Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus trichocarpa)
Abstract
:1. Introduction
2. Results
2.1. Genome-Wide Identification and Evolutionary Relationship of HXKs
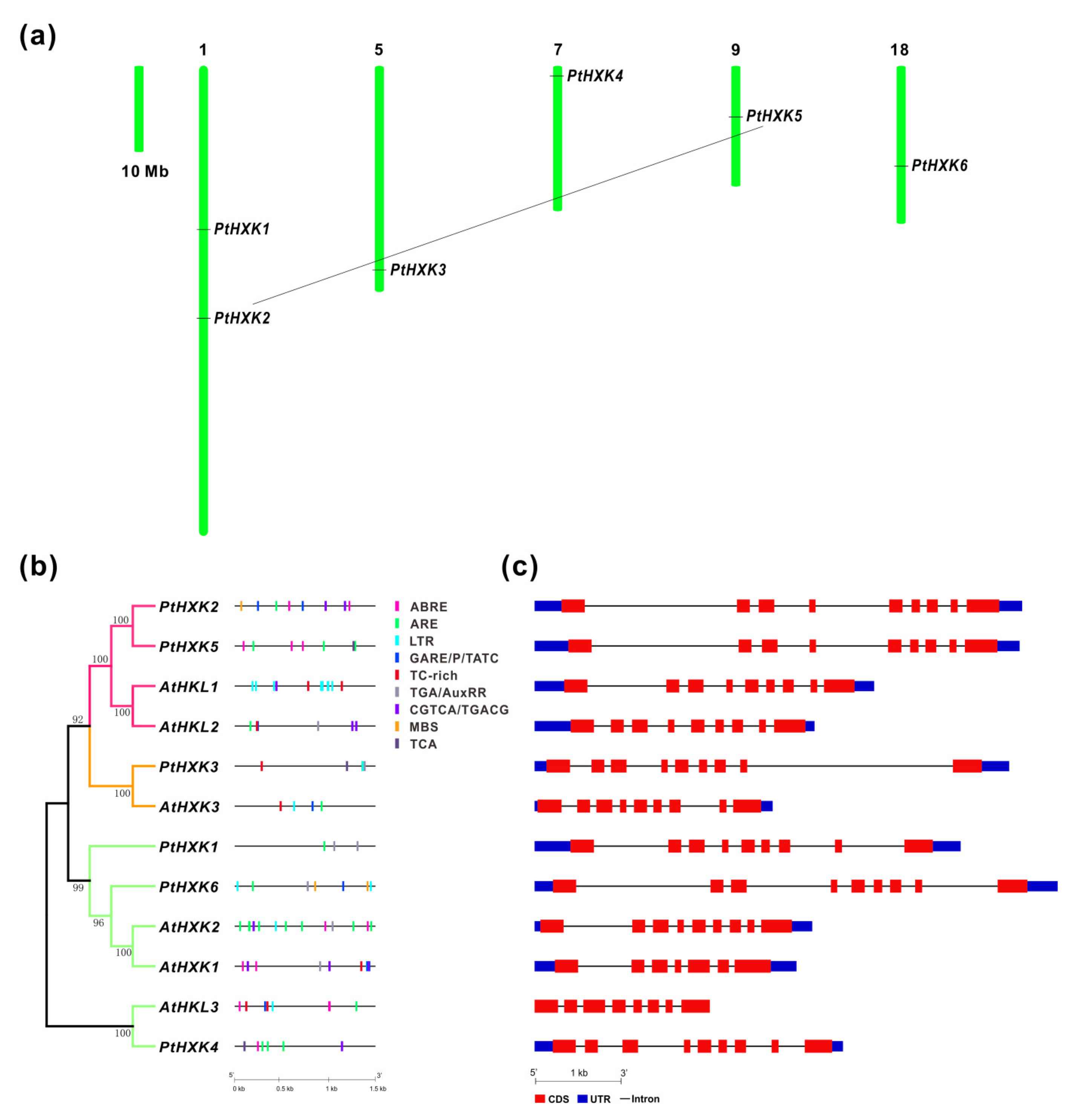
2.2. Chromosomal Location, Cis-Regulatory Elements, and Genomic Structure of HXKs
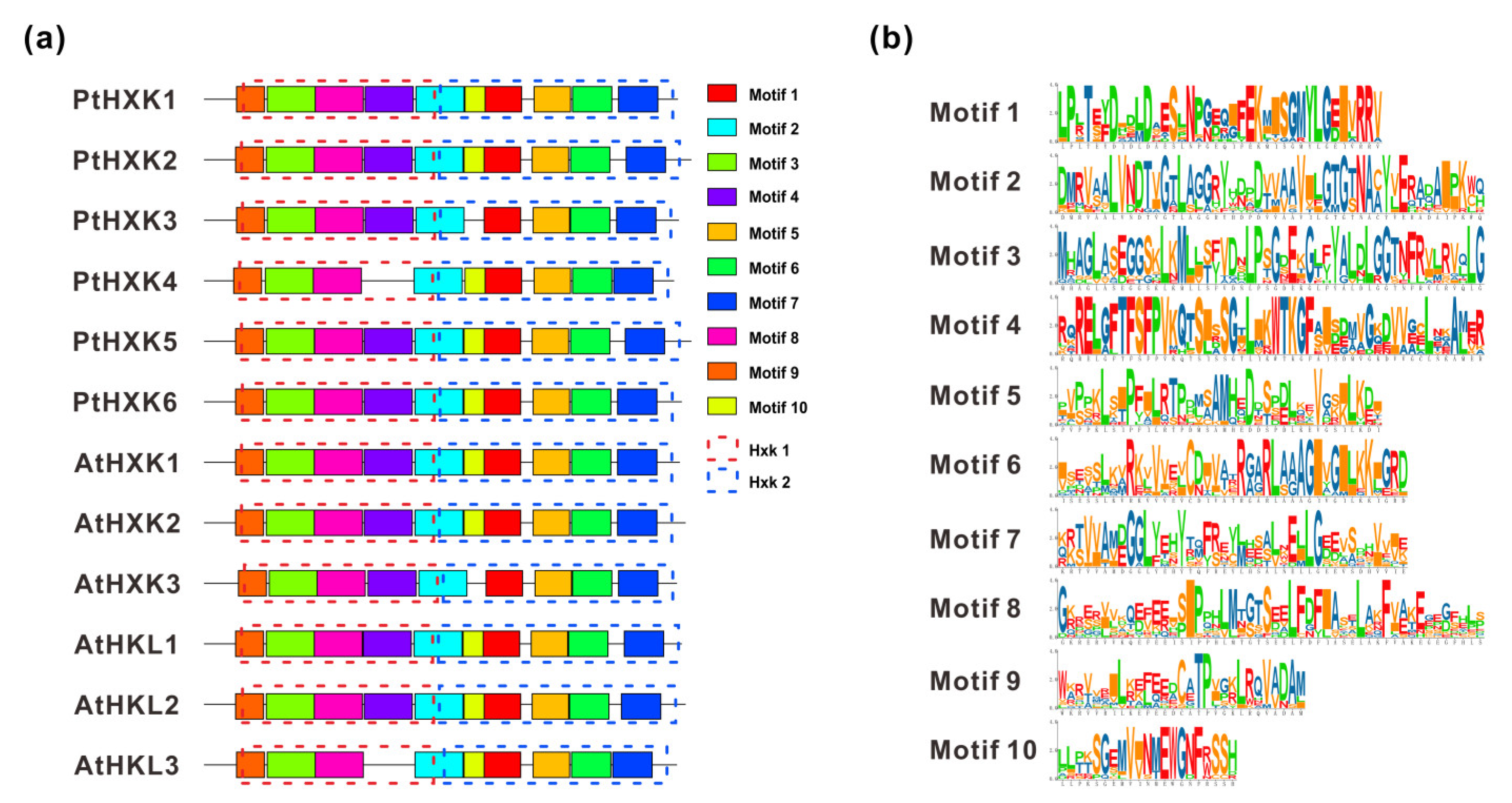
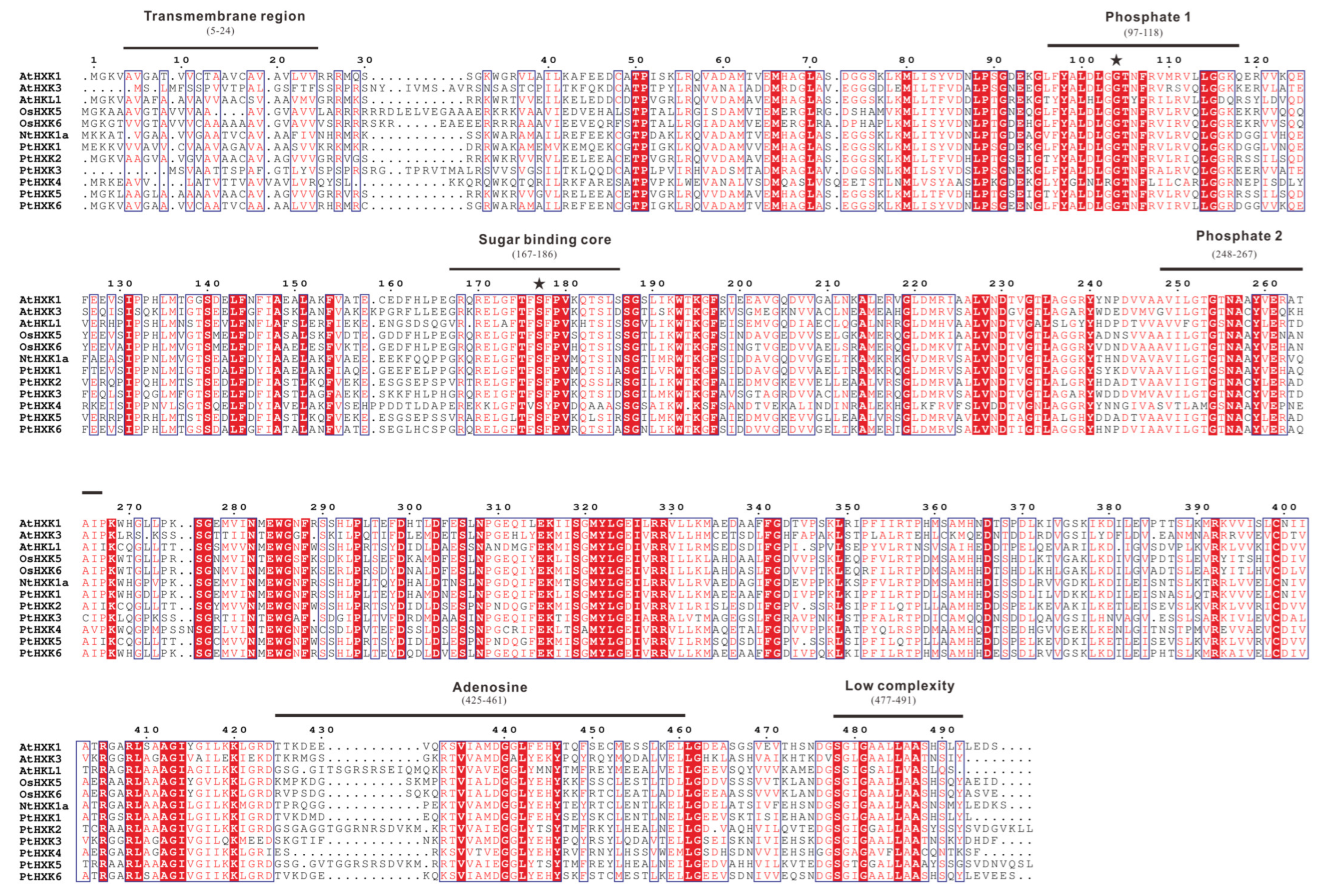
2.3. Conserved Motifs and Domains in Protein Sequences of PtHXKs
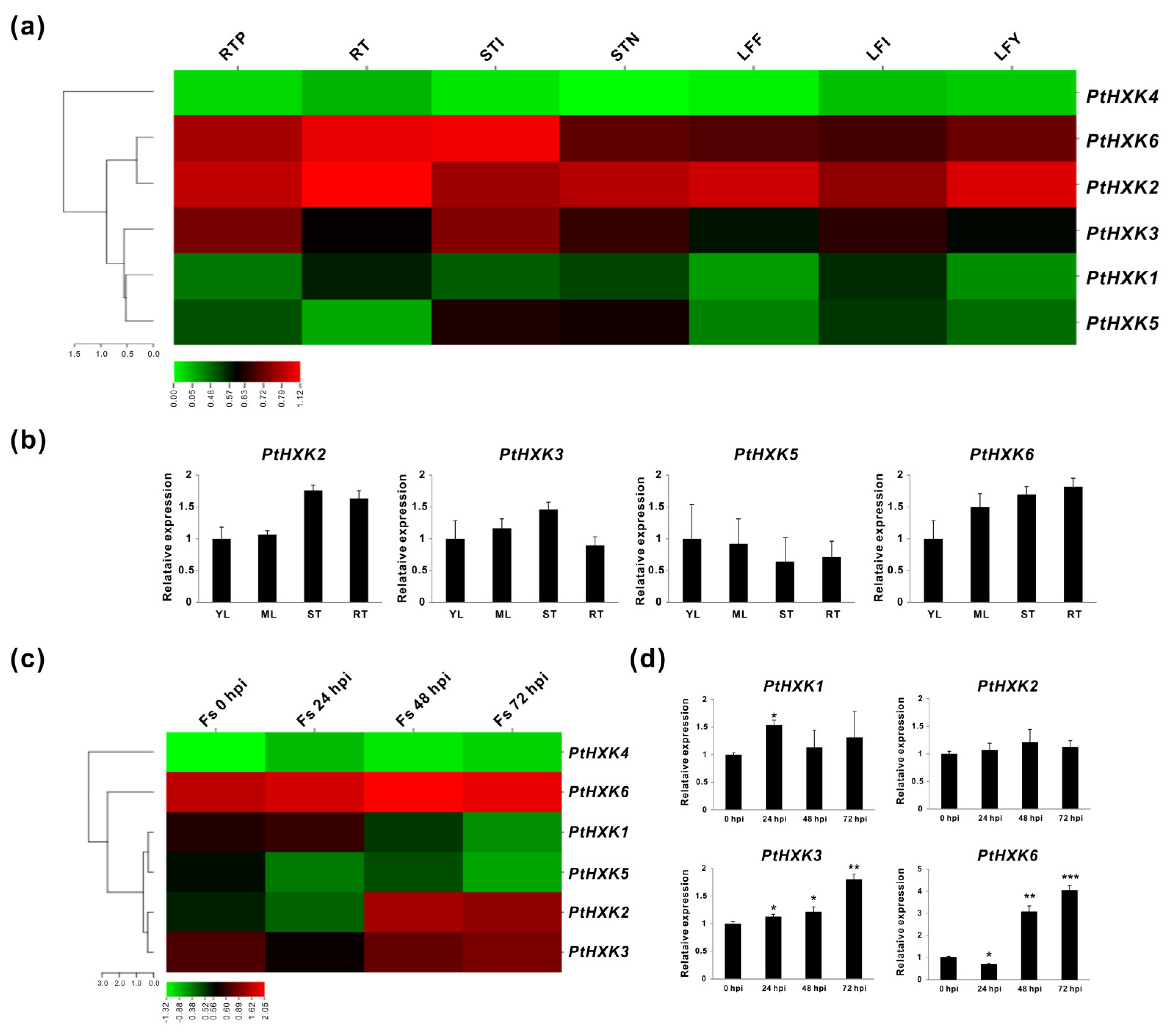
2.4. Transcriptomics of PtHXKs in Vegetative Tissues and upon Pathogenic Fungi Infection
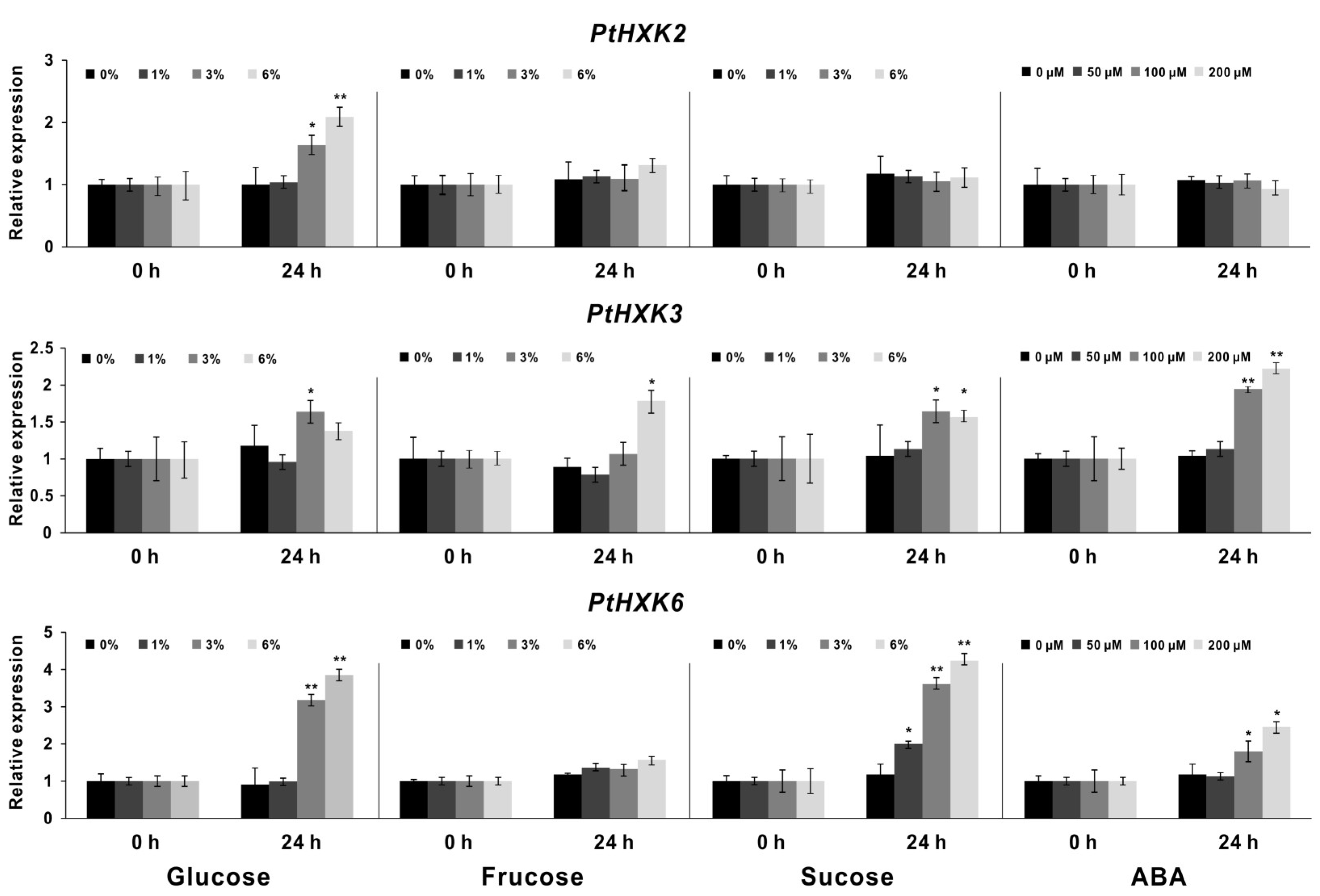
2.5. Effects of PtHXKs Expression Responding to Sugars and ABA treatments
3. Discussion
3.1. The HXKs Family and Conserved Profiles in Plant Species
3.2. The Regulatory Role of HXKs in Defense and Stress Acclimation
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Treatments
4.2. Sequence Mining, Identification, and Genomic Analyses of HXKs
4.3. Phylogenetic Tree Construction and Analyses of Conserved Motifs
4.4. Transcriptome and Expression Validation by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karve, R.; Lauria, M.; Virnig, A.; Xia, X.; Rauh, B.L.; Moore, B.D. Evolutionary Lineages and Functional Diversification of Plant Hexokinases. Mol. Plant 2010, 3, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y.-L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; David-Schwartz, R.; Kelly, G. Hexose Kinases and Their Role in Sugar-Sensing and Plant Development. Front. Plant Sci. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claeyssen, É.; Dorion, S.; Clendenning, A.; He, J.Z.; Wally, O.; Chen, J.; Auslender, E.L.; Moisan, M.-C.; Jolicoeur, M.; Rivoal, J. The Futile Cycling of Hexose Phosphates Could Account for the Fact That Hexokinase Exerts a High Control on Glucose Phosphorylation but Not on Glycolytic Rate in Transgenic Potato (Solanum tuberosum) Roots. PLoS ONE 2013, 8, e53898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pego, J.V.; Smeekens, S.C.M. Plant fructokinases: A sweet family get-together. Trends Plant Sci. 2000, 5, 531–536. [Google Scholar] [CrossRef]
