Extending the Shelf Life of Fresh Khalal Barhi Dates via an Optimized Postharvest Ultrasonic Treatment
Abstract
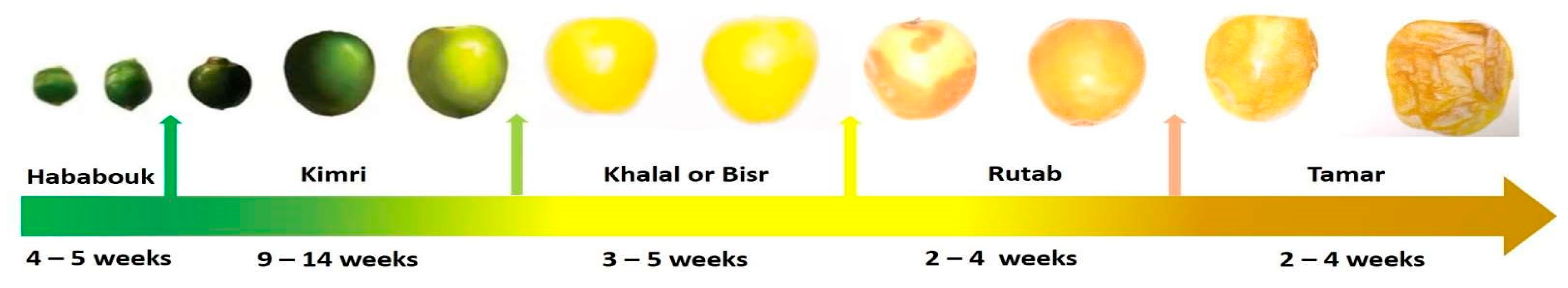
:1. Introduction
2. Results and Discussion
2.1. Model Fitting
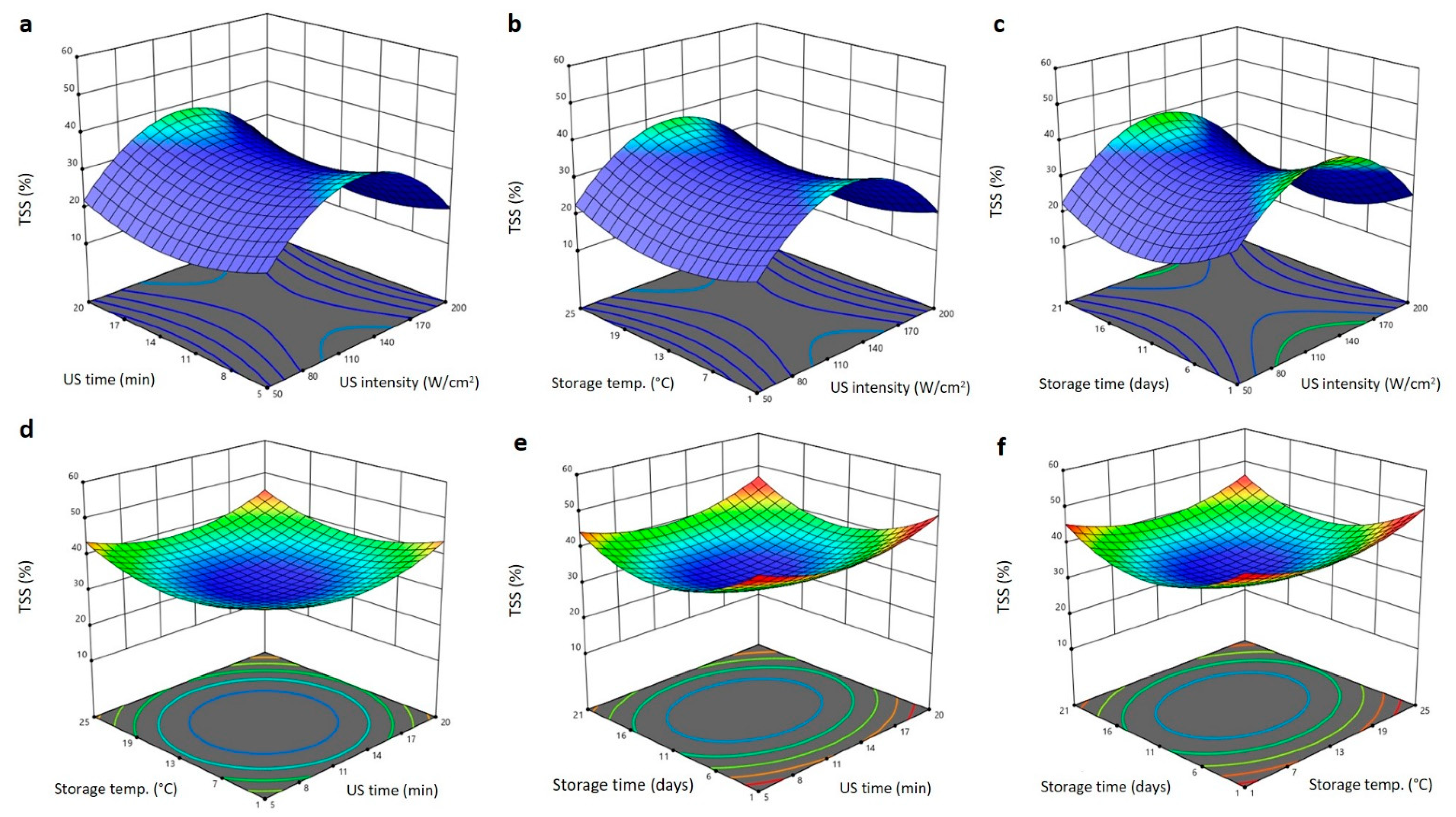
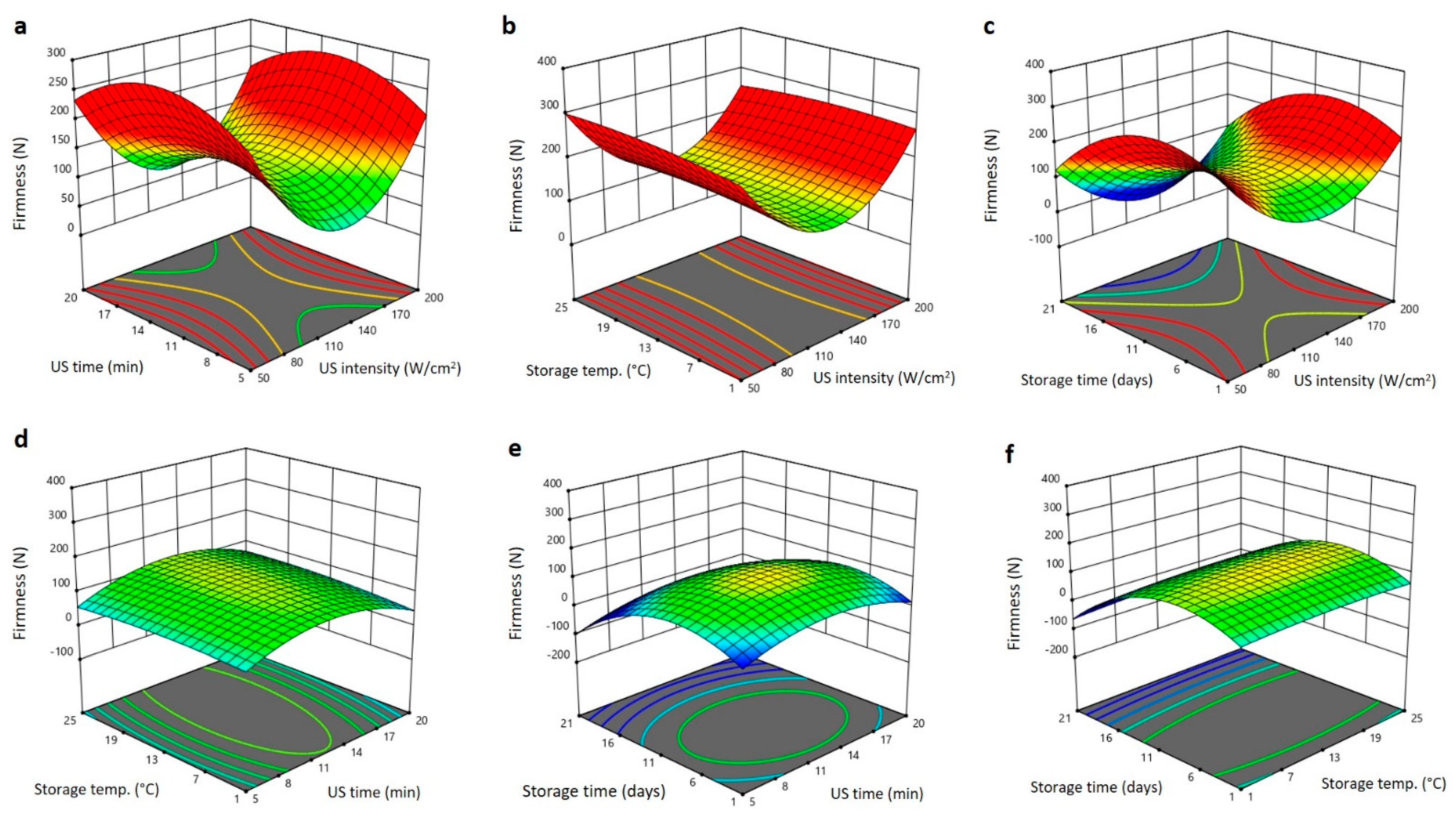
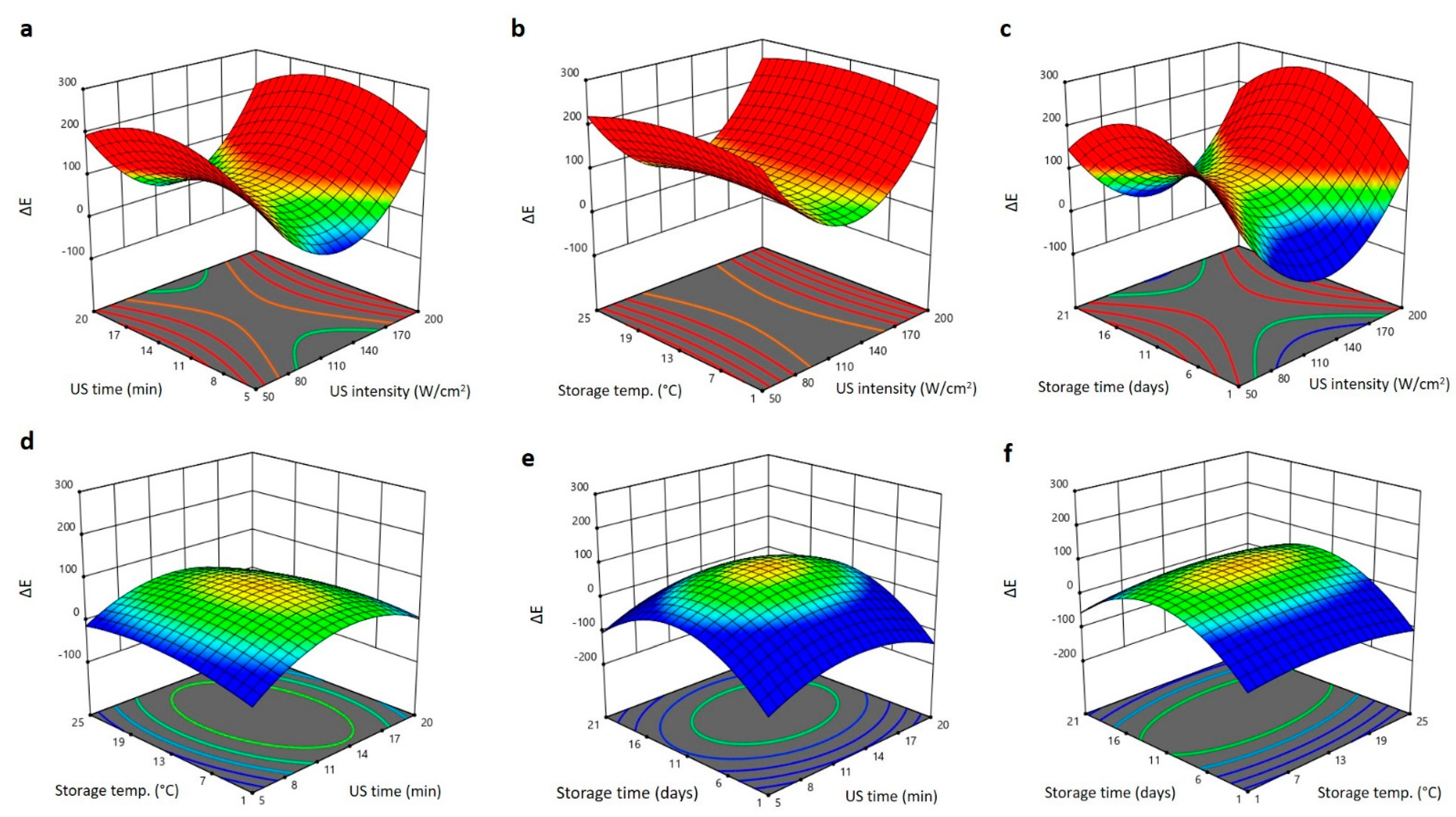
2.2. Effect of US Treatment and Storage Environments on Physical Properties of Barhi Dates
2.3. Effect of US Treatment and Storing Conditions on Total Viable Count (TVC) of Barhi Dates
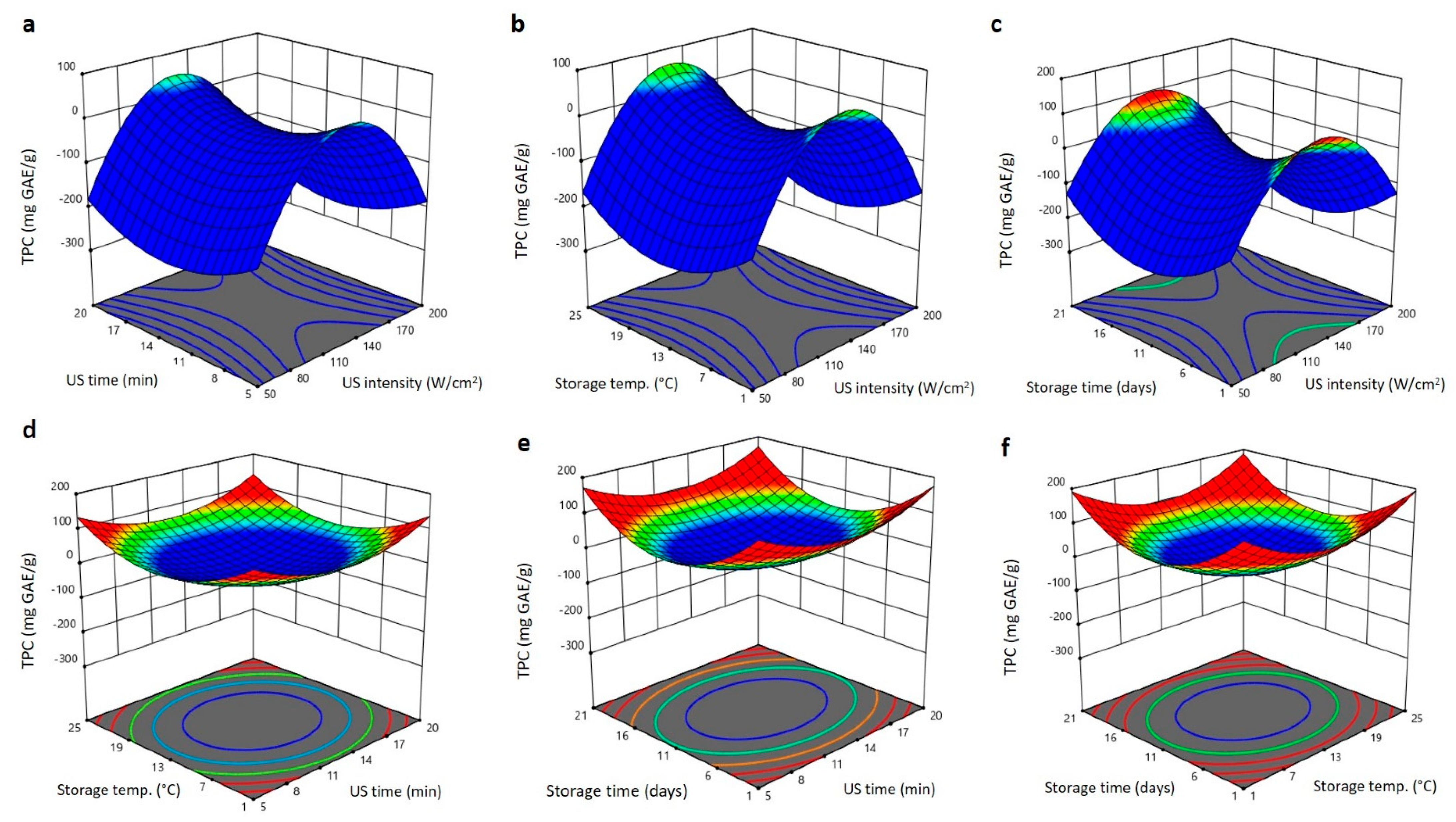
2.4. Effect of US Treatment and Storing Conditions on Bioactive Features of Barhi Dates
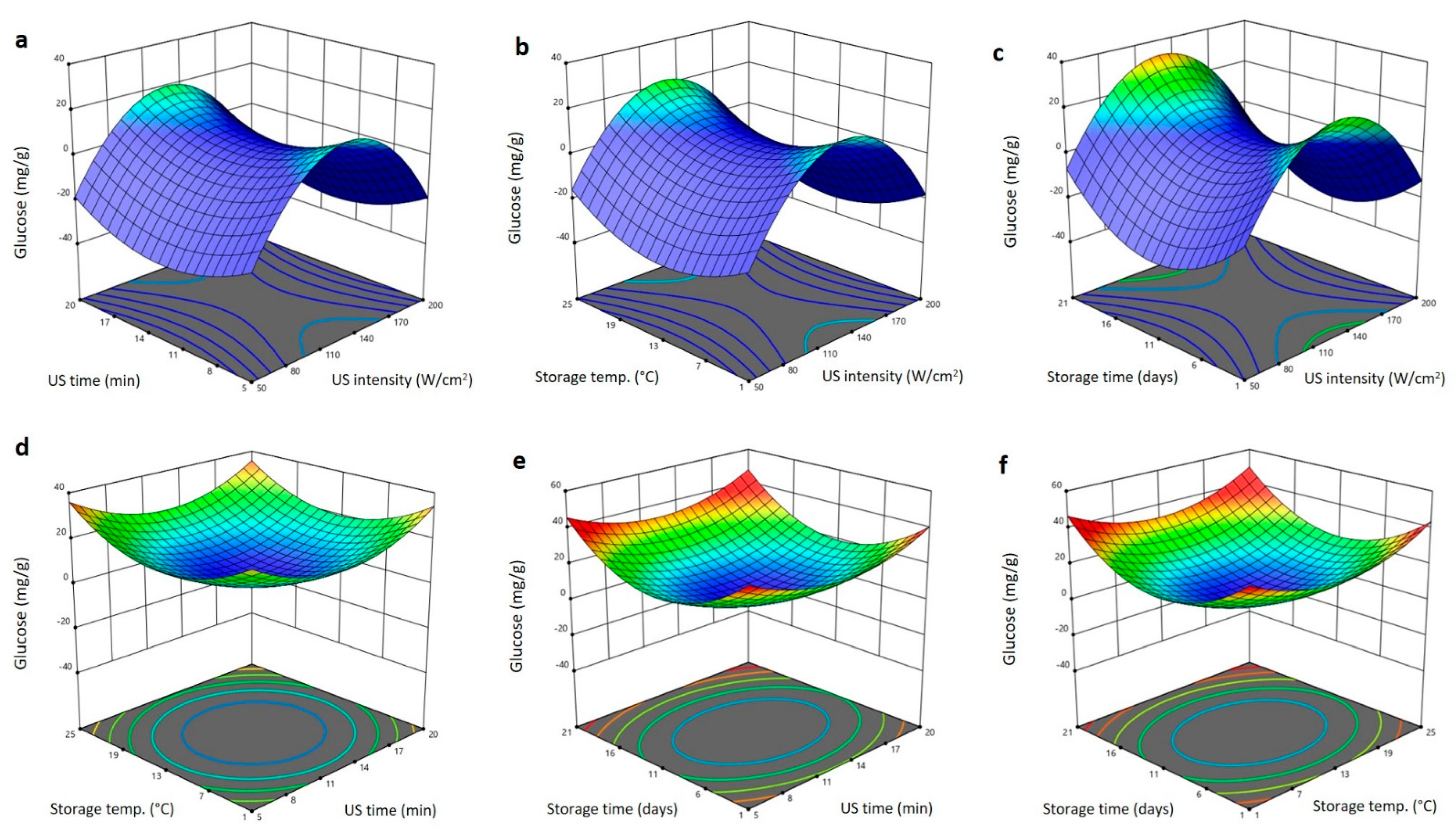
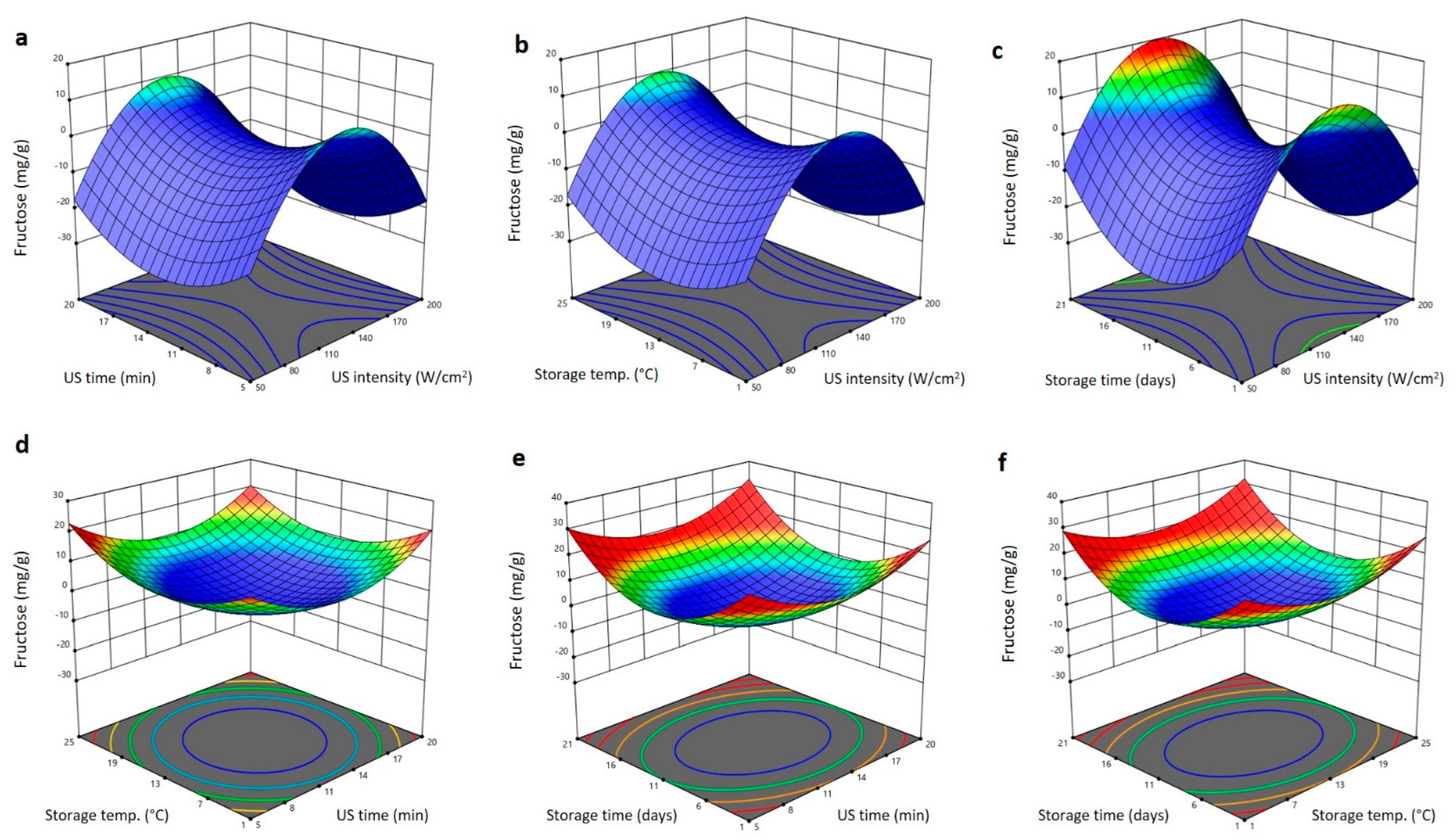
2.5. Effect of US Treatment and Storing Conditions on Glucose and Fructose Content of Barhi Dates
3. Materials and Methods
3.1. Barhi Date Fruits
3.2. Ultrasonic Treatment
3.3. Designing Experiment
3.4. Determination of Quality Features of Barhi Dates
3.4.1. Determination of TSS
3.4.2. Determination of Firmness
3.4.3. Determination of Surface Color
3.4.4. Determination of Total Viable Count (TVC)
3.4.5. Determination of Total Phenolic Content (TPC) and DPPH Antiradical Activity
3.4.6. Determination of Glucose and Fructose
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahia, E.M.; Lobo, M.G.; Kader, A.A. Harvesting and Postharvest Technology of Dates. In Dates: Postharvest Science, Processing Technology and Health Benefits; Siddiq, M., Aleid, S.M., Kader, A.A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 105–135. [Google Scholar]
