Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress
Abstract
:1. Introduction
2. Results
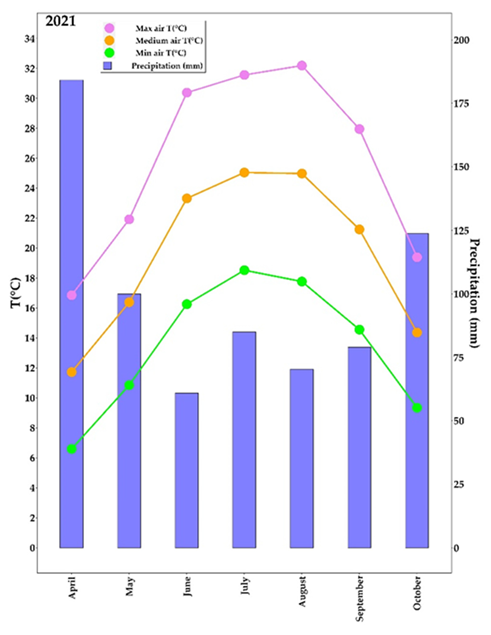
2.1. Meteorological Parameters
2.2. Leaf Gas Exchange, Chlorophyll Fluorescence, Water Potential (Leaf, Stem, and Pre-Dawn), Relative Water Content, Plant Hydraulic Conductance, Intercepted Photosynthetically Active Radiation, and Leaf Temperature
2.3. Grape Composition
2.4. Pearson’s Correlation
2.5. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Location, Meteorological Parameters, and Experiment Design
4.2. Leaf Gas Exchange, Chlorophyll Fluorescence, Water Potential (Leaf, Stem, and Pre-Dawn), Relative Water Content, Plant Hydraulic Conductance, Intercepted Photosynthetically Active Radiation, and Leaf Temperature
4.3. Technological Parameters of Berries
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Prior, S.A.; Runion, G.B.; Torbert, H.A.; Tian, H.; Lu, C.; Venterea, R.T. Effects of elevated carbon dioxide and increased temperature on methane and nitrous oxide fluxes: Evidence from field experiments. Front. Ecol. Environ. 2012, 10, 520–527. [Google Scholar] [CrossRef]
- Hu, X.; Xie, J.; Cai, W.; Wang, R.; Davarpanah, A. Thermodynamic effects of cycling carbon dioxide injectivity in shale reservoirs. J. Pet. Sci. Eng. 2020, 195, 107717. [Google Scholar] [CrossRef]
- Singh, R.K.; Arrighi, J.; de Perez, E.C.; Warrick, O.; Suarez, P.; Koelle, B.; Jjemba, E.; van Aalst, M.K.; Roberts, D.C.; Pörtner, H.O.; et al. International conference on climate risk management, inputs for the intergovernmental panel on climate change’s sixth assessment report. Clim. Dev. 2019, 11, 655–658. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Qiu, X.; Liu, B.; Chang, X.; Liu, L.; Zhang, L.; Cao, W.; Zhu, Y. Impacts of 1.5 and 2.0 °C global warming on rice production across China. Agric. For. Meteorol. 2020, 284, 107900. [Google Scholar] [CrossRef]
- Mammola, S.; Piano, E.; Cardoso, P.; Vernon, P.; Domínguez-Villar, D.; Culver, D.C.; Pipan, T.; Isaia, M. Climate change going deep: The effects of global climatic alterations on cave ecosystems. Anthr. Rev. 2019, 6, 98–116. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Rayne, S.; Forest, K. Rapidly changing climatic conditions for wine grape growing in the Okanagan Valley region of British Columbia, Canada. Sci. Total Environ. 2016, 556, 169–178. [Google Scholar] [CrossRef]
- Cardell, M.F.; Amengual, A.; Romero, R. Future effects of climate change on the suitability of wine grape production across Europe. Reg. Environ. Chang. 2019, 19, 2299–2310. [Google Scholar] [CrossRef]
- Gbejewoh, O.; Keesstra, S.; Blancquaert, E. The 3Ps (profit, planet, and people) of sustainability amidst climate change: A South African grape and wine perspective. Sustainability 2021, 13, 2910. [Google Scholar] [CrossRef]
- Bonfante, A.; Monaco, E.; Langella, G.; Mercogliano, P.; Bucchignani, E.; Manna, P.; Terribile, F. A dynamic viticultural zoning to explore the resilience of terroir concept under climate change. Sci. Total Environ. 2018, 624, 294–308. [Google Scholar] [CrossRef]
- Jones, G.V. The climate component of terroir. Elem. Int. Mag. Mineral. Geochem. Petrol. 2018, 14, 167–172. [Google Scholar] [CrossRef]
- de Oliveira, J.B.; Egipto, R.; Laureano, O.; de Castro, R.; Pereira, G.E.; Ricardo-da-Silva, J.M. Climate effects on physicochemical composition of Syrah grapes at low and high altitude sites from tropical grown regions of Brazil. Food Res. Int. 2019, 121, 870–879. [Google Scholar] [CrossRef]
- Cabré, F.; Nuñez, M. Impacts of climate change on viticulture in Argentina. Reg. Environ. Chang. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Fraga, H.; Nakas, C.T.; Koundouras, S. A study on the effects of climate change on viticulture on Santorini Island. OENO One 2022, 56, 259–273. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Pszczólkowski, P.; Cañón, P.; Taquichiri, M.; Peñarrieta, J.M. UV-B Radiation as a Factor that Deserves Further Research in Bolivian Viticulture: A Review. S. Afr. J. Enol. Vitic. 2021, 42, 201–212. [Google Scholar] [CrossRef]
- Nimac, I.; Herceg-Bulić, I.; Cindrić Kalin, K.; Perčec Tadić, M. Changes in extreme air temperatures in the mid-sized European city situated on southern base of a mountain (Zagreb, Croatia). Theor. Appl. Climatol. 2021, 146, 429–441. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aranjuelo, I.; Pascual, I.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Araus, J.L.; Morales, F. Is vegetative area, photosynthesis, or grape C uploading involved in the climate change-related grape sugar/anthocyanin decoupling in Tempranillo? Photosynth. Res. 2018, 138, 115–128. [Google Scholar] [CrossRef]
- Candar, S.; Bahar, E.; Korkutal, I. Impacts of Leaf Area on the Physiological Activity and Berry Maturation of Merlot (Vitis vinifera L.). Appl. Ecol. Environ. Res. 2020, 18, 1523–1538. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. A Review on the Observed Climate Change in Europe and Its Impacts on Viticulture. Atmosphere 2022, 13, 837. [Google Scholar] [CrossRef]
- Gohari, A.; Mirchi, A.; Madani, K. System dynamics evaluation of climate change adaptation strategies for water resources management in central Iran. Water Resour. Manag. 2017, 31, 1413–1434. [Google Scholar] [CrossRef] [Green Version]
- Ostad-Ali-Askari, K.; Ghorbanizadeh Kharazi, H.; Shayannejad, M.; Zareian, M.J. Effect of climate change on precipitation patterns in an arid region using GCM models: Case study of Isfahan-Borkhar Plain. Nat. Hazards Rev. 2020, 21, 04020006. [Google Scholar] [CrossRef]
