Spectroscopic Evidence of Thermal Changes in Plant Oils during Deep-Frying—Chemical and Infrared Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Properties
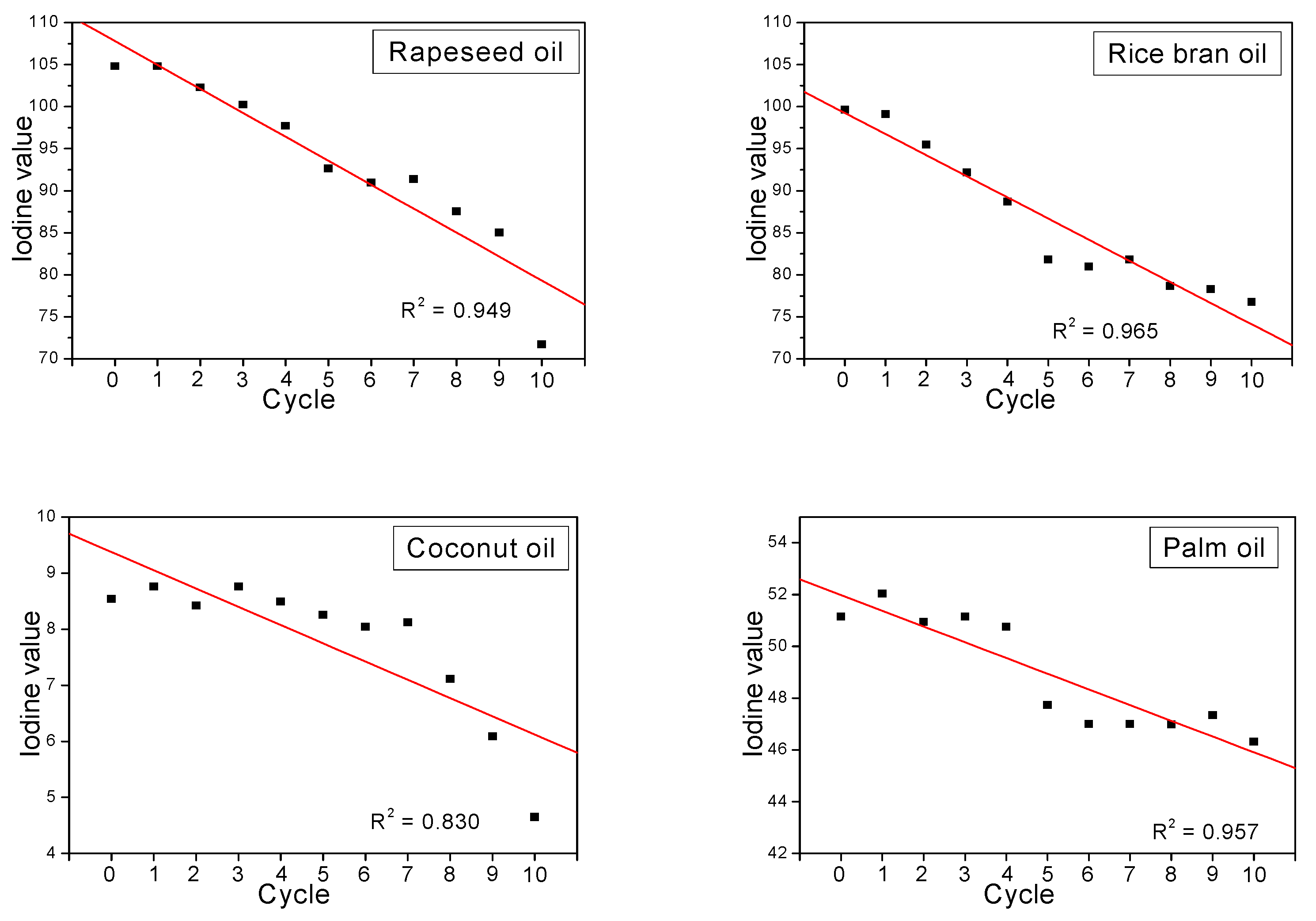
2.2. Heating–Time