- Paul, V.; Pandey, R.; Srivastava, G.C. The fading distinctions between classical patterns of ripening in climacteric and non-climacteric fruit and the ubiquity of ethylene—An overview. J. Food Sci. Technol. 2012, 49, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Deroanne, C.; Drira, N.; Attia, H. Date flesh: Chemical composition and characteristics of the dietary fiber. Food Chem. 2008, 111, 676–682. [Google Scholar] [CrossRef]
- Biglari, F.; AlKarkhi, A.F.M.; Easa, M.A. Cluster analysis of antioxidant compounds in dates (Phoenix dactylifera): Effect of long-term cold storage. Food Chem. 2009, 112, 998–1001. [Google Scholar] [CrossRef]
- Al-Turki, S.; Shahba, M.A.; Stushnoff, C. Diversity of antioxidant properties and phenolic content of date palm (Phoenix dactylifera L.) fruits as affected by cultivar and location. J. Food Agric. Environ. 2010, 8, 253–260. [Google Scholar]
- Ramadan, B.R.; Selim, M.A.A.; Nagy, K.S.A.; Mohamed, Z.S. Influence of Packaging and Cold Storage Conditions on the Physiochemical Properties of Barhi Date Fruits. Assiut J. Agric. Sci. 2020, 51, 79–91. [Google Scholar]
- Atia, A.; Abdelkarim, D.; Younis, M.; Alhamdan, A. Effects of Gibberellic Acid (GA3) and Salicylic Acid (SA) postharvest treatments on the quality of fresh barhi dates at different ripening levels in the Khalal maturity stage during controlled atmosphere storage. Int. J. Agric. Biol. Eng. 2018, 11, 211–219. [Google Scholar] [CrossRef] [Green Version]
