Chemical Constituents from the Aerial Parts of Artemisia iwayomogi and Their Anti-Neuroinflammatory Activities
Abstract
:1. Introduction
2. Results and Discussion
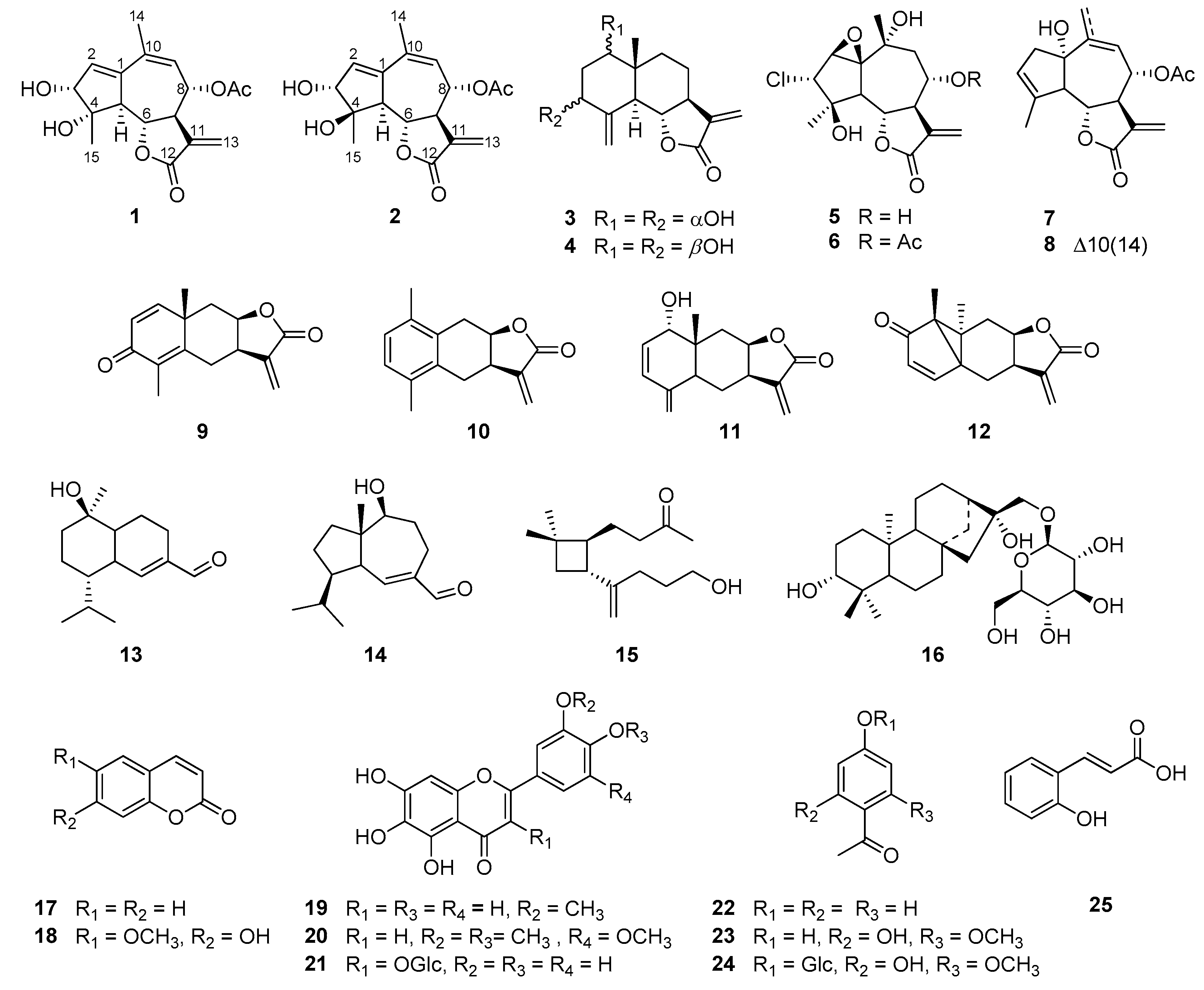
2.1. Structure Elucidation of the Compounds Isolated from A. iwayomogi
2.2. Inhibitory Effects of the Isolates on NO Production
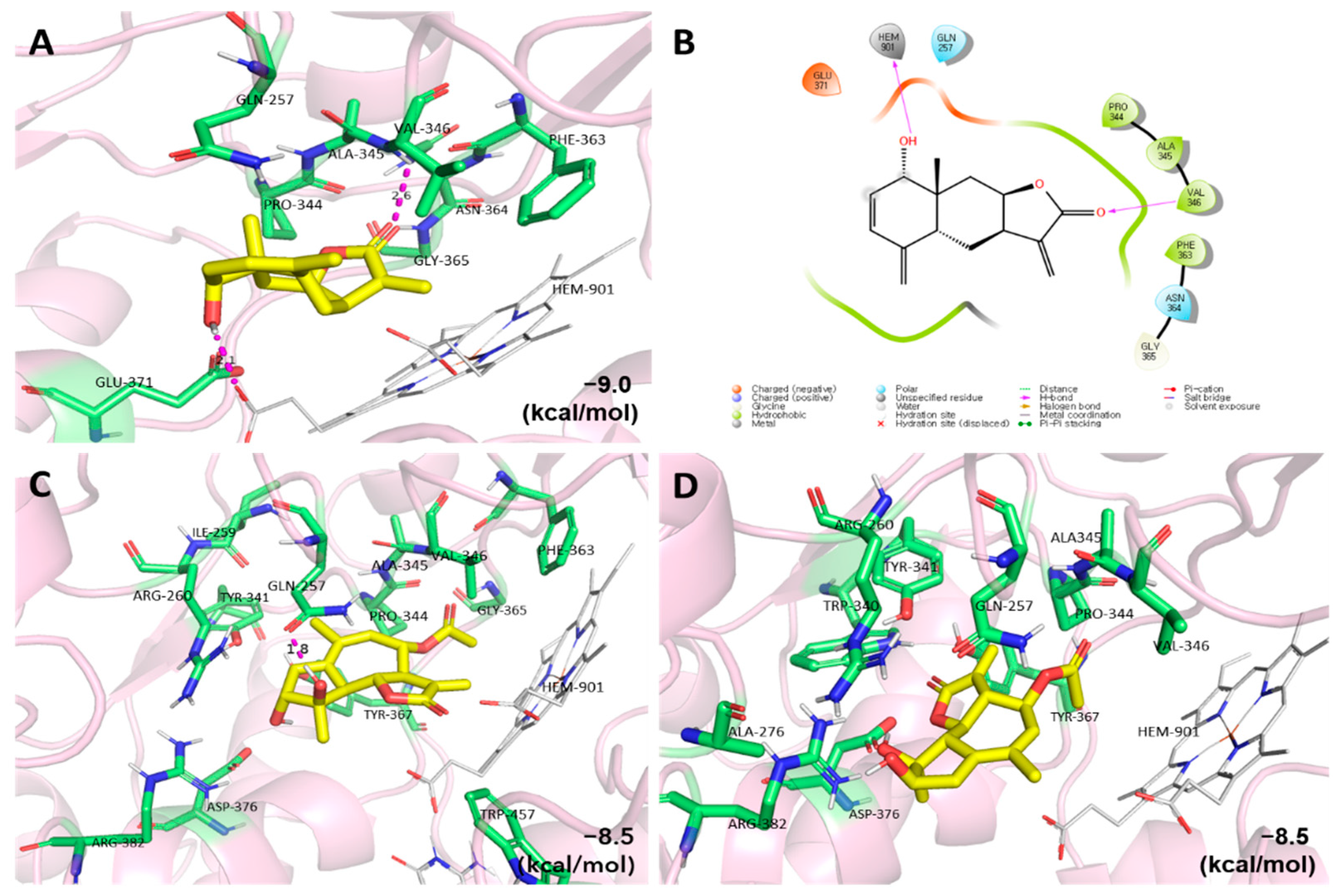
2.3. Molecular Docking Studies of the Active Compounds
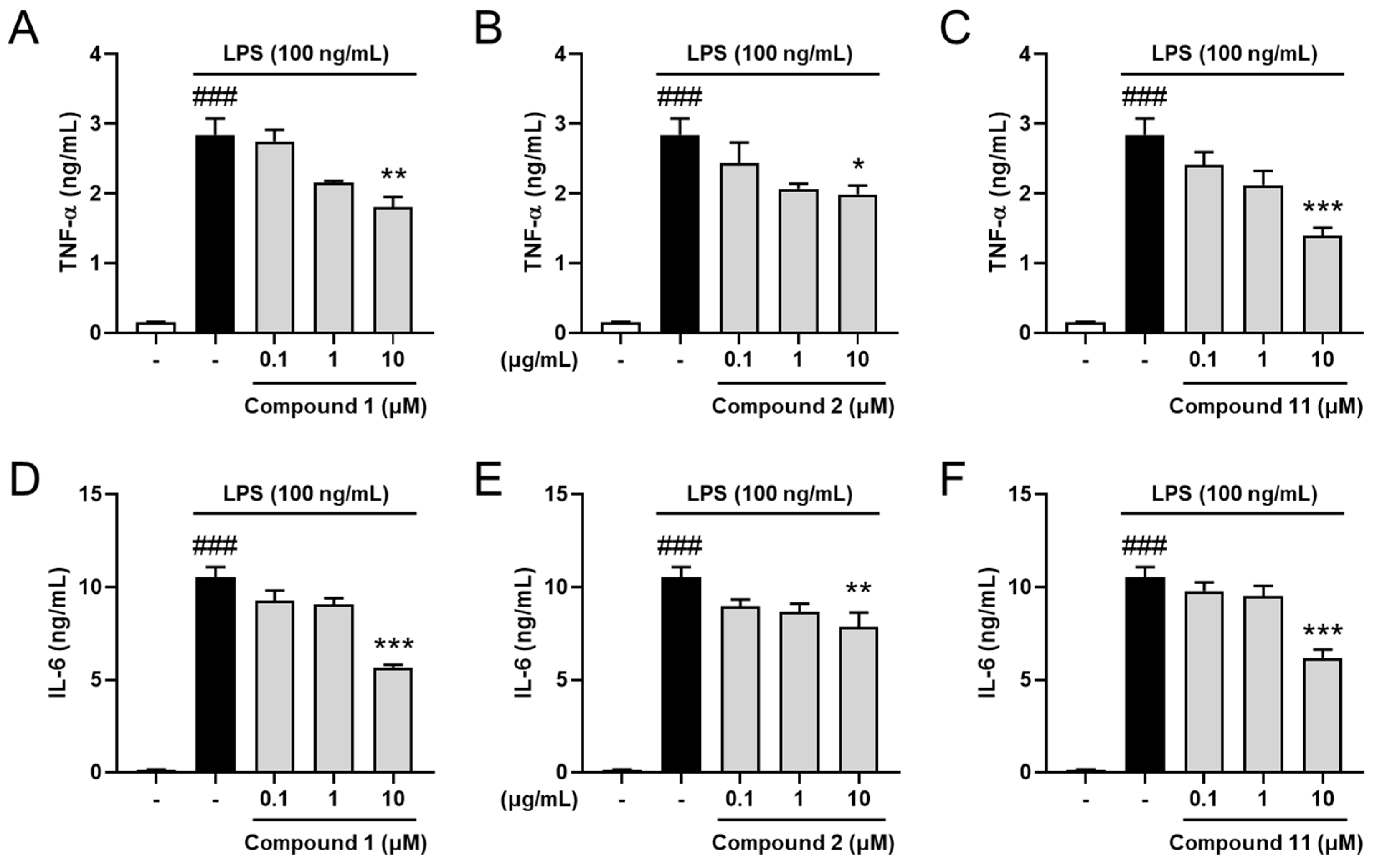
2.4. Inhibitory Effects on Pro-Inflammatory Cytokines
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. Iwayomogin A (1)
3.3.2. Iwayomogin B (2)
3.4. Computational Methods for the ECD Spectrum
3.5. Cell Culture and Treatment
3.6. Measurement of Pro-Inflammatory Mediators
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar]
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-induced neuroinflammation as a bridge to understand neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar]
- Kempuraj, D.; Thangavel, R.; Natteru, P.; Selvakumar, G.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.; Zaheer, A. Neuroinflammation induces neurodegeneration. Neurospine. 2016, 1, 1003. [Google Scholar]
- Kim, S.M.; Vetrivel, P.; Kim, H.H.; Ha, S.E.; Venkatarame Gowda Saralamma, V.; Kim, G.S. Artemisia iwayomogi (Dowijigi) inhibits lipopolysaccharide-induced inflammation in RAW264. 7 macrophages by suppressing the NF-κB signaling pathway. Exp. Ther. Med. 2020, 19, 2161–2170. [Google Scholar]