Dependence of the Iodine Values
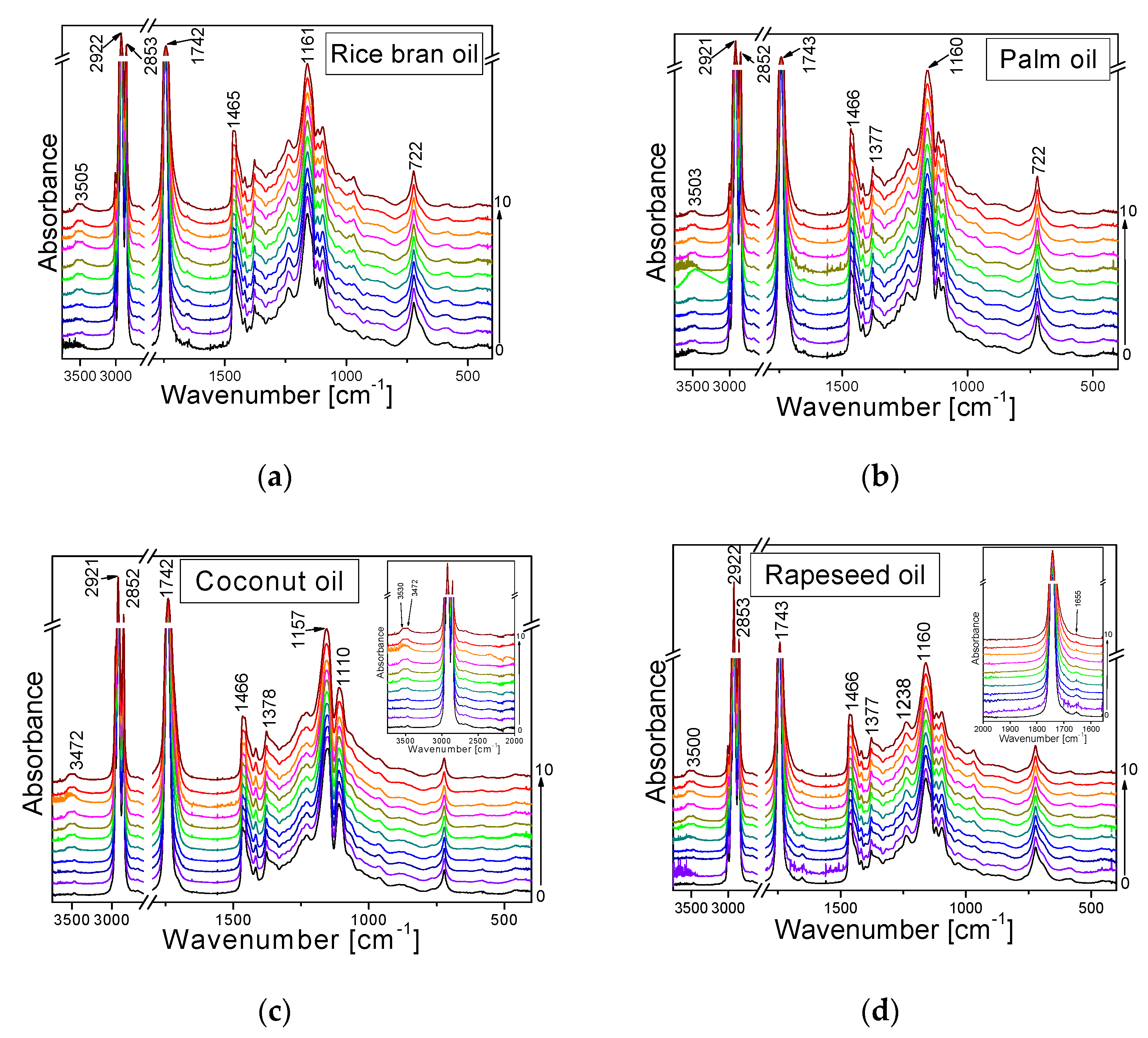
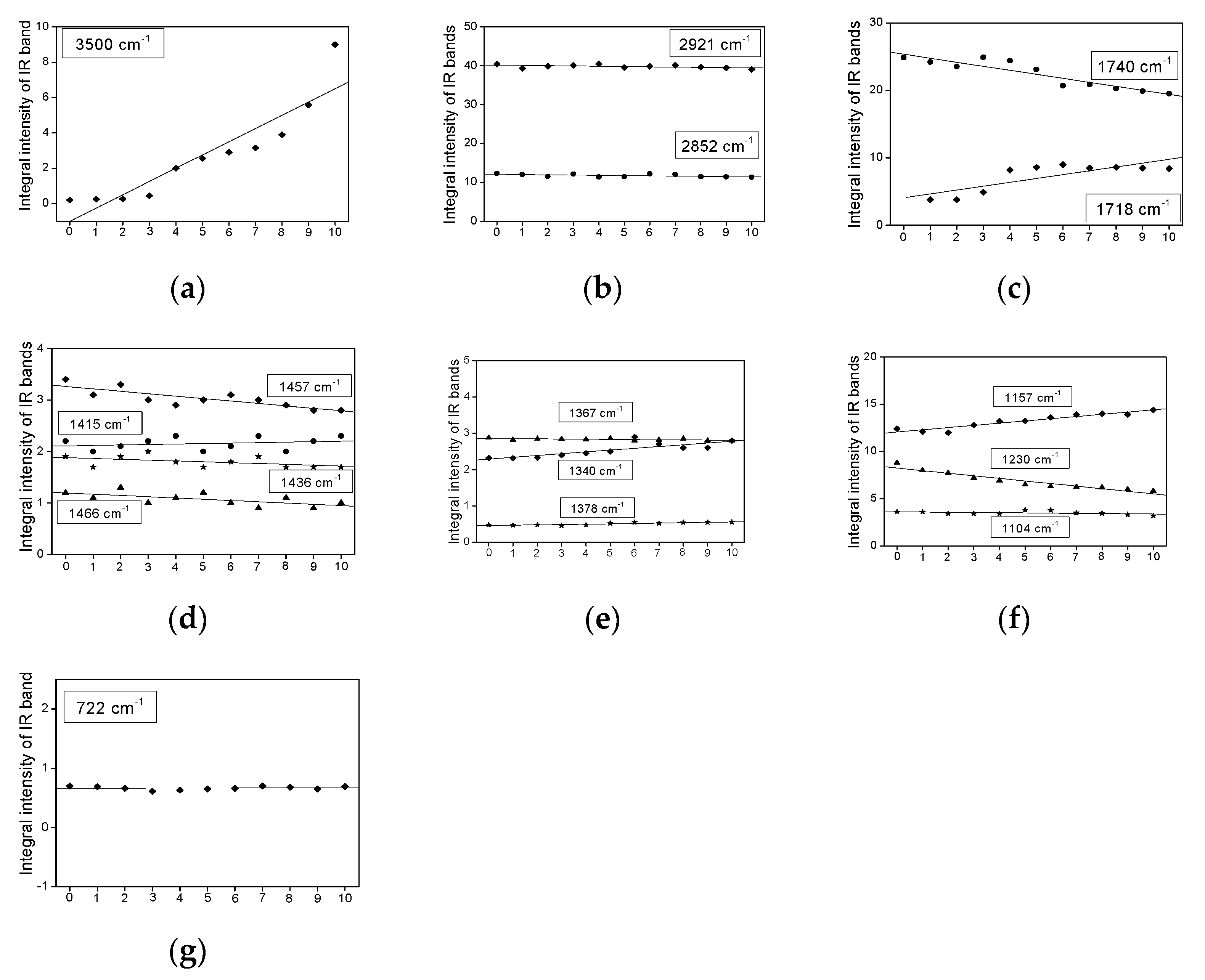