- Granot, D.; Kelly, G.; Stein, O.; David-Schwartz, R. Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J. Exp. Bot. 2014, 65, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Alvarado, G.P.; Sánchez-Nieto, S. Plant Hexokinases are Multifaceted Proteins. Plant Cell Physiol. 2017, 58, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Sheen, J. Master regulators in plant glucose signaling networks. J. Plant Biol. 2014, 57, 67–79. [Google Scholar] [CrossRef]
- Karve, A.; Rauh, B.L.; Xia, X.; Kandasamy, M.; Meagher, R.B.; Sheen, J.; Moore, B.D. Expression and evolutionary features of the hexokinase gene family in Arabidopsis. Planta 2008, 228, 411–425. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-H.; Yoo, S.-D.; Sheen, J. Regulatory Functions of Nuclear Hexokinase1 Complex in Glucose Signaling. Cell 2006, 127, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-B.; Cho, J.-I.; Ryoo, N.; Shin, D.-H.; Park, Y.-I.; Hwang, Y.-S.; Lee, S.-K.; An, G.; Jeon, J.-S. Role of rice cytosolic hexokinase OsHXK7 in sugar signaling and metabolism. J. Integr. Plant Biol. 2016, 58, 127–135. [Google Scholar] [CrossRef]
- Xu, F.-Q.; Li, X.-R.; Ruan, Y.-L. RNAi-mediated suppression of hexokinase gene OsHXK10 in rice leads to non-dehiscent anther and reduction of pollen germination. Plant Sci. 2008, 175, 674–684. [Google Scholar] [CrossRef]
- Cho, J.-I.; Ryoo, N.; Eom, J.-S.; Lee, D.-W.; Kim, H.-B.; Jeong, S.-W.; Lee, Y.-H.; Kwon, Y.-K.; Cho, M.-H.; Bhoo, S.H.; et al. Role of the Rice Hexokinases OsHXK5 and OsHXK6 as Glucose Sensors. Plant Physiol. 2009, 149, 745–759. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, J.; Chen, Y.; Li, R.; Wang, H.; Ding, L.; Wei, J. Isolation, structural analysis, and expression characteristics of the maize (Zea mays L.) hexokinase gene family. Mol. Biol. Rep. 2014, 41, 6157–6166. [Google Scholar] [CrossRef]
- Geng, M.-T.; Yao, Y.; Wang, Y.-L.; Wu, X.-H.; Sun, C.; Li, R.-M.; Fu, S.-P.; Duan, R.-J.; Liu, J.; Hu, X.-W.; et al. Structure, Expression, and Functional Analysis of the Hexokinase Gene Family in Cassava. Int. J. Mol. Sci. 2017, 18, 1041. [Google Scholar] [CrossRef] [Green Version]