- Seo, B.; Kwon, D.; Choi, H.; Lee, J.; Oh, M.S.; Bu, Y. Medicinal Herbology; Younglim-Sa: Seoul, Korea, 2012. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar]
- Noh, D.; Choi, J.G.; Hong, S.-S.; Oh, M.S. Comparison of Anti-inflammatory effects between Artemisia capillaris and Artemisia iwayomogi by extraction solvents. Korean J. Herbol. 2018, 33, 55–61. [Google Scholar]
- Choi, Y.; Yanagawa, Y.; Kim, S.; Whang, W.K.; Park, T. Artemisia iwayomogi extract attenuates high-fat diet-induced obesity by decreasing the expression of genes associated with adipogenesis in mice. Evid. Based Complement. Altern. Med. 2013, 2013, 915953. [Google Scholar]
- Ding, Y.; Kim, J.-A.; Yang, S.-Y.; Kim, W.-K.; Lee, S.-H.; Jang, H.-D.; Kim, Y.-H. Antioxidative sesquiterpenes from Artemisia iwayomogi. Bull. Korean Chem. Soc. 2011, 32, 3493–3496. [Google Scholar]
- Wang, J.-H.; Choi, M.-K.; Shin, J.-W.; Hwang, S.-Y.; Son, C.-G. Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J. Ethnopharmacol. 2012, 140, 179–185. [Google Scholar]
- Choi, W.-S.; Kim, C.-J.; Park, B.-S.; Lee, S.-E.; Takeoka, G.R.; Kim, D.-G.; LanPiao, X.; Kim, J.-H. Inhibitory effect on proliferation of vascular smooth muscle cells and protective effect on CCl4-induced hepatic damage of HEAI extract. J. Ethnopharmacol. 2005, 100, 176–179. [Google Scholar]
- Kim, S.-H.; Choi, C.-H.; Kim, S.-Y.; Eun, J.-S.; Shin, T.-Y. Anti-allergic effects of Artemisia iwayomogi on mast cell-mediated allergy model. Exp. Biol. Med. 2005, 230, 82–88. [Google Scholar]