- Al-Qurashi, A.D.; Awad, M.A. Quality characteristics of bisir ‘Barhee’ dates during cold storage as affected by postharvest dipping in gibberellic acid, naphthalene acetic acid and benzyladenine. Fruits 2011, 66, 343–352. [Google Scholar] [CrossRef]
- Kentish, M.; Ashokkumar, S. The physical and chemical effects of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 1–12. [Google Scholar]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonic in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, D.W. Innovative applications of power ultrasound during food freezing processes—A review. Trend Food Sci. Technol. 2006, 17, 16–23. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Cryogenic freezing of fresh date fruits for quality preservation during frozen storage. J. Saudi Soc. Agric. Sci. 2018, 17, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Abdelkarim, D.O.; Ahmed, K.A.; Younis, M.; Yehia, H.M.; El-Abedein, A.I.Z.; Alhamdan, A.; Ahmed, I.A.M. Optimization of Infrared Postharvest Treatment of Barhi Dates Using Response Surface Methodology (RSM). Horticulturae 2022, 8, 342. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al Juhaimi, F.Y.; Fadimu, G.J.; Osman, M.A.; Al Maiman, S.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT-Food Sci. Technol. 2021, 140, 110816. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Muzaffar, S.; Ahmad, M.; Wani, S.M.; Adil, G.; Baba, W.N.; Shah, U.; Khan, A.A.; Masoodi, F.A.; Gani, A.; Wani, T.A. Ultrasound treatment: Effect on physicochemical, microbial and antioxidant properties of cherry (Prunus avium). J. Food Sci. Technol. 2016, 53, 2752–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.; Zhang, M.; Jiang, F. Ultrasound treatment to modified atmospheric packaged fresh-cut cucumber: Influence on microbial inhibition and storage quality. Ultrason. Sonochem. 2019, 54, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.M.; Nadeem, M.; Maken, F.; Tayyaba, A.; Majeed, H.; Munir, M. Influence of ultrasound on the functional characteristics of indigenous varieties of mango (Mangifera indica L.). Ultrason. Sonochem. 2020, 64, 104987. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, L. Effect of ultrasound treatment on microbial inhibition and quality maintenance of green asparagus during cold storage. Ultrason. Sonochem. 2019, 58, 104631. [Google Scholar] [CrossRef] [PubMed]
- Vivek, K.; Subbarao, K.V.; Srivastava, B. Optimization of postharvest ultrasonic treatment of kiwifruit using RSM. Ultrason. Sonochem. 2016, 32, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ee, C.T. Drying Kinetics and Quality of Dried Kedondong Fruits Undergoing Hybrid Drying Processes. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L.M. Influence of postharvest ultrasounds treatments on tomato (Solanum lycopersicum, cv. Zinac) quality and microbial load during storage. Ultrason. Sonochem. 