- Boo, Y.C.; Jung, J. Water deficit—induced oxidative stress and antioxidative defenses in rice plants. J. Plant Physiol. 1999, 155, 255–261. [Google Scholar]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of drought stress on lipid peroxidation, osmotic adjustment and antioxidant enzyme activity of leaves and roots of Lycium ruthenicum Murr. seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, P. Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.; Ruan, H. Response of plants to water stress: A meta-analysis. Front. Plant Sci. 2020, 11, 978. [Google Scholar] [CrossRef]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef]
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants 2019, 25, 667–681. [Google Scholar] [CrossRef]
- Galat Giorgi, E.; Sadras, V.O.; Keller, M.; Perez Peña, J. Interactive effects of high temperature and water deficit on Malbec grapevines. Aust. J. Grape Wine Res. 2019, 25, 345–356. [Google Scholar] [CrossRef]
- Li, D.; Wang, M.; Zhang, T.; Chen, X.; Li, C.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants. Photosynth. Res. 2021, 147, 301–315. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yue, X.F.; Zhao, X.F.; Zhao, H.; Fang, Y.L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Tombesi, S.; Frioni, T.; Grisafi, F.; Sabbatini, P.; Poni, S.; Palliotti, A. The Decrease of Leaf Dark Respiration during Water Stress Is Related to Leaf Non-Structural Carbohydrate Pool in Vitis vinifera L. Plants 2021, 11, 36. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [Green Version]
- Gambetta, G.A.; Herrera, J.C.; Dayer, S.; Feng, Q.; Hochberg, U.; Castellarin, S.D. The physiology of drought stress in grapevine: Towards an integrative definition of drought tolerance. J. Exp. Bot. 2020, 71, 4658–4676. [Google Scholar] [CrossRef]
- Ratajczak, Z.; Carpenter, S.R.; Ives, A.R.; Kucharik, C.J.; Ramiadantsoa, T.; Stegner, M.A.; Williams, J.W.; Zhang, J.; Turner, M.G. Abrupt change in ecological systems: Inference and diagnosis. Trends Ecol. Evol. 2018, 33, 513–526. [Google Scholar] [CrossRef]
- Powell, E.B.; Krause, J.R.; Martin, R.M.; Nikinmaa, L.; Lindner, M.; Cantarello, E.; Jump, A.S.; Seidl, R.; Winkel, G.; Muys, B. Reviewing the Use of Resilience Concepts in Forest Sciences. Curr. For. Rep. 2020, 6, 61–80. [Google Scholar]
- Holling, C.S. Resilience and stability of ecological systems. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.P.; De Kauwe, M.G.; Medlyn, B.E.; Zaehle, S.; Iversen, C.M.; Asao, S.; Guenet, B.; Harper, A.; Hickler, T.; Hungate, B.A. Decadal biomass increment in early secondary succession woody ecosystems is increased by CO2 enrichment. Nat. Commun. 2019, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Albrich, K.; Rammer, W.; Seidl, R. Climate change causes critical transitions and irreversible alterations of mountain forests. Glob. Chang. Biol. 2020, 26, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch-Thömmes, A.; Stocker, T.F.; Joos, F. Hysteresis of the Earth system under positive and negative CO2 emissions. Environ. Res. Lett. 2020, 15, 124026. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Precision Viticulture: Managing vineyard variability for improved quality outcomes. In Managing Wine Quality; Volume One: Viticulture and Wine Quality Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Thorston, UK, 2022; pp. 541–586. [Google Scholar]
- Rehakova, M.; Čuvanová, S.; Dzivak, M.; Rimár, J.; Gaval’Ova, Z. Agricultural and agrochemical uses of natural zeolite of the clinoptilolite type. Curr. Opin. Solid State Mater. Sci. 2004, 8, 397–404. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite clinoptilolite: Therapeutic virtues of an ancient mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [Green Version]
- Wambu, E.W.; Attahiru, S.; Shiundu, P.M.; Wabomba, J. Removal of heavy-metals from wastewater using a hydrous Alumino-silicate mineral from Kenya. Bull. Chem. Soc. Ethiop. 2018, 32, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Jakkula, V.S.; Wani, S.P. Zeolites: Potential soil amendments for improving nutrient and water use efficiency and agriculture productivity. Sci. Rev. Chem. Commun. 2018, 8, 1–15. [Google Scholar]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Bogdanović, G.D.; Antonijević, M.M. Application of natural zeolite in wastewater treatment: A review. J. Min. Metall. A Min. 2019, 55, 67–79. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sprynskyy, M.; Terzyk, A.P.; Lebedynets, M.; Namieśnik, J.; Buszewski, B. Porous structure of natural and modified clinoptilolites. J. Colloid Interface Sci. 2006, 297, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, J.; Ding, X.; Chu, H.; Zhang, J. Frost resistance of internal curing concrete with calcined natural zeolite particles. Constr. Build. Mater. 2021, 288, 123062. [Google Scholar] [CrossRef]
- Lavoie-Lamoureux, A.; Sacco, D.; Risse, P.A.; Lovisolo, C. Factors influencing stomatal conductance in response to water availability in grapevine: A meta-analysis. Physiol. Plant 2017, 159, 468–482. [Google Scholar] [CrossRef] [PubMed]
- Oddo, E.; Abbate, L.; Inzerillo, S.; Carimi, F.; Motisi, A.; Sajeva, M.; Nardini, A. Water relations of two Sicilian grapevine cultivars in response to potassium availability and drought stress. Plant Physiol. Biochem. 2020, 148, 282–290. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Wang, W.; Ran, H.; Song, T.; Guo, Y. Evaluation of the crop water stress index as an indicator for the diagnosis of grapevine water deficiency in greenhouses. Horticulturae 2020, 6, 86. [Google Scholar] [CrossRef]
- Degu, A.; Hochberg, U.; Wong, D.C.; Alberti, G.; Lazarovitch, N.; Peterlunger, E.; Castellarin, S.D.; Herrera, J.C.; Fait, A. Swift metabolite changes and leaf shedding are milestones in the acclimation process of grapevine under prolonged water stress. BMC Plant Biol. 2019, 19, 69. [Google Scholar] [CrossRef]