- Ahn, H.; Kim, J.Y.; Lee, H.J.; Kim, Y.K.; Ryu, J.H. Inhibitors of inducible nitric oxide synthase expression from Artemisia iwayomogi. Arch. Pharm. Res. 2003, 26, 301–305. [Google Scholar]
- Ju, I.G.; Huh, E.; Kim, N.; Lee, S.; Choi, J.G.; Hong, J.; Oh, M.S. Artemisiae Iwayomogii Herba inhibits lipopolysaccharide-induced neuroinflammation by regulating NF-κB and MAPK signaling pathways. Phytomedicine 2021, 84, 153501. [Google Scholar]
- Huang, Z.S.; Pei, Y.H.; Liu, C.M.; Lin, S.; Tang, J.; Huang, D.S.; Song, T.F.; Lu, L.H.; Gao, Y.P.; Zhang, W.D. Highly oxygenated guaianolides from Artemisia dubi. Planta. Med. 2010, 76, 1710–1716. [Google Scholar]
- Lee, K.; Geissman, T. Sesquiterpene lactones of artemisia constituents of A ludoviciana SSP. Mexicana. Phytochemistry 1970, 9, 403–408. [Google Scholar]
- Irwin, M.; Geissman, T. Ridentin-β: An eudesmanolide from Artemisia tripartita ssp. rupicola. Phytochemistry 1973, 12, 871–873. [Google Scholar]
- Trifunovic, S.A.; Aljančic, I.; Vajs, V.; Macura, S.; Milosavljevic, S. Sesquiterpene lactones and flavonoids of Achillea depressa. Biochem. Syst. Ecol. 2005, 33, 317. [Google Scholar]
- Trifunović, S.; Milosavljević, S.; Vajs, V.; Macura, S.; Todorović, N. Stereochemistry and conformations of natural 1,2-epoxy-guaianolides based on 1D and 2D NMR data and semiempirical calculations. Magn. Reson. Chem. 2008, 46, 427–431. [Google Scholar]
- Milosavjević, S.; Aljančić, I.; Macura, S.; Milinkovic, D.; Stefanović, M. Sesquiterpene lactones from Achillea crithmifolia. Phytochemistry 1991, 30, 3464–3466. [Google Scholar]