2015, 27, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Alsawmahi, O.N.; Al-Juhaimi, F.Y.; Alhamdan, A.M.; Ghafoor, K.; Mohamed Ahmed, I.A.; Hassan, B.H.; Ehmed, K.A.; Abdelkarim, D.; Younis, M.; Alashmawe, N.; et al. Enzyme activity, sugar composition, microbial growth and texture of fresh Barhi dates as affected by modified atmosphere packaging. J. Food Sci. Technol. 2018, 55, 4492–4504. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkh, K. Modification of food ingredients by ultrasound to improve functionality, a preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Z.; Pang, B.; Wang, H.; Xie, H.; Wu, F. Effect of ultrasound treatment on fruit decay and quality maintenance in strawberry after harvest. Food Control 2010, 21, 529–532. [Google Scholar] [CrossRef]
- Vega Gálvez, A.; Uribe, E.; Poblete, J.; García, V.; Pastén, A.; Aguilera, L.E.; Stucken, K. Comparative study of dehydrated papaya (Vasconcellea pubescens) by different drying methods: Quality attributes and effects on cells viability. J. Food Meas. Charact. 2021, 15, 2524–2530. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Sugars and organic acids in Japanese plums (Prunus salicina Lindell) as influenced by maturation, harvest date, storage temperature and period. Int. J. Food Sci. Technol. 2009, 44, 1973–1982. [Google Scholar] [CrossRef]
- Alhamdan, A.; Hassan, B.; Alkahtani, H.; Abdelkarim, D.; Younis, M. Freezing of fresh Barhi dates for quality preservation during frozen storage. Saudi J. Biol. Sci. 2018, 25, 1552–1561. [Google Scholar] [CrossRef] [Green Version]