- Dou, L.; Li, Z.; Wang, H.; Li, H.; Xiao, G.; Zhang, X. The hexokinase Gene Family in Cotton: Genome-Wide Characterization and Bioinformatics Analysis. Front. Plant Sci. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Y.; Zhang, Q.; Wu, R.; Wang, X.; Feng, S.; Chen, S.; Lu, C.; Du, L. Genome-Wide Identification and Characterization of Hexokinase Genes in Moso Bamboo (Phyllostachys edulis). Front. Plant Sci. 2020, 11, 600. [Google Scholar] [CrossRef]
- Nilsson, A.; Olsson, T.; Ulfstedt, M.; Thelander, M.; Ronne, H. Two novel types of hexokinases in the moss Physcomitrella patens. BMC Plant Biol. 2011, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Xin, H.; Guo, J.; Gao, Y.; Liu, C.; Dai, D.; Tang, L. Genome-wide screening of hexokinase gene family and functional elucidation of HXK2 response to cold stress in Jatropha curcas. Mol. Biol. Rep. 2019, 46, 1649–1660. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, H.; Zhou, X.; Liu, H.; Liu, Y.; Li, J.; Han, S.; Wang, Y. Subcellular localization of rice hexokinase (OsHXK) family members in the mesophyll protoplasts of tobacco. Biol. Plant. 2011, 55, 173–177. [Google Scholar] [CrossRef]
- Jang, J.C.; León, P.; Zhou, L.; Sheen, J. Hexokinase as a sugar sensor in higher plants. Plant Cell 1997, 9, 5–19. [Google Scholar]
- Dai, N.; Schaffer, A.; Petreikov, M.; Shahak, Y.; Giller, Y.; Ratner, K.; Levine, A.; Granot, D. Overexpression of Arabidopsis Hexokinase in Tomato Plants Inhibits Growth, Reduces Photosynthesis, and Induces Rapid Senescence. Plant Cell 1999, 11, 1253–1266. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.; Sheen, J.; Jang, J.C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 2000, 44, 451–461. [Google Scholar] [CrossRef]
- Feng, J.; Zhao, S.; Chen, X.; Wang, W.; Dong, W.; Chen, J.; Shen, J.-R.; Liu, L.; Kuang, T. Biochemical and structural study of Arabidopsis hexokinase 1. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 367–375. [Google Scholar] [CrossRef]
- Karve, A.; Moore, B.D. Function of Arabidopsis hexokinase-like1 as a negative regulator of plant growth. J. Exp. Bot. 2009, 60, 4137–4149. [Google Scholar] [CrossRef] [Green Version]
