Vitro Cytotoxicity (%) | ||
|---|---|---|---|---|
| HePG-2 | PC3 | WI-38 | ||
| R. tingitana | 100 | 93.14 | 91.41 | 9.55 |
| 50 | 88.34 | 81.62 | 8.33 | |
| 25 | 85.07 | 75.31 | 5.58 | |
| 12.5 | 68.52 | 58.22 | 3.67 | |
| 6.25 | 50.59 | 41.72 | 1.48 | |
| 3.125 | 38.99 | 25.38 | 1.32 | |
| 1.56 | 25.62 | 22.93 | 0.95 | |
| IC50 | 29.977 | 40.479 | >100 | |
| Doxorubicin | 100 | 63.15 | 70.07 | 12.23 |
| 50 | 54.03 | 46.83 | 9.52 | |
| 25 | 49.58 | 37.61 | 6.31 | |
| 12.5 | 41.39 | 32.42 | 4.98 | |
| 6.25 | 34.56 | 24.27 | 3.32 | |
| 3.125 | 23.14 | 14.65 | 2.45 | |
| 1.56 | 6.47 | 3.87 | 1.13 | |
| IC50 | 5.274 | 8.303 | >100 | |
| Conc. (mg L−1) | R. tingitana | ||
|---|---|---|---|
| 24 h Post-Treatment | 48 h Post-Treatment | 72 h Post-Treatment | |
| 300 | 32.54 ± 1.41 A | 41.28 ± 2.03 A | 50.81 ± 2.37 A |
| 250 | 28.33 ± 1.23 A | 31.51 ± 1.39 B | 42.17 ± 1.83 AB |
| 200 | 18.16 ± 0.79 B | 29.37 ± 1.25 B | 34.62 ± 1.44 BC |
| 150 | 11.32 ± 0.42 C | 19.09 ± 0.83 C | 28.06 ± 1.07 C |
| 100 | 6.35 ± 0.31 CD | 10.13 ± 0.44 CD | 15.74 ± 0.52 D |
| Control | 1.05 ± 0.05 D | 1.05 ± 0.05 D | 1.05 ± 0.05 E |
| F-value | 166.13 *** | 110.75 *** | 142.10 *** |
| p-value | <0.0001 *** | <0.0001 *** | <0.0001 *** |
| LC50 | 46.85 | 35.75 | 29.38 |
| LC90 | 82.66 | 63.82 | 53.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, S.A.; Al-Faifi, Z.E.; El-Amier, Y.A. Chemical Composition of Reichardia tingitana Methanolic Extract and Its Potential Antioxidant, Antimicrobial, Cytotoxic and Larvicidal Activity. Plants 2022, 11, 2028. https://doi.org/10.3390/plants11152028
Salama SA, Al-Faifi ZE, El-Amier YA. Chemical Composition of Reichardia tingitana Methanolic Extract and Its Potential Antioxidant, Antimicrobial, Cytotoxic and Larvicidal Activity. Plants. 2022; 11(15):2028. https://doi.org/10.3390/plants11152028
Chicago/Turabian StyleSalama, Salama A., Zarraq E. Al-Faifi, and Yasser A. El-Amier. 2022. "Chemical Composition of Reichardia tingitana Methanolic Extract and Its Potential Antioxidant, Antimicrobial, Cytotoxic and Larvicidal Activity" Plants 11, no. 15: 2028. https://doi.org/10.3390/plants11152028
APA StyleSalama, S. A., Al-Faifi, Z. E., & El-Amier, Y. A. (2022). Chemical Composition of Reichardia tingitana Methanolic Extract and Its Potential Antioxidant, Antimicrobial, Cytotoxic and Larvicidal Activity. Plants, 11(15), 2028. https://doi.org/10.3390/plants11152028