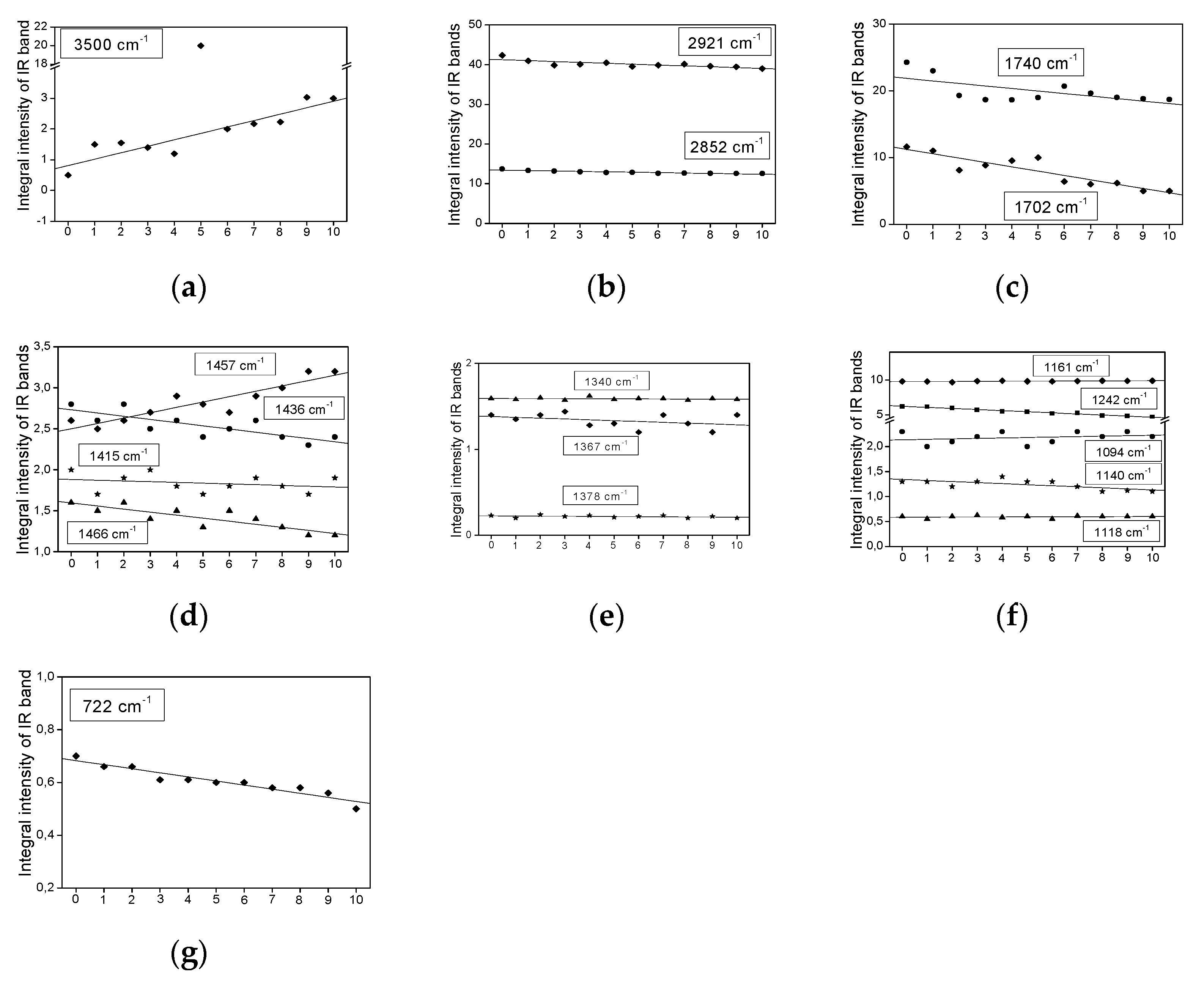
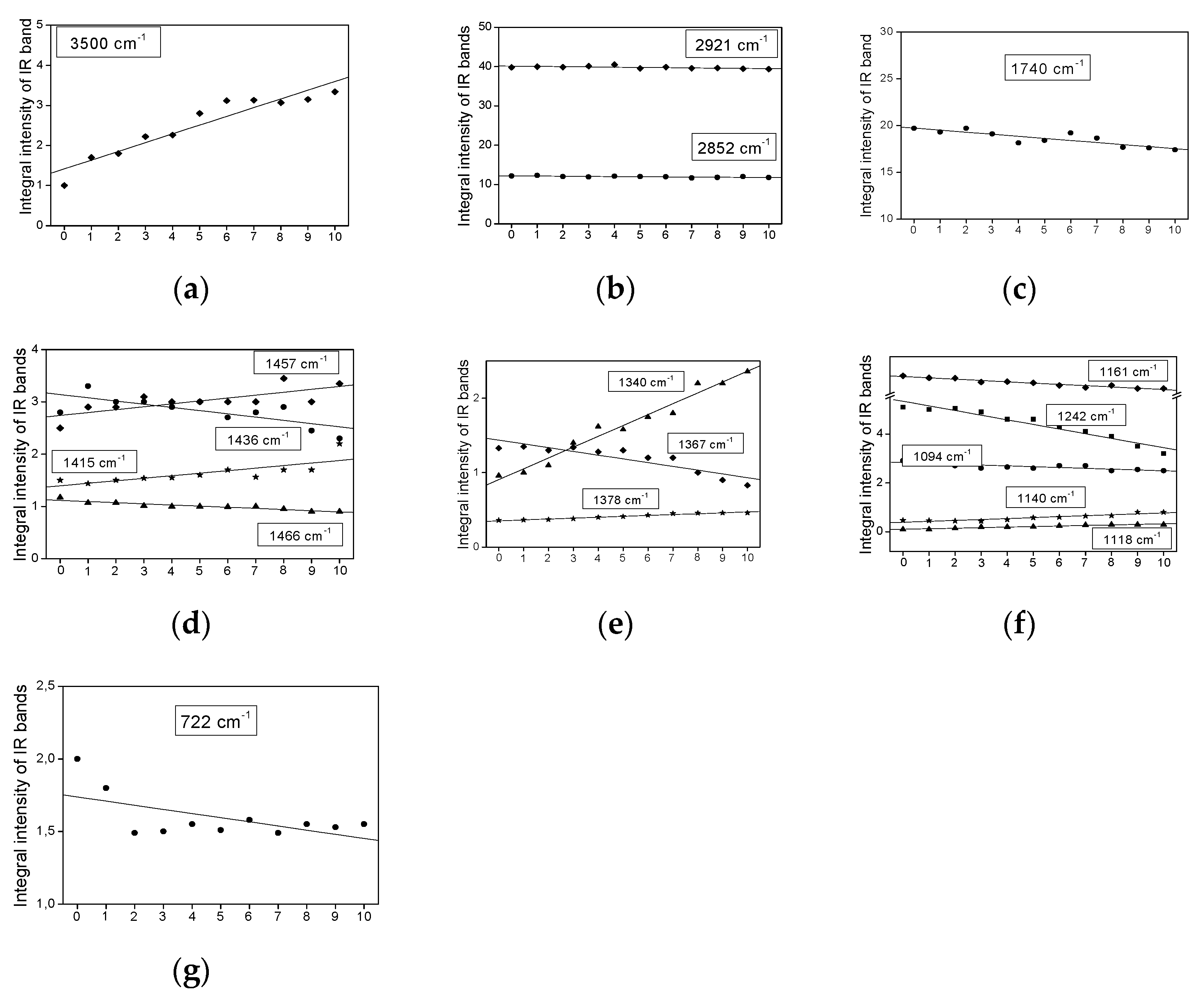
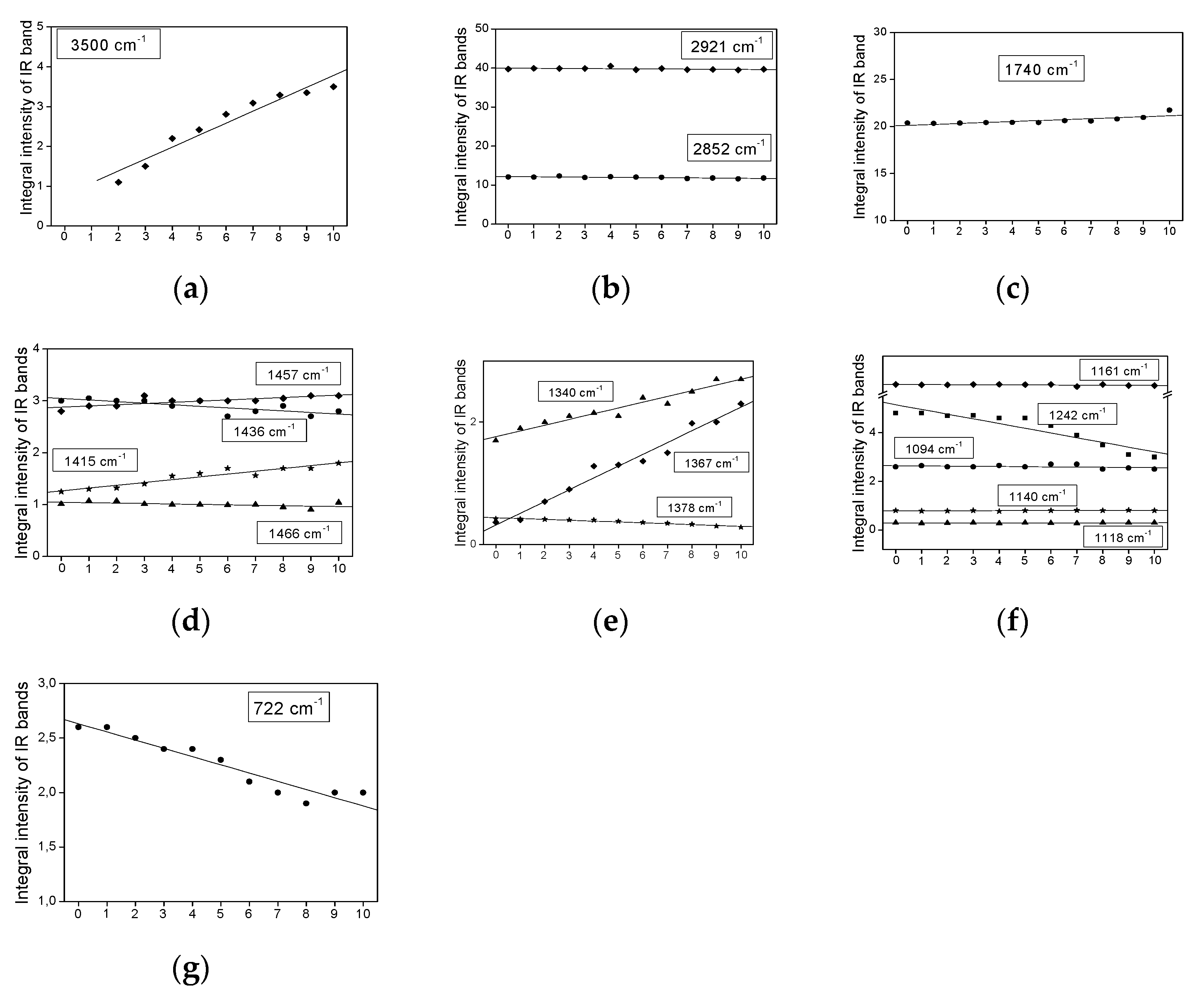