- de Campos Bernardi, A.C.; Oliviera, P.P.A.; de Melo Monte, M.B.; Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 2013, 167, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Gül, A.; Eroğul, D.; Ongun, A.R. Comparison of the use of zeolite and perlite as substrate for crisp-head lettuce. Sci. Hortic. 2005, 106, 464–471. [Google Scholar] [CrossRef]
- Valente, S.; Burriesci, N.; Cavallaro, S.; Galvagno, S.; Zipelli, C. Utilization of zeolites as soil conditioner in tomato-growing. Zeolites 1982, 2, 271–274. [Google Scholar] [CrossRef]
- Xiubin, H.; Zhanbin, H. Zeolite application for enhancing water infiltration and retention in loess soil. Resour. Conserv. Recycl. 2001, 34, 45–52. [Google Scholar] [CrossRef]
- Domenico, P. Comparison between sterilized zeolite and natural zeolite in the Cactus Pear (Opuntia Ficus-Indica L. Mill.) growing. GSC Adv. Res. Rev. 2020, 5, 007–014. [Google Scholar] [CrossRef]
- De Smedt, C.; Someus, E.; Spanoghe, P. Potential and actual uses of zeolites in crop protection. Pest Manag. Sci. 2015, 71, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Nakhli, S.A.A.; Delkash, M.; Bakhshayesh, B.E.; Kazemian, H. Application of zeolites for sustainable agriculture: A review on water and nutrient retention. Water Air Soil Pollut. 2017, 228, 464. [Google Scholar] [CrossRef]
- Ozbahce, A.; Tari, A.F.; Gönülal, E.; Simsekli, N.; Padem, H. The effect of zeolite applications on yield components and nutrient uptake of common bean under water stress. Arch. Agron. Soil Sci. 2015, 61, 615–626. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Swaefy, H.M. Comparison between commercial and nano NPK in presence of nano zeolite on sage plant yield and its components under water stress. Agriculture 2020, 66, 24–39. [Google Scholar] [CrossRef]
- Gholizadeh, A.; Mohd Soom, M.A.; Abdul Rahim, A.; Saberioon, M.M. Water stress and natural zeolite impacts on phisiomorphological characteristics of moldavian balm (Dracocephalum moldavica L.). Aust. J. Basic Appl. Sci. 2010, 4, 5184–5190. [Google Scholar]
- Valadabadi, S.A.; Shiranirad, A.H.; Farahani, H.A. Ecophysiological influences of zeolite and selenium on water deficit stress tolerance in different rapeseed cultivars. J. Ecol. Nat. Environ. 2013, 2, 154–159. [Google Scholar]
- Baghbani-Arani, A.; Jami, M.G.; Namdari, A.; Karami Borz-Abad, R. Influence of irrigation regimes, zeolite, inorganic and organic manures on water use efficiency, soil fertility and yield of sunflower in a sandy soil. Commun. Soil Sci. Plant Anal. 2020, 51, 711–725. [Google Scholar] [CrossRef]
- Baninasab, B. Effects of the application of natural zeolite on the growth and nutrient status of radish (Raphanus sativus L.). J. Hortic. Sci. Biotechnol. 2009, 84, 13–16. [Google Scholar] [CrossRef]
- Flexas, J.; Scoffoni, C.; Gago, J.; Sack, L. Leaf mesophyll conductance and leaf hydraulic conductance: An introduction to their measurement and coordination. J. Exp. Bot. 2013, 64, 3965–3981. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.M.; Woodruff, D.R.; McCulloh, K.A.; Meinzer, F.C. Leaf hydraulic conductance, measured in situ, declines and recovers daily: Leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol. 2009, 29, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Garcia-Forner, N. Water potential regulation, stomatal behaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2017, 40, 962–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Storchi, P.; Mattii, G.B. Eco-physiological traits and phenylpropanoid profiling on potted Vitis vinifera L. cv Pinot noir subjected to Ascophyllum nodosum treatments under post-veraison low water availability. Appl. Sci. 2020, 10, 4473. [Google Scholar] [CrossRef]
- Cataldo, E.; Salvi, L.; Mattii, G.B. Effects of irrigation on ecophysiology, sugar content and thiol precursors (3-S-cysteinylhexan-1-ol and 3-S-glutathionylhexan-1-ol) on Vitis vinifera cv. Sauvignon Blanc. Plant Physiol. Biochem. 2021, 164, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Janoš, P.; Vávrová, J.; Herzogová, L.; Pilařová, V. Effects of inorganic and organic amendments on the mobility (leachability) of heavy metals in contaminated soil: A sequential extraction study. Geoderma 2010, 159, 335–341. [Google Scholar] [CrossRef]
- Cataldo, E.C.; Salvi, L.S.; Paoli, F.P.; Fucile, M.F.; Masciandaro, G.M.; Manzi, D.M.; Masini, C.M.M.; Mattii, G.B.M. Effects of natural clinoptilolite on physiology, water stress, sugar, and anthocyanin content in Sanforte (Vitis vinifera L.) young vineyard. J. Agric. Sci. 2021, 159, 488–499. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Ghaffari, S.; Reynard, J.S.; Rienth, M. Single berry reconstitution prior to RNA-sequencing reveals novel insights into transcriptomic remodeling by leafroll virus infections in grapevines. Sci. Rep. 2020, 10, 12905. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Civardi, S.; Gatti, M.; Porro, D.; Camin, F. Performance and water-use efficiency (single-leaf vs. whole-canopy) of well-watered and half-stressed split-root Lambrusco grapevines grown in Po Valley (Italy). Agric. Ecosyst. Environ. 2009, 129, 97–106. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Zuecco, G.; Amin, A.; Frentress, J.; Engel, M.; Marchina, C.; Anfodillo, T.; Borga, M.; Carraro, V.; Scandellari, F.; Tagliavini, M.; et al. A Comparative Study of Plant Water Extraction Methods for Isotopic Analyses: Scholander-Type Pressure Chamber vs. Cryogenic Vacuum Distillation. Hydrol. Earth Syst. Sci. Discuss 2020, 1–23. Available online: https://hess.copernicus.org/preprints/hess-2020-446/ (accessed on 9 June 2022).