- Ryu, J.-H.; Lee, H.J.; Jeong, Y.S.; Ryu, S.Y.; Han, Y.N. Yomogin, an inhibitor of nitric oxide production in LPS-activated macrophages. Arch. Pharm. Res. 1998, 21, 481–484. [Google Scholar]
- Jakupovic, J.; Schuster, A.; Bohlmann, F.; Dillon, M. Lumiyomogin, ferreyrantholide, fruticolide and other sesquiterpene lactones from Ferreyranthus fruticosus. Phytochemistry 1988, 27, 1113–1120. [Google Scholar]
- Iijima, T.; Yaoita, Y.; Kikuchi, M. Five New Sesquiterpenoids and a New Diterpenoid from Erigeron annuus (L.) P ERS., Erigeron philadelphicus L. and Erigeron sumatrensis R ETZ. Chem. Pharm. Bull. 2003, 51, 545–549. [Google Scholar]
- Xie, W.-D.; Niu, Y.-F.; Lai, P.-X.; Row, K.H. Sesquiterpenoids and other constituents from Senecio argunensis. Chem. Pharm. Bull. 2010, 58, 991–994. [Google Scholar]
- Ahmed, A.A.; Gáti, T.; Hussein, T.A.; Ali, A.T.; Tzakou, O.A.; Couladis, M.A.; Mabry, T.J.; Tóth, G. Ligustolide A and B, two novel sesquiterpenes with rare skeletons and three 1, 10-seco-guaianolide derivatives from Achillea ligustica. Tetrahedron 2003, 59, 3729–3735. [Google Scholar]
- Ding, Y.; Liang, C.; Yang, S.-Y.; Kim, J.-H.; Lee, Y.-M.; Kim, Y.-H. Iwayoside A, a new diterpene glycoside from Artemisia iwayomogi kitamura that enhances IL-2 secretion. Bull. Korean Chem. Soc. 2010, 31, 2422–2423. [Google Scholar]
- Sankar, S.; Gilbert, R.; Fornes, R. 13C NMR studies of some hydroxycoumarins and related compounds. Org. Magn. Reson. 1982, 19, 222–224. [Google Scholar]