- Kushwah, S.; Jones, A.M.; Laxmi, A. Cytokinin Interplay with Ethylene, Auxin, and Glucose Signaling Controls Arabidopsis Seedling Root Directional Growth. Plant Physiol. 2011, 156, 1851–1866. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Singh, M.; Laxmi, A. Interaction between Glucose and Brassinosteroid during the Regulation of Lateral Root Development in Arabidopsis. Plant Physiol. 2015, 168, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Smeekens, S.; Hellmann, H.A. Sugar sensing and signaling in plants. Front. Plant Sci. 2014, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Mayordomo, I.; Sanz, P. Hexokinase PII: Structural analysis and glucose signalling in the yeast Saccharomyces cerevisiae. Yeast 2001, 18, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Sami, F.; Siddiqui, H.; Hayat, S. Interaction of glucose and phytohormone signaling in plants. Plant Physiol. Biochem. 2019, 135, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Cao, D.; Fichtner, F.; Weiste, C.; Perez-Garcia, M.; Caradeuc, M.; Le Gourrierec, J.; Sakr, S.; Beveridge, C.A. HEXOKINASE1 signalling promotes shoot branching and interacts with cytokinin and strigolactone pathways. New Phytol. 2021, 231, 1088–1104. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, Q.; Prunier, F.; Mazubert, C.; de Bont, L.; Garmier, M.; Lugan, R.; Benhamed, M.; Bergounioux, C.; Raynaud, C.; Delarue, M. Involvement of Arabidopsis Hexokinase1 in Cell Death Mediated by Myo -Inositol Accumulation. Plant Cell 2015, 27, 1801–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-Q.; Zheng, L.-L.; Lin, H.; Yu, F.; Sun, L.-H.; Li, L.-M. Grape hexokinases are involved in the expression regulation of sucrose synthase- and cell wall invertase-encoding genes by glucose and ABA. Plant Mol. Biol. 2017, 94, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Lugassi, N.; Kelly, G.; Fidel, L.; Yaniv, Y.; Attia, Z.; Levi, A.; Alchanatis, V.; Moshelion, M.; Raveh, E.; Carmi, N.; et al. Expression of Arabidopsis Hexokinase in Citrus Guard Cells Controls Stomatal Aperture and Reduces Transpiration. Front. Plant Sci. 2015, 6, 1114. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G.; Sade, N.; Doron-Faigenboim, A.; Lerner, S.; Shatil-Cohen, A.; Yeselson, Y.; Egbaria, A.; Kottapalli, J.; Schaffer, A.A.; Moshelion, M.; et al. Sugar and hexokinase suppress expression of PIP aquaporins and reduce leaf hydraulics that preserves leaf water potential. Plant J. 2017, 91, 325–339. [Google Scholar] [CrossRef]