- Maskan, M. Kinetics of color change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists—International, Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bouhlali, E.T.; Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Alem, C. Nutritional, mineral and organic acid composition of syrups produced from six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. Food Compos. Anal. 2020, 93, 103591. [Google Scholar] [CrossRef]


| Factors | TSS | Hardness | ΔE | TVC | TPC | DPPH | Fructose | Glucose |
|---|---|---|---|---|---|---|---|---|
| Intercept | ||||||||
| β0 | 27.677 * | 332.997 ** | 156.733 *** | 32.134 ** | 4.962 * | 63.455 ** | 14.580 * | 24.782 ** |
| Linear | ||||||||
| X1 (β1) | 0.677 * | −7.010 ** | −8.115 *** | −1.258 ** | 10.360 | 0.660 ** | 1.232 | 1.728 |
| X2 (β2) | −2.717 | 25.638 | 33.753 | 3.148 | −31.550 | −1.259 | −5.101 * | −5.826 * |
| X3 (β3) | −0.988 | 1.9432 | 5.038 ** | 1.381 * | −25.005 ** | 0.693 ** | −1.634 * | −2.312 * |
| X4 (β4) | −2.513 ** | 18.583 *** | 27.525 ** | 4.476 * | −34.684 ** | −1.611 ** | −4.345 | −5.322 |
| Interaction | ||||||||
| X1X2 (β12) | 0.016 * | 0.049 | −0.042 | 0.015 * | −0.013 | −0.027 | −0.029 | −0.091 |
| X1X3 (β13) | 0.010 | −0.100 * | 0.058 ** | 0.040 | 0.012 | −0.028 ** | −0.071 | −0.012 |
| X1X4 (β14) | 0.020 ** | 0.024 | 0.063 ** | 0.012 ** | −0.021 | 0.012 | 0.077 | 0.014 |
| X2X3 (β23) | 0.055 | 0.085 * | −0.154 ** | −0.024 *** | 0.017 | 0.125 | −0.013 | −0.091 |