- Williams, L.E.; Araujo, F.J. Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera. J. Am. Soc. Hortic. Sci. 2002, 127, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of irrigation and crop level on growth, yield and wine quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 132–139. [Google Scholar]
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 2006, 44, 151–154. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, lilliefors and Anderson-darling tests. J. Stat. Modeling Anal. 2011, 2, 21–33. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of Levene’s test of equality of variances on statistical theory and practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Roman, I.A.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Analysis of Physico-Chemical and Organoleptic Fruit Parameters Relevant for Tomato Quality. Agronomy 2022, 12, 1232. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Tassinari, A.; Stefanello, L.O.; Schwalbert, R.A.; Vitto, B.B.; Kulmann, M.S.D.S.; Santos, J.P.J.; Arruda, W.S.; Schwalbert, R.; Tiecher, T.L.; Ceretta, C.A.; et al. Nitrogen Critical Level in Leaves in ‘Chardonnay’and ‘Pinot Noir’Grapevines to Adequate Yield and Quality Must. Agronomy 2022, 12, 1132. [Google Scholar] [CrossRef]
- Horton, N.J.; Kleinman, K. Using R and RStudio for Data Management, Statistical Analysis, and Graphics; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
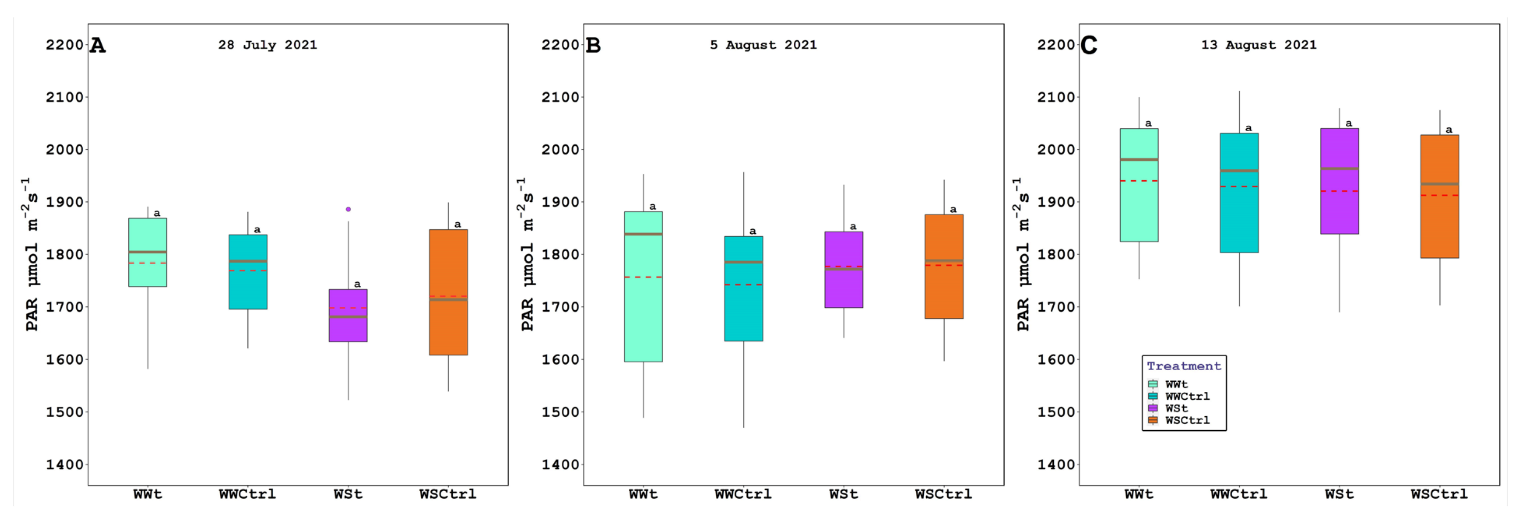
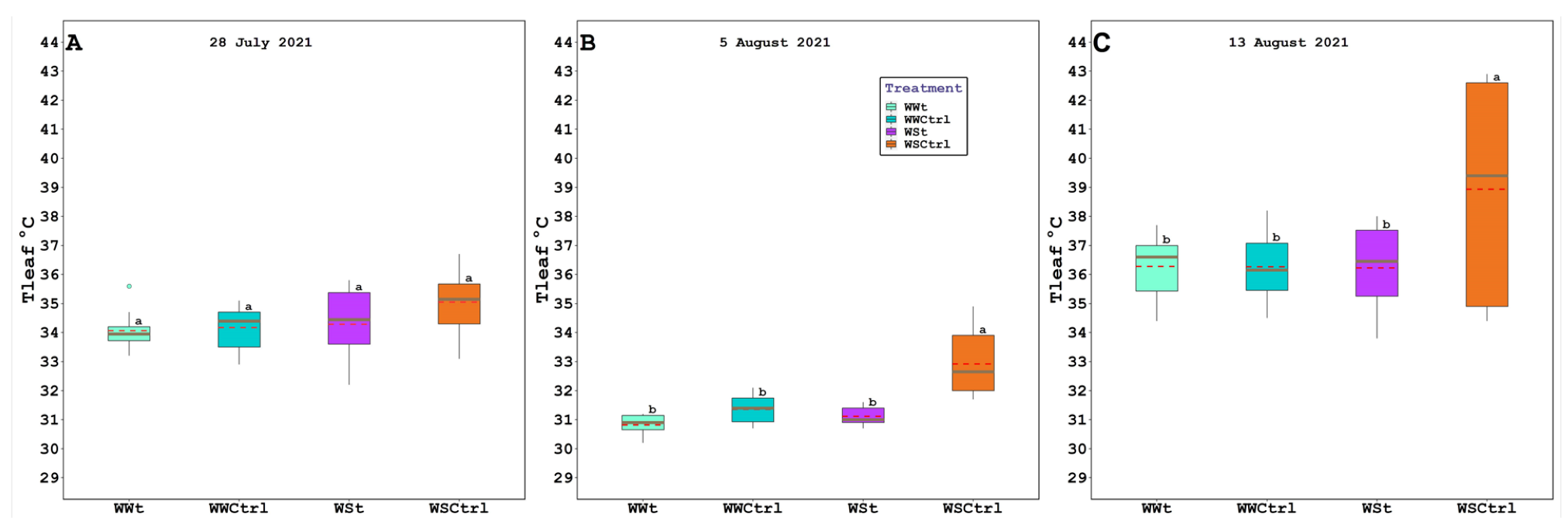

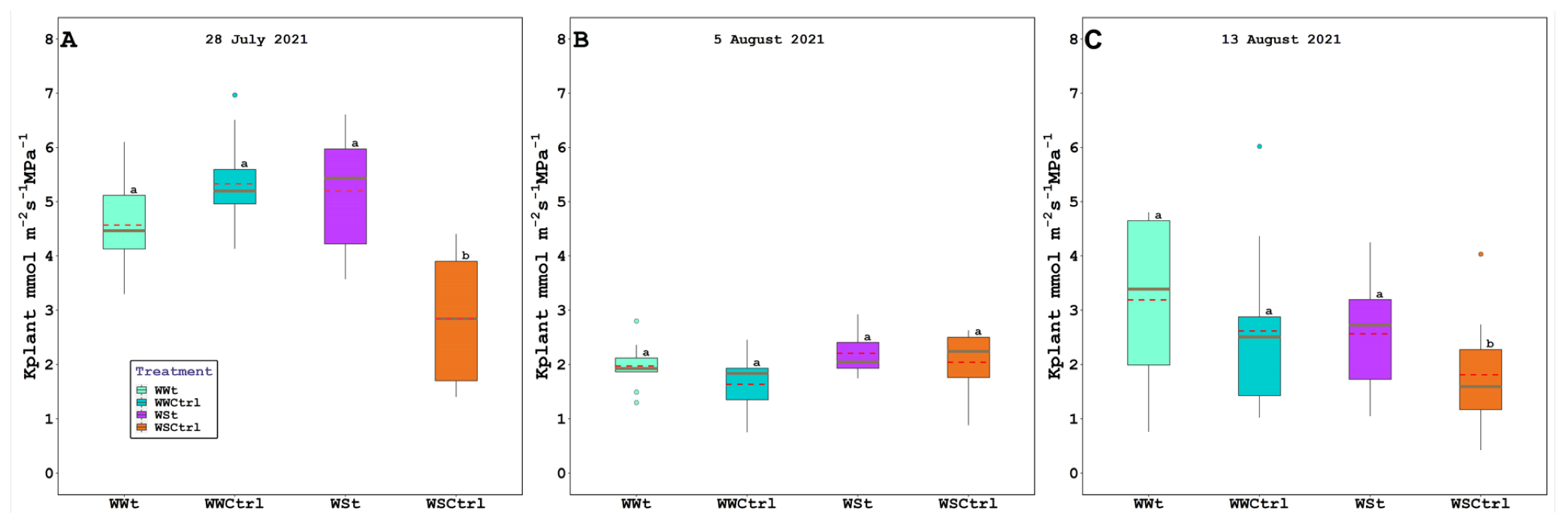
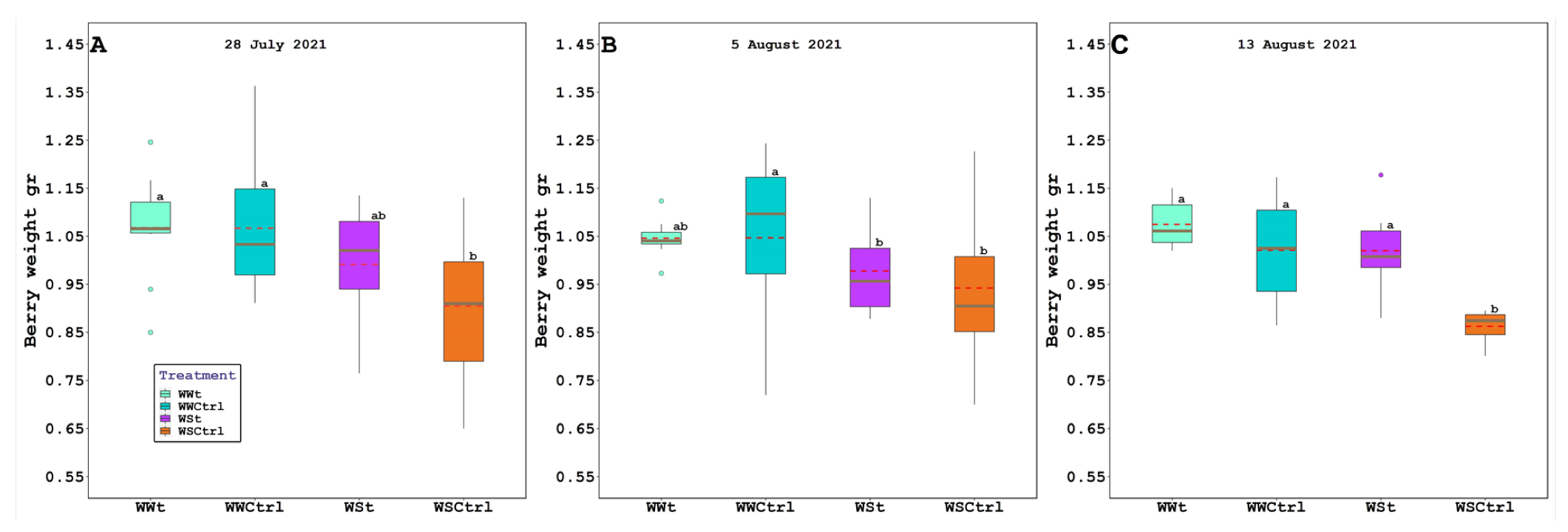
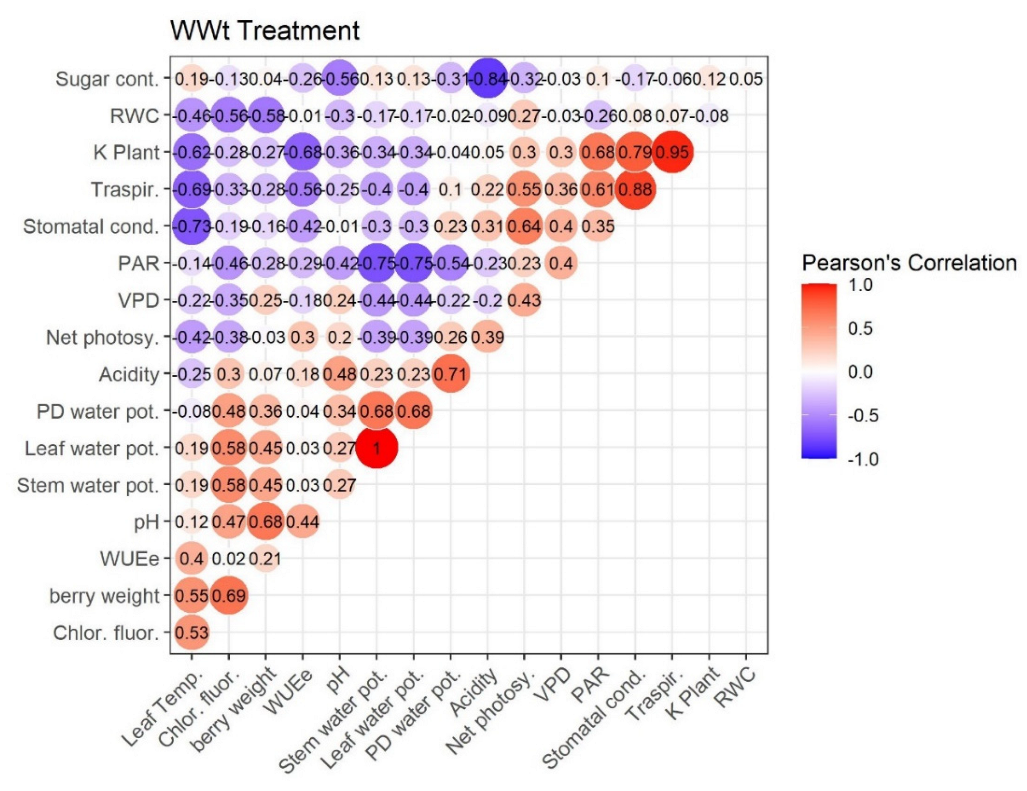
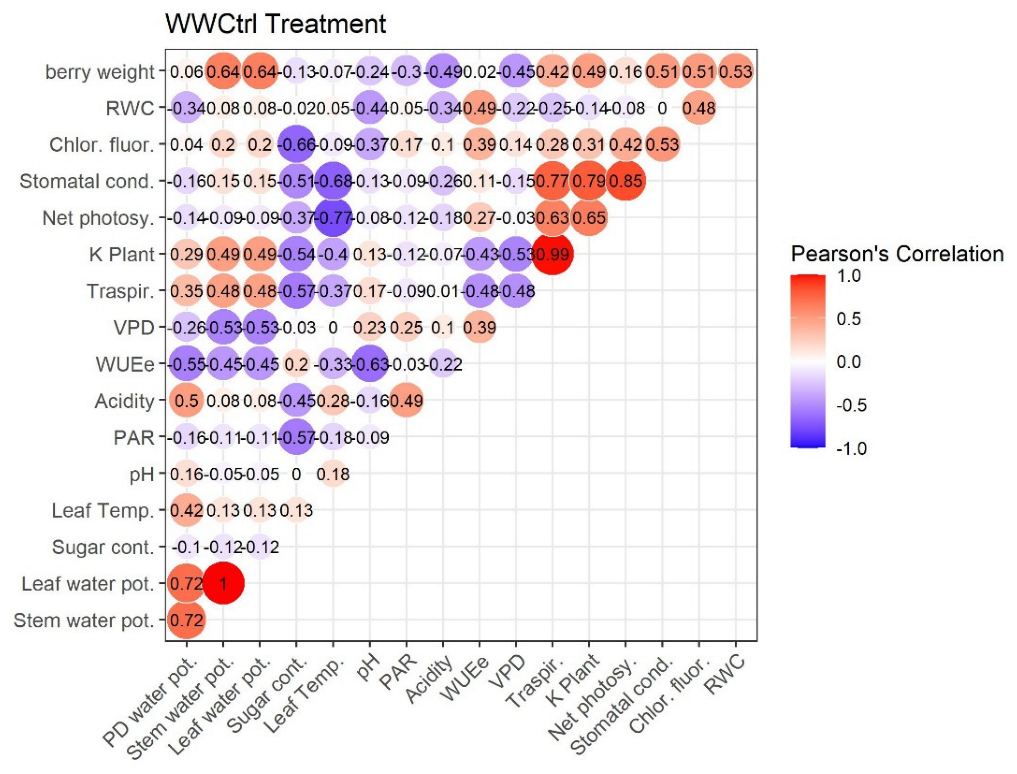

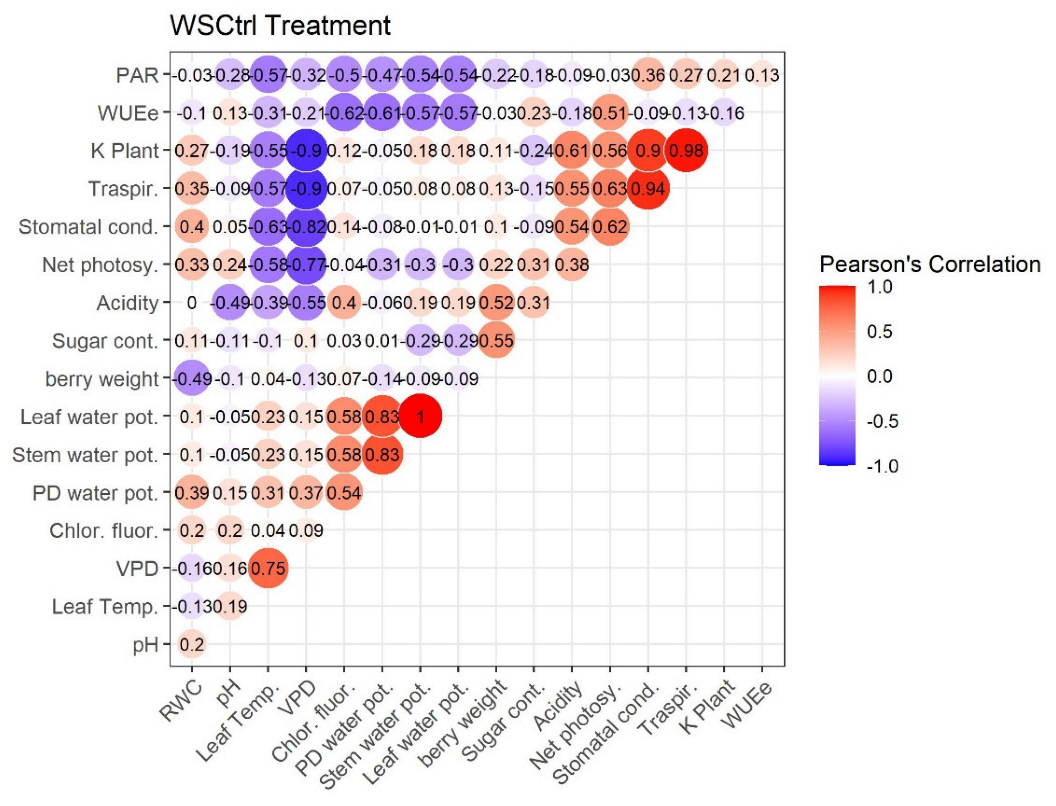
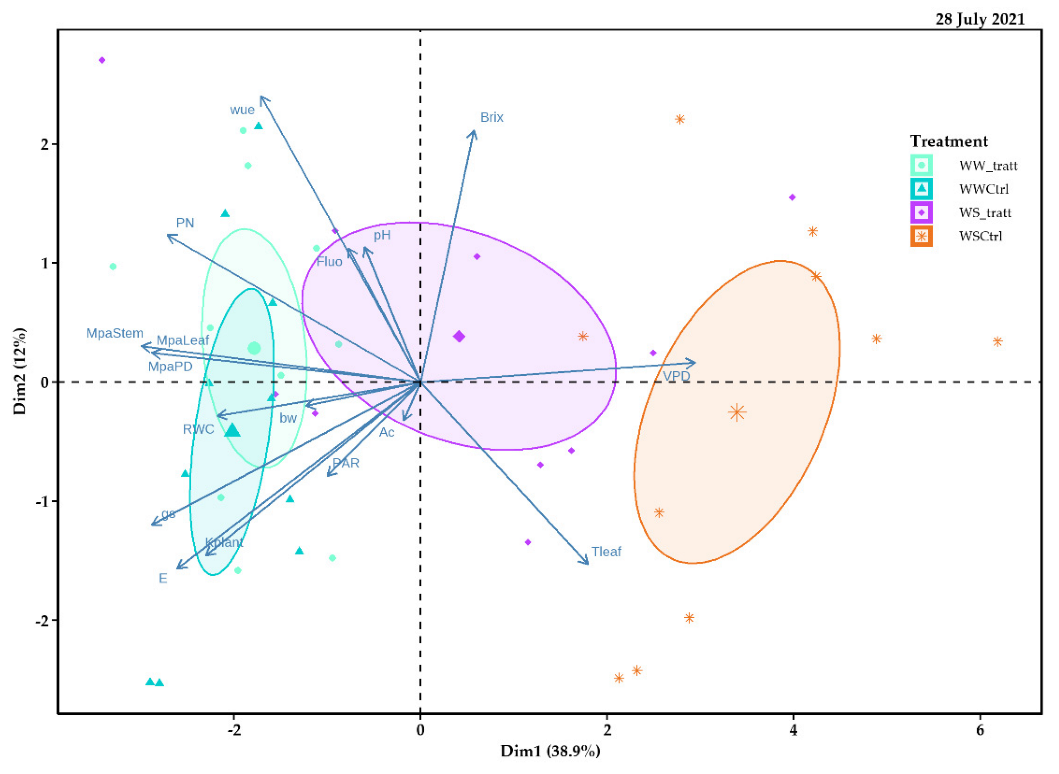
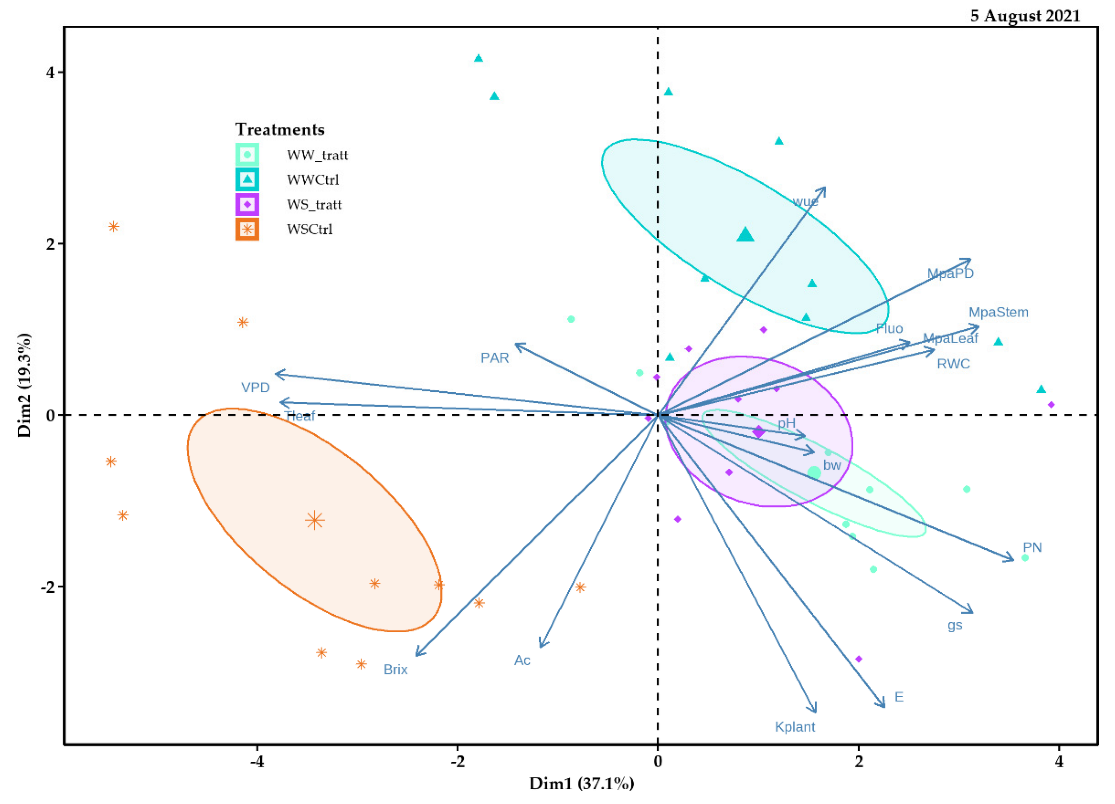
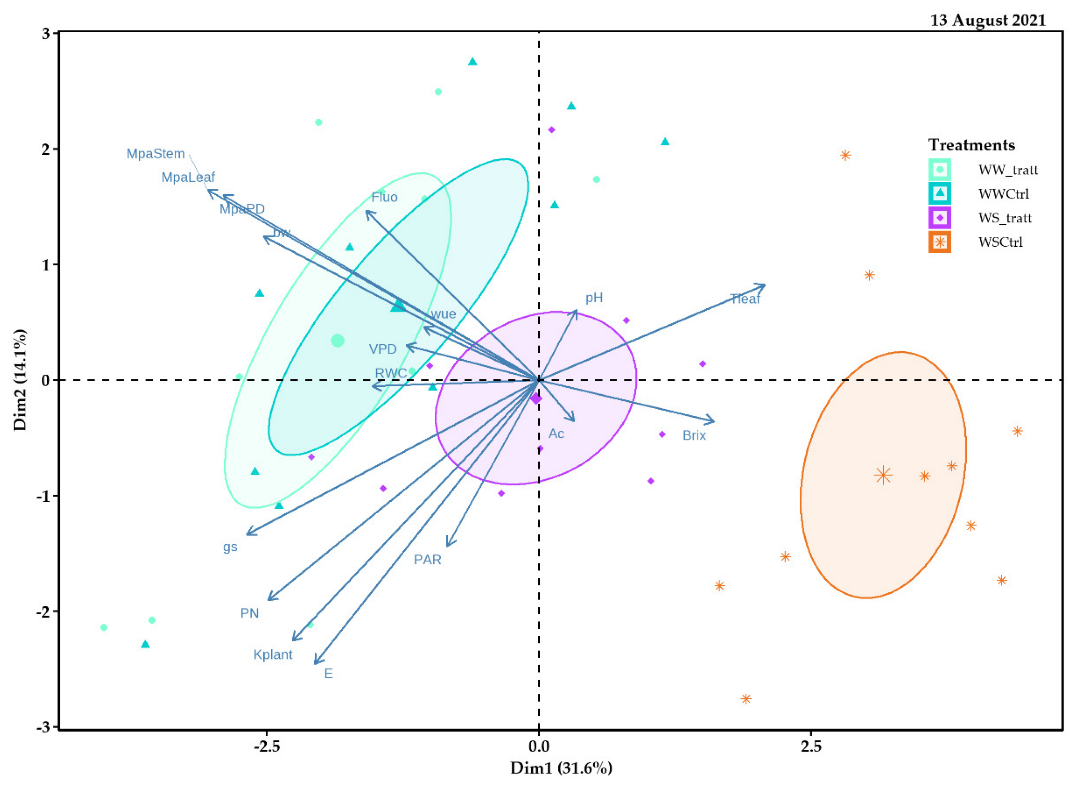
| Parameter | gs | E | WUEe | Fluo | VPD |
|---|---|---|---|---|---|
| Unit | mmol m−2 s−1 | mmol m−2 s−1 | µmol mmol−1 | Fv/Fm | kPa |
| 28 July 2021 | |||||
| Treatments: | |||||
| WWt | 174.70 ± 55.39 a | 4.62 ± 1.07 a | 3.21 ± 1.16 a | 0.77 ± 0.04 a | 3.17 ± 0.23 b |
| WSt | 145.40 ± 39.00 a | 4.57 ± 1.17 a | 3.00 ± 1.10 a | 0.76 ± 0.05 a | 3.50 ± 0.49 ab |
| Irrigation regime: | |||||
| WWCtrl | 189.60 ± 57.25 a | 5.34 ± 1.49 a | 2.67 ± 0.95 ab | 0.78 ± 0.03 a | 3.11 ± 0.45 b |
| WSCtrl | 82.60 ± 14.29 b | 2.82 ± 0.29 b | 1.64 ± 0.35 b | 0.75 ± 0.03 a | 3.76 ± 0.17 a |