- Zheng, S.; Lu, J.; Yu, D.; Li, J.; Zhou, H.; Jiang, D.; Liu, Z.; Zhuang, C. Hexokinase gene OsHXK1 positively regulates leaf senescence in rice. BMC Plant Biol. 2021, 21, 580. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond—Emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef]
- Li, J.; Chen, G.; Zhang, J.; Shen, H.; Kang, J.; Feng, P.; Xie, Q.; Hu, Z. Suppression of a hexokinase gene, SlHXK1, leads to accelerated leaf senescence and stunted plant growth in tomato. Plant Sci. 2020, 298, 110544. [Google Scholar] [CrossRef]
- Sarowar, S.; Lee, J.Y.; Ahn, E.R.; Pai, H.S. A role of hexokinases in plant resistance to oxidative stress and pathogen infection. J. Plant Biol. 2008, 51, 341–346. [Google Scholar] [CrossRef]
- Atanasov, V.; Fürtauer, L.; Nägele, T. Indications for a Central Role of Hexokinase Activity in Natural Variation of Heat Acclimation in Arabidopsis thaliana. Plants 2020, 9, 819. [Google Scholar] [CrossRef]
- Lugassi, N.; Yadav, B.S.; Egbaria, A.; Wolf, D.; Kelly, G.; Neuhaus, E.; Raveh, E.; Carmi, N.; Granot, D. Expression of Arabidopsis Hexokinase in Tobacco Guard Cells Increases Water-Use Efficiency and Confers Tolerance to Drought and Salt Stress. Plants 2019, 8, 613. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G.; Egbaria, A.; Khamaisi, B.; Lugassi, N.; Attia, Z.; Moshelion, M.; Granot, D. Guard-Cell Hexokinase Increases Water-Use Efficiency Under Normal and Drought Conditions. Front. Plant Sci. 2019, 10, 1499. [Google Scholar] [CrossRef] [Green Version]
- Lehretz, G.G.; Sonnewald, S.; Lugassi, N.; Granot, D.; Sonnewald, U. Future-Proofing Potato for Drought and Heat Tolerance by Overexpression of Hexokinase and SP6A. Front. Plant Sci. 2021, 11, 614534. [Google Scholar] [CrossRef]
- Küstner, L.; Fürtauer, L.; Weckwerth, W.; Nägele, T.; Heyer, A.G. Subcellular dynamics of proteins and metabolites under abiotic stress reveal deferred response of the Arabidopsis thaliana hexokinase-1 mutant gin2-1 to high light. Plant J. 2019, 100, 456–472. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; Uddin, S.; Chakraborty, R.; Van Anh, D.T.; Macoy, D.M.; Park, S.O.; Ryu, G.R.; Kim, Y.H.; Cha, J.; Kim, W.