- Nam, Y.; Choi, M.; Hwang, H.; Lee, M.G.; Kwon, B.M.; Lee, W.H.; Suk, K. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother. Res. 2013, 27, 404–411. [Google Scholar]
- Marco, J.A.; Barberá, O.; Rodríguez, S.; Domingo, C.; Adell, J. Flavonoids and other phenolics from Artemisia hispanica. Phytochemistry 1988, 27, 3155–3159. [Google Scholar]
- Bylka, W. A new acylated flavonol diglycoside from Atriplex littoralis. Acta Physiol. Plant. 2004, 26, 393–398. [Google Scholar]
- Dhami, K.; Stothers, J. 13C NMR studies: Part iii. carbon-13 NMR spectra of substituted acetophenones. Can. J. Chem. 1965, 43, 479–497. [Google Scholar]
- Singh, A.K.; Pathak, V.; Agrawal, P.K. Annphenone, a phenolic acetophenone from Artemisia annua. Phytochemistry 1997, 44, 555–557. [Google Scholar]
- Kowczyk-Sadowy, M.; Świsłocka, R.; Lewandowska, H.; Piekut, J.; Lewandowski, W. Spectroscopic (FT-IR, FT-Raman, 1H-and 13C-NMR), theoretical and microbiological study of trans o-coumaric acid and alkali metal o-coumarates. Molecules 2015, 20, 3146–3169. [Google Scholar]
- Subedi, L.; Gaire, B.P.; Parveen, A.; Kim, S.-Y. Nitric Oxide as a Target for Phytochemicals in Anti-Neuroinflammatory Prevention Therapy. Int. J. Mol. Sci. 2021, 22, 4771. [Google Scholar]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar]
- West, P.K.; Viengkhou, B.; Campbell, I.L.; Hofer, M.J. Microglia responses to interleukin-6 and type I interferons in neuroinflammatory disease. Glia 2019, 67, 1821–1841. [Google Scholar]
 ) and HMBC (
) and HMBC (  ) NMR spectra of 1 and 2.