| Significance: | |||||
| Treatments | 0.097 | 0.112 | 0.003 | 1.000 | 0.391 |
| Irrigation regime | 0.000 | 0.000 | 0.044 | 0.131 | 0.000 |
| Treat. × Irr. regime | 0.009 | 0.000 | 0.178 | 0.445 | 0.174 |
| 5 August 2021 | |||||
| Treatments: | |||||
| WWt | 89.20 ± 22.42 a | 2.28 ± 0.44 a | 3.86 ± 0.53 b | 0.77 ± 0.03 a | 2.66 ± 0.21 c |
| WSt | 69.90 ± 56.28 ab | 1.85 ± 1.37 ab | 4.75 ± 0.68 a | 0.76 ± 0.05 a | 2.81 ± 1.22 bc |
| Irrigation regime: | |||||
| WWCtrl | 61.00 ± 18.10 b | 1.61 ± 0.32 b | 4.80 ± 0.87 a | 0.76 ± 0.06 a | 2.92 ± 0.35 b |
| WSCtrl | 54.70 ± 52.66 b | 1.81 ± 1.56 ab | 3.63 ± 1.80 b | 0.69 ± 0.04 b | 3.30 ± 1.02 a |
| Significance: | |||||
| Treatments | 0.000 | 0.007 | 0.639 | 0.010 | 0.000 |
| Irrigation regime | 0.038 | 0.376 | 0.422 | 0.007 | 0.000 |
| Treat. × Irr. regime | 0.281 | 0.015 | 0.000 | 0.090 | 0.098 |
| 13 August 2021 | |||||
| Treatments: | |||||
| WWt | 115.00 ± 74.64 a | 3.14 ± 1.35 a | 2.91 ± 1.31 a | 0.77 ± 0.01 ab | 4.80 ± 1.00 a |
| WSt | 109.42 ± 23.12 a | 2.27 ± 0.99 a | 3.71 ± 0.48 a | 0.76 ± 0.10 ab | 3.54 ± 0.35 b |
| Irrigation regime: | |||||