-Y.; et al. Molecular characterization of HEXOKINASE1 in plant innate immunity. Appl. Biol. Chem. 2020, 63, 76. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Sun, F.; Guo, W.; Wang, W.; Li, X.; Lan, Y.; Du, L.; Li, S.; Fan, Y.; et al. ARGONAUTE 2 increases rice susceptibility to rice black-streaked dwarf virus infection by epigenetically regulating HEXOKINASE 1 expression. Mol. Plant Pathol. 2021, 22, 1029–1040. [Google Scholar] [CrossRef]
- Yu, J.; Li, X.; Wang, W.; Gu, K.; Sun, C.; You, C.; Hu, D. Glucose sensor MdHXK1 activates an immune response to the fungal pathogen Botryosphaeria dothidea in apple. Physiol. Plant. 2022, 174, e13596. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Y.; Li, Q.; Liu, S.; He, F.; An, Y.; Zhou, Y.; Liu, C.; Yin, W.; Xia, X. PdGNC confers drought tolerance by mediating stomatal closure resulting from NO and H 2 O 2 production via the direct regulation of PdHXK1 expression in Populus. New Phytol. 2021, 230, 1868–1882. [Google Scholar] [CrossRef]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.-H.; Liu, Y.-X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis Glucose Sensor HXK1 in Nutrient, Light, and Hormonal Signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef] [Green Version]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef]
- Polle, A.; Douglas, C. The molecular physiology of poplars: Paving the way for knowledge-based biomass production. Plant Biol. 2010, 12, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Grotewold, E.; Stam, M. Cis-regulatory sequences in plants: Their importance, discovery, and future challenges. Plant Cell 2022, 34, 718–741. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Zhang, Y.; Jia, X.; Zhou, M. Hexokinase plays a critical role in deoxynivalenol (DON) production and fungal development in Fusarium graminearum. Mol. Plant Pathol. 2016, 17, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Bork, P.; Sander, C.; Valencia, A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc. Natl. Acad. Sci. USA 1992, 89, 7290–7294. [Google Scholar] [CrossRef] [Green Version]