) NMR spectra of 1 and 2.

| Position a | 1 c | 2 c | ||
|---|---|---|---|---|
| δHb | δC | δHb | δC | |
| 1 | 143.4 | 143.5 | ||
| 2 | 6.21 t (2.5) | 134.2 | 6.19 t (2.5) | 134.9 |
| 3 | 3.95 d (3.0) | 80.4 | 4.09 d (3.0) | 83.4 |
| 4 | 80.2 | 82.2 | ||
| 5 | 3.20 dd (11.5, 2.5) | 56.3 | 3.05 dd (11.0, 2.5) | 59.6 |
| 6 | 4.35 dd (11.5, 9.0) | 78.7 | 4.41 (11.0, 9.5) | 79.2 |
| 7 | 3.27 m | 50.0 | 3.34 m | 49.5 |
| 8 | 5.50 dd (11.5, 2.0) | 74.0 | 5.47 dd (11.0, 2.0) | 74.0 |
| 9 | 5.52 br d | 128.1 | 5.50 d (2.0) | 129.8 |
| 10 | 132.5 | 132.3 | ||
| 11 | 138.6 | 138.6 | ||
| 12 | 171.6 | 171.7 | ||
| 13 | 5.76 d (3.0) 6.22 d (3.0) | 123.5 | 5.78 d (3.0) 6.22 d (3.0) | 123.6 |
| 14 | 2.00 s | 25.4 | 2.03 s | 26.0 |
| 15 | 1.32 s | 21.8 | 1.54 s | 23.1 |
| 8-OCOCH3 | 2.17 s | 21.2 | 2.17 s | 21.2 |
| 8-OCOCH3 | 172.0 | 172.0 | ||
| Compound | IC50 (μM) a | Compound | IC50 (μM) a |
|---|---|---|---|
| 1 | 10.59 | 15 | >30 |
| 2 | 12.85 | 16 | >30 |
| 3 | 3.83 | 17 | >30 |
| 4 | 13.59 | 18 | 19.91 |
| 5 | 8.59 | 19 | 19.25 |
| 6 | 8.29 | 20 | 8.08 |
| 7 | 7.38 | 21 | >30 |
| 8 | 14.50 | 22 | >30 |
| 9 | 5.24 | 23 | 22.52 |
| 11 | 1.78 | 24 | >30 |
| 13 | >30 | 25 | >30 |
| 14 | >30 | Quercetin b | 8.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, S.-R.; Ju, I.G.; Kim, J.; Park, K.-T.; Oh, M.S.; Jang, D.S. Chemical Constituents from the Aerial Parts of Artemisia iwayomogi and Their Anti-Neuroinflammatory Activities. Plants 2022, 11, 1954. https://doi.org/10.3390/plants11151954
Son S-R, Ju IG, Kim J, Park K-T, Oh MS, Jang DS. Chemical Constituents from the Aerial Parts of Artemisia iwayomogi and Their Anti-Neuroinflammatory Activities. Plants. 2022; 11(15):1954. https://doi.org/10.3390/plants11151954
Chicago/Turabian StyleSon, So-Ri, In Gyong Ju, Jinhee Kim, Keon-Tae Park, Myung Sook Oh, and Dae Sik Jang. 2022. "Chemical Constituents from the Aerial Parts of Artemisia iwayomogi and Their Anti-Neuroinflammatory Activities" Plants 11, no. 15: 1954. https://doi.org/10.3390/plants11151954
APA StyleSon, S.-R., Ju, I. G., Kim, J., Park, K.-T., Oh, M. S., & Jang, D. S. (2022). Chemical Constituents from the Aerial Parts of Artemisia iwayomogi and Their Anti-Neuroinflammatory Activities. Plants, 11(15), 1954. https://doi.org/10.3390/plants11151954