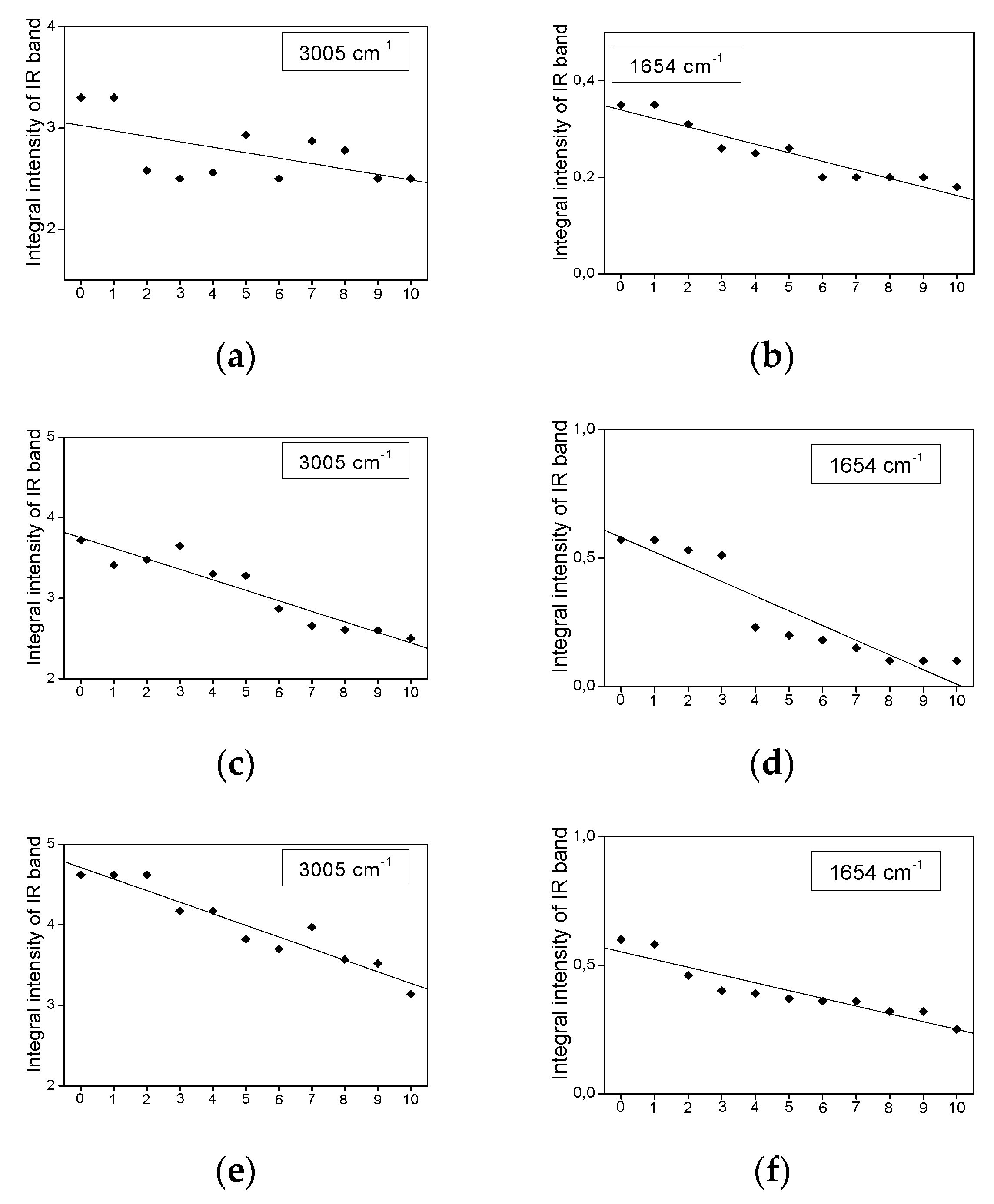
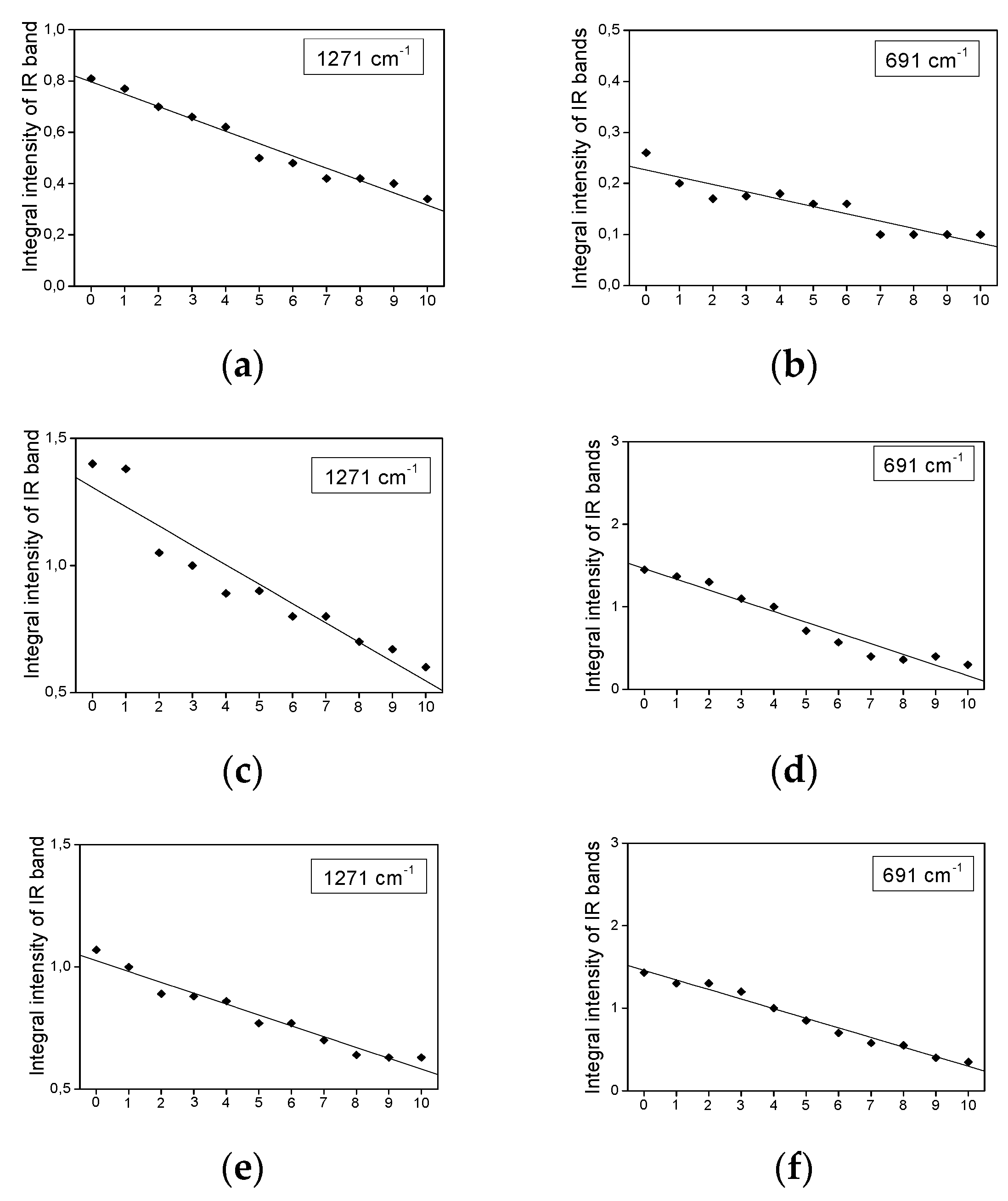
2.3. Infrared Spectra Measurements
- The bands do not change their position and intensity during the periodical heating of the oils.