| X2X4 (β24) | 0.084 | −0.029 | 0.079 ** | 0.011 ** | −0.075 | −0.025 | 0.012 | 0.072 |
| X3X4 (β34) | −0.018 | 0.040 | 0.075 ** | 0.031 | −0.044 | −0.011 | 0.038 | 1.091 |
| Quadratic | ||||||||
| X1² (β11) | −0.029 | 0.028 | 0.033 * | 0.050 | −0.041 ** | −0.030 | −0.049 | −0.068 |
| X2² (β22) | 0.100 | −1.083 | −1.228 | −0.129 | 1.248 ** | 0.023 | 0.208 | 0.242 |
| X3² (β33) | 0.039 | −0.069 | −0.179 * | −0.043 | 0.619 ** | −0.072 | 0.075 | 0.104 |
| X4² (β44) | 0.092 | −1.134 | −1.254 | −0.209 * | 1.256 ** | 0.061 | 0.195 | 0.244 |
| Model F-value | 3.55 | 15.59 | 29.57 | 9.47 | 5.76 | 10.42 | 2.74 | 5.69 |
| p-value | 0.019 | 0.001 | 0.0002 | 0.005 | 0.019 | 0.004 | 0.016 | 0.010 |
| Mean | 37.97 | 99.71 | 35.88 | 2.28 | 31.90 | 60.14 | 12.21 | 21.24 |
| C.V. % | 3.61 | 2.516 | 1.877 | 1.779 | 3.593 | 1.901 | 1.088 | 3.731 |
| Adeq. precision | 9.341 | 11.489 | 16.535 | 8.654 | 13.370 | 10.506 | 15.250 | 7.913 |
| R² | 0.934 | 0.984 | 0.992 | 0.974 | 0.958 | 0.977 | 0.926 | 0.925 |
| Adjusted R2 | 0.871 | 0.921 | 0.958 | 0.871 | 0.921 | 0.883 | 0.882 | 0.875 |
| Std. Dev. | 1.37 | 15.12 | 6.73 | 1.32 | 5.08 | 5.42 | 2.55 | 3.68 |
| F-value (Lack of Fit) | 2.38 | 4.19 | 2.92 | 0.230 | 8.11 | 1.37 | 0.981 | 5.129 |
| p-value (Lack of Fit) | 0.075 | 0.096 | 0.148 | 0.652 | 0.086 | 0.295 | 0.062 | 0.064 |
| Independent Variables | Level | ||||
|---|---|---|---|---|---|
| US Intensity, W/cm2 (X1) | 50 (−1) | 100 (−0.333) | 150 (0.333) | 200 (1) | |