- Rocca, J.D.; Hall, E.K.; Lennon, J.T.; Evans, S.E.; Waldrop, M.P.; Cotner, J.B.; Nemergut, D.R.; Graham, E.B.; Wallenstein, M.D. Relationships between protein-encoding gene abundance and corresponding process are commonly assumed yet rarely observed. ISME J. 2015, 9, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.; Khokhar, W.; Jabre, I.; Reddy, A.S.N.; Byrne, L.J.; Wilson, C.M.; Syed, N.H. Alternative splicing and protein diversity: Plants versus animals. Front. Plant Sci. 2019, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Xu, X.; Li, X.; Xu, M.; Hu, M.; Xiong, Y.; Feng, J.; Wu, H.; Zhu, H.; Su, T. New Insight into Aspartate Metabolic Pathways in Populus: Linking the Root Responsive Isoenzymes with Amino Acid Biosynthesis during Incompatible Interactions of Fusarium solani. Int. J. Mol. Sci. 2022, 23, 6368. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granot, D.; Kelly, G. Evolution of Guard-Cell Theories: The Story of Sugars. Trends Plant Sci. 2019, 24, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Papacek, M.; Christmann, A.; Grill, E. Interaction network of ABA receptors in grey poplar. Plant J. 2017, 92, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estravis-Barcala, M.; Mattera, M.G.; Soliani, C.; Bellora, N.; Opgenoorth, L.; Heer, K.; Arana, M.V. Molecular bases of responses to abiotic stress in trees. J. Exp. Bot. 2020, 71, 3765–3779. [Google Scholar] [CrossRef]
- Kelly, G.; Moshelion, M.; David-Schwartz, R.; Halperin, O.; Wallach, R.; Attia, Z.; Belausov, E.; Granot, D. Hexokinase mediates stomatal closure. Plant J. 2013, 75, 977–988. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Min, J.; Zhou, H.; Zhang, Q.; Zhao, J.; Fang, Y. Functional Characterization of Invertase Inhibitors PtC/VIF1 and 2 Revealed Their Involvements in the Defense Response to Fungal Pathogen in Populus trichocarpa. Front. Plant Sci. 2020, 10, 1654. [Google Scholar] [CrossRef]
- Su, T.; Zhou, B.; Cao, D.; Pan, Y.; Hu, M.; Zhang, M.; Wei, H.; Han, M. Transcriptomic Profiling of Populus Roots Challenged with Fusarium Reveals Differential Responsive Patterns of Invertase and Invertase Inhibitor-Like Families within Carbohydrate Metabolism. J. Fungi 2021, 7, 89. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- De Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Han, M.; Heppel, S.C.; Su, T.; Bogs, J.; Zu, Y.; An, Z.; Rausch, T. Enzyme Inhibitor Studies Reveal Complex Control of Methyl-D-Erythritol 4-Phosphate (MEP) Pathway Enzyme Expression in Catharanthus roseus. PLoS ONE 2013, 8, e62467. [Google Scholar] [CrossRef] [Green Version]