| WWCtrl | 112.30 ± 54.33 a | 2.32 ± 0.84 a | 3.72 ± 1.83 a | 0.79 ± 0.09 a | 5.04 ± 0.88 a |
| WSCtrl | 47.30 ± 21.36 a | 1.99 ± 0.95 a | 2.26 ± 0.73 a | 0.69 ± 0.10 b | 3.98 ± 0.31 b |
| Significance: | |||||
| Treatments | 0.078 | 0.187 | 0.498 | 0.347 | 0.209 |
| Irrigation regime | 0.056 | 0.150 | 0.484 | 0.024 | 0.000 |
| Treat. × Irr. regime | 0.105 | 0.522 | 0.020 | 0.035 | 0.711 |
| Parameter | ΨPD | Ψleaf | Ψstem | RWC |
|---|---|---|---|---|
| Unit | MPa | MPa | MPa | % |
| 28 July 2021 | ||||
| Treatments: | ||||
| WWt | −0.27 ± 0.12 a | −1.28 ± 0.10 a | −1.00 ± 0.11 a | 90.99 ± 1.76 a |
| WSt | −1.42 ± 0.22 b | −1.66 ± 0.14 b | −1.42 ± 0.16 a | 88.49 ± 3.23 ab |
| Irrigation regime: | ||||
| WWCtrl | −0.28 ± 0.34 a | −1.28 ± 0.30 a | −1.00 ± 0.33 a | 90.32 ± 3.16 ab |
| WSCtrl | −0.91 ± 0.13 b | −1.90 ± 0.11 c | −1.69 ± 0.12 b | 87.32 ± 12.36 b |
| Significance: | ||||
| Treatments | 0.344 | 0.070 | 0.070 | 0.319 |
| Irrigation regime | 0.000 | 0.000 | 0.000 | 0.004 |
| Treat. × Irr. regime | 0.437 | 0.070 | 0.070 | 0.789 |
| 5 August 2021 | ||||
| Treatments: | ||||
| WWt | −0.51 ± 0.12 a | −1.69 ± 0.15 a | −1.45 ± 0.17 b | 87.59 ± 4.70 a |
| WSt | −0.64 ± 0.22 a | −1.49 ± 0.17 a | −1.23 ± 0.19 a | 82.25 ± 19.86 ab |