- The bands show an increase in their intensity with increasing heating-time of the oils.
- The bands show a decrease in their intensity with increasing heating time.
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boskou, G.; Salta, F.N.; Chiou, A.; Troullidou, E.; Andrikopoulos, N.K. Content oftrans,trans-2,4-decadienal in deep-fried and pan-fried potatoes. Eur. J. Lipid Sci. Technol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Babylatha, R.; Pavithra, A.S.; Khatoon, S. Thermal degradation of groundnut oil during continuous and intermittent frying. J. Food Sci. Technol. 2013, 50, 1186–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.P.; Man, Y.B.C.; Jinap, S.; Yusoff, M.S.A. Effects of microwave heating on changes in chemical and thermal properties of vegetable oils. J. Am. Oil Chem. Soc. 2001, 78, 1227–1232. [Google Scholar] [CrossRef]
- Yoshida, H.; Tatsumi, M.; Kajimoto, G. Influence of fatty acids on the tocopherol stability in vegetable oils during microwave heating. J. Am. Oil Chem. Soc. 1992, 69, 119–125. [Google Scholar] [CrossRef]
- Yoshida, H.; Kondo, I.; Kajimoto, G. Participation of free fatty acids in the oxidation of purified soybean oil during microwave heating. J. Am. Oil Chem. Soc. 1992, 69, 1136–1140. [Google Scholar] [CrossRef]
- Yoshida, H.; Kajimoto, G. Microwave Heating Affects Composition and Oxidative Stability of Sesame (Sesamum indicum) Oil. J. Food Sci. 1994, 59, 613–616. [Google Scholar] [CrossRef]
- Yáńez-Limŏn, J.M.; Mayen-Mondragŏn, R.; Martinez-Flores, O.; Flores-Farias, R.; Ruiz, F.; Araujo-Andrade, C.; Martinez, J.R. Thermal diffusivity studies in edible commercial oils using thermal lens spectroscopy. Superf. Vacío 2005, 18, 31–37. [Google Scholar]
- Sharma, H.K.; Kaur, B.; Sarkar, B.C.; Singh, C. Thermal behavior of pure rice bran oil, sunflower oil and their model blends during deep fat frying. Grasas Aceites 2006, 57, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Reena, M.B.; Reddy, S.R.Y.; Lokesh, B.R. Changes in triacylglycerol molecular species and thermal properties of blended and interesterified mixtures of coconut oil or palm oil with rice bran oil or sesame oil. Eur. J. Lipid Sci. Technol. 2009, 111, 346–357. [Google Scholar] [CrossRef]
- Tekin, L.; Aday, M.S.; Yilmaz, E. Physicochemical Changes in Hazelnut, Olive Pomace, Grapeseed and Sunflower Oils Heated at Frying Temperatures. Food Sci. Technol. Res. 2009, 15, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Ulusoy, B.O.; Karabulut, I.; Turan, S. Performance of some edible oils during heating in a steam pressure cooker. J. Food Lipids 2004, 11, 234–241. [Google Scholar] [CrossRef]
- Attya, M.; Benabdelkamel, H.; Perri, E.; Russo, A.; Sindona, G. Effects of Conventional Heating on the Stability of Major Olive Oil Phenolic Compounds by Tandem Mass Spectrometry and Isotope Dilution Assay. Molecules 2010, 15, 8734–8746. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Marinova, E.M.; Seizova, K.A.; Totseva, I.R.; Panayotova, S.S.; Marekov, I.N.; Momchilova, S. Oxidative changes in some vegetable oils during heating at frying temperature. Bulg. Chem. Commun. 2012, 44, 57–63. [Google Scholar]
- Tiwari, M.R.; Tiwari, K.K.; Toliwal, S.D. Studies on Thermal Stability of Palm -Sesame oil blends during Deep Fat Frying. J. Sci. Ind. Res. 2014, 73, 153–156. [Google Scholar]
- Bastos Fernandes, J.C. Thermal Stability of Soybean Oil: When must we discard it? MOJ Food Process. Technol. 2016, 2, 170–174. [Google Scholar] [CrossRef]
- Reda, S.Y. Evaluation of antioxidants stability by thermal analysis and its protective effect in heated edible vegetable oil. Food Sci. Technol. 2011, 31, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Mujeeda, B.; Prasad, N. Siddaramaiah Effect of antioxidant on thermal stability of vegetable oils by using ultrasonic studies. Int. Food Res. J. 2016, 23, 528–536. [Google Scholar]