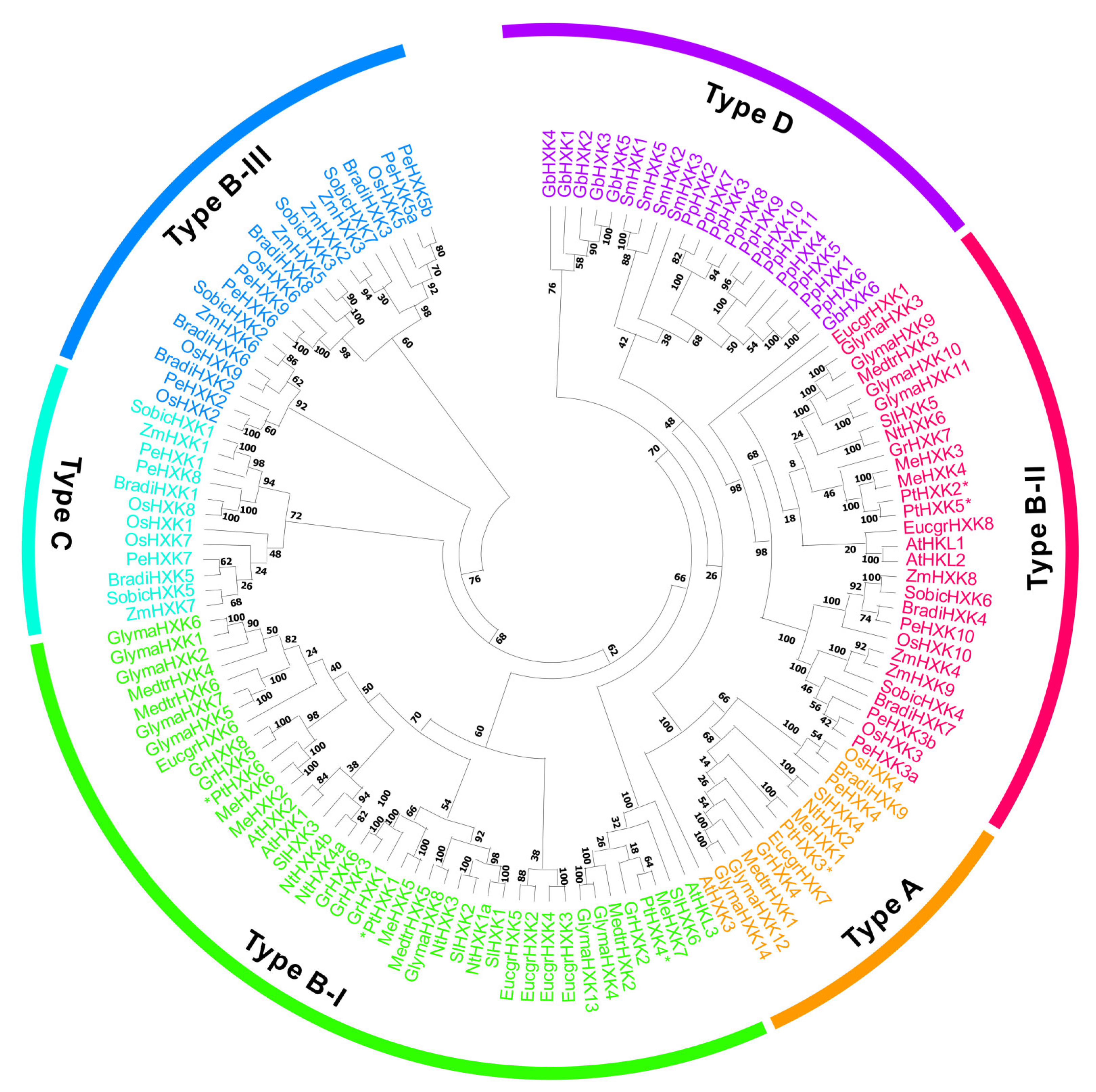
| Groups | Species | Type A | Type B | Type C | Type D | Total | ||
|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||
| Bryophytes | Physcomitrella patens | 11 | 11 | |||||
| Lycophytes | Selaginella moellendorffii | 4 | 4 | |||||
| Gymnosperms | Ginkgo biloba | 6 | 6 | |||||
| Monocots | Brachypodium distachyon | 1 | 2 | 4 | 2 | 9 | ||
| Oryza sativa | 1 | 2 | 4 | 3 | 10 | |||
| Phyllostachys edulis | 1 | 3 | 5 | 3 | 12 | |||
| Sorghum bicolor | 2 | 3 | 2 | 7 | ||||
| Zea mays | 3 | 4 | 2 | 9 | ||||
| Eudicots | Arabidopsis thaliana | 1 | 3 | 2 | 6 | |||
| Eucalyptus grandis | 1 | 5 | 2 | 8 | ||||
| Glycine max | 2 | 8 | 4 | 14 | ||||
| Gossypium raimondii | 1 | 6 | 1 | 8 | ||||
| Manihot esculenta | 1 | 4 | 2 | 7 | ||||
| Medicago truncatula | 1 | 4 | 1 | 6 | ||||
| Nicotiana tabacum | 1 | 4 | 1 | 6 | ||||
| Populus trichocarpa | 1 | 3 | 2 | 6 | ||||
| Solanum lycopersicum | 1 | 4 | 1 | 6 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, M.; Xu, X.; Xiong, Y.; Wei, H.; Yao, K.; Huang, T.; Long, Y.; Su, T. Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus trichocarpa). Plants 2022, 11, 2025. https://doi.org/10.3390/plants11152025
Han M, Xu X, Xiong Y, Wei H, Yao K, Huang T, Long Y, Su T. Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus trichocarpa). Plants. 2022; 11(15):2025. https://doi.org/10.3390/plants11152025
Chicago/Turabian StyleHan, Mei, Xianglei Xu, Yuan Xiong, Haikun Wei, Kejun Yao, Tingting Huang, Yingle Long, and Tao Su. 2022. "Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus trichocarpa)" Plants 11, no. 15: 2025. https://doi.org/10.3390/plants11152025
APA StyleHan, M., Xu, X., Xiong, Y., Wei, H., Yao, K., Huang, T., Long, Y., & Su, T. (2022). Genome-Wide Survey and Expression Analyses of Hexokinase Family in Poplar (Populus trichocarpa). Plants, 11(15), 2025. https://doi.org/10.3390/plants11152025