| US time, min (X2) | 5 (−1) | 10 (−0.333) | 15 (0.333) | 20 (1) | |
| Storage temperature, °C (X3) | 1 (−1) | 5 (−0.667) | 15 (0.167) | 25 (1) | |
| Storage time, days (X4) | 1 (−1) | 6 (−0.5) | 11 (0) | 16 (0.5) | 21 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelkarim, D.O.; Mohamed Ahmed, I.A.; Ahmed, K.A.; Younis, M.; Yehia, H.M.; Zein El-Abedein, A.I.; Alhamdan, A. Extending the Shelf Life of Fresh Khalal Barhi Dates via an Optimized Postharvest Ultrasonic Treatment. Plants 2022, 11, 2029. https://doi.org/10.3390/plants11152029
Abdelkarim DO, Mohamed Ahmed IA, Ahmed KA, Younis M, Yehia HM, Zein El-Abedein AI, Alhamdan A. Extending the Shelf Life of Fresh Khalal Barhi Dates via an Optimized Postharvest Ultrasonic Treatment. Plants. 2022; 11(15):2029. https://doi.org/10.3390/plants11152029
Chicago/Turabian StyleAbdelkarim, Diaeldin O., Isam A. Mohamed Ahmed, Khaled A. Ahmed, Mahmoud Younis, Hany M. Yehia, Assem I. Zein El-Abedein, and Abdulla Alhamdan. 2022. "Extending the Shelf Life of Fresh Khalal Barhi Dates via an Optimized Postharvest Ultrasonic Treatment" Plants 11, no. 15: 2029. https://doi.org/10.3390/plants11152029
APA StyleAbdelkarim, D. O., Mohamed Ahmed, I. A., Ahmed, K. A., Younis, M., Yehia, H. M., Zein El-Abedein, A. I., & Alhamdan, A. (2022). Extending the Shelf Life of Fresh Khalal Barhi Dates via an Optimized Postharvest Ultrasonic Treatment. Plants, 11(15), 2029. https://doi.org/10.3390/plants11152029