- Falade, A.O.; Oboh, G. Thermal Oxidation Induces Lipid Peroxidation and Changes in the Physicochemical Properties and β -Carotene Content of Arachis Oil. Int. J. Food Sci. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Majchrzak, T.; Lubinska, M.; Różańska, A.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Thermal degradation assessment of canola and olive oil using ultra-fast gas chromatography coupled with chemometrics. Monatshefte Für Chemie—Chem. Mon. 2017, 148, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Assessment of conventional and microwave heating induced degradation of carotenoids in olive oil by VIS Raman spectroscopy and classical methods. Food Res. Int. 2010, 43, 694–700. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Valcarcel, M.; Ayora-Cañada, M.J. Two-dimensional correlation spectroscopy and multivariate curve resolution for the study of lipid oxidation in edible oils monitored by FTIR and FT-Raman spectroscopy. Anal. Chim. Acta 2007, 593, 54–67. [Google Scholar] [CrossRef]
- Vaskova, H.; Bučková, M. Thermal Degradation of Vegetable Oils: Spectroscopic Measurement and Analysis. Procedia Eng. 2015, 100, 630–635. [Google Scholar] [CrossRef] [Green Version]
- Moya Moreno, M.C.; Mendoza Olivares, D.; Amézquita López, F.J.; Gimeno Adelantado, J.V.; Bosch Reig, F. Analytical evaluation of polyunsaturated fatty acids degradation during thermal oxidation of edible oils by Fourier transform infrared spectroscopy. Talanta 1999, 50, 269–275. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Some of the most significant changes in the Fourier transform infrared spectra of edible oils under oxidative conditions. J. Sci. Food Agric. 2000, 80, 2028–2036. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Fourier transform infrared spectra data versus peroxide and anisidine values to determine oxidative stability of edible oils. Food Chem. 2002, 77, 503–510. [Google Scholar] [CrossRef]
- Dymińska, L.; Albegar, A.M.; Zając, A.; Czuj, T.; Sąsiadek, W.; Lorenc, J.; Hanuza, J. Applying additivity rule to determine physico-chemical properties of edible oil blends based on known parameters of component oils. Zywnosc Nauk. Technol. Jakosc/Food Sci. Technol. Qual. 2021, 28, 133–149. [Google Scholar] [CrossRef]
- Dymińska, L.; Calik, M.; Albegar, A.M.M.; Zając, A.; Kostyń, K.; Lorenc, J.; Hanuza, J. Quantitative determination of the iodine values of unsaturated plant oils using infrared and Raman spectroscopy methods. Int. J. Food Prop. 2017, 20, 2003–2015. [Google Scholar] [CrossRef] [Green Version]
- Okolo, J.C.; Adejumo, B.A. Effect of Bleaching on Some Quality Attributes of Crude Palm Oil. IOSR J. Eng. (IOSRJEN) 2014, 4, 25–28. [Google Scholar]
- Orthoefer, F.T. Rice Bran Oil. In Edible Oil and Fat Products: Edible Oils, Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 2. [Google Scholar]
- Martín-Hernández, C.; Bénet, S.; Obert, L. Determination of Proteins in Refined and Nonrefined Oils. J. Agric. Food Chem. 2008, 56, 4348–4351. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Benjakul, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J. Food Sci. 2018, 83, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, V.G.; Braiman, M.S. Vibrational Spectroscopy of Biological and Polymeric Materials; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2006. [Google Scholar]
- ISO 3961; Animal and Vegetable Fats and Oils—Determination of Iodine Value. International Organization for Standardization: Geneva, Switzerland, 2013.
| Oils | SFA | MUFA | PUFA |
|---|---|---|---|
| Palm | 46.10 | 44.73 | 9.17 |
| Coconut | 94.60 | 4.55 | 0.85 |
| Rice bran | 16.62 | 45.54 | 37.84 |
| Rapeseed | 8.91 | 63.08 | 28.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dymińska, L.; Albegar, A.M.M.; Sąsiadek, W.; Kucharska, E.; Zając, A.; Hanuza, J. Spectroscopic Evidence of Thermal Changes in Plant Oils during Deep-Frying—Chemical and Infrared Studies. Plants 2022, 11, 1813. https://doi.org/10.3390/plants11141813
Dymińska L, Albegar AMM, Sąsiadek W, Kucharska E, Zając A, Hanuza J. Spectroscopic Evidence of Thermal Changes in Plant Oils during Deep-Frying—Chemical and Infrared Studies. Plants. 2022; 11(14):1813. https://doi.org/10.3390/plants11141813
Chicago/Turabian StyleDymińska, Lucyna, Abduladhim Moamer Moftah Albegar, Wojciech Sąsiadek, Edyta Kucharska, Adam Zając, and Jerzy Hanuza. 2022. "Spectroscopic Evidence of Thermal Changes in Plant Oils during Deep-Frying—Chemical and Infrared Studies" Plants 11, no. 14: 1813. https://doi.org/10.3390/plants11141813
APA StyleDymińska, L., Albegar, A. M. M., Sąsiadek, W., Kucharska, E., Zając, A., & Hanuza, J. (2022). Spectroscopic Evidence of Thermal Changes in Plant Oils during Deep-Frying—Chemical and Infrared Studies. Plants, 11(14), 1813. https://doi.org/10.3390/plants11141813