| Irrigation regime: | ||||
| WWCtrl | −0.54 ± 0.21 a | −1.57 ± 0.21 a | −1.32 ± 0.24 a | 82.97 ± 5.97 ab |
| WSCtrl | −0.94 ± 0.15 b | −1.85 ± 0.15 b | −1.63 ± 0.16 c | 66.70 ± 10.08 c |
| Significance: | ||||
| Treatments | 0.000 | 0.05 | 0.005 | 0.000 |
| Irrigation regime | 0.000 | 0.329 | 0.329 | 0.000 |
| Treat. × Irr. regime | 0.001 | 0.000 | 0.000 | 0.057 |
| 13 August 2021 | ||||
| Treatments: | ||||
| WWt | −0.36 ± 0.17 a | −1.32 ± 0.16 a | −1.05 ± 0.17 a | 34.55 ± 8.22 a |
| WSt | −0.69 ± 0.13 b | −1.59 ± 0.11 b | −1.35 ± 0.12 b | 28.74 ± 5.71 a |
| Irrigation regime: | ||||
| WWCtrl | −0.49 ± 0.21 a | −1.38 ± 0.31 a | −1.12 ± 0.34 a | 29.96 ± 6.59 a |
| WSCtrl | −0.99 ± 0.18 c | −2.14 ± 0.18 c | −1.94 ± 0.20 c | 26.09 ± 5.81 a |
| Significance: | ||||
| Treatments | 0.000 | 0.000 | 0.000 | 0.144 |
| Irrigation regime | 0.000 | 0.000 | 0.000 | 0.069 |
| Treat. × Irr. regime | 0.135 | 0.000 | 0.000 | 0.662 |
| Parameter | Sugar Content | Acidity | pH |
|---|---|---|---|
| Unit | °Brix | g L−1 tartaric ac. | pH |
| 28 July 2021 | |||
| Treatments: | |||
| WWt | 15.93 ± 0.80 b | 13.56 ± 1.35 a | 3.12 ± 0.04 a |
| WSt | 16.73 ± 0.77 a | 13.13 ± 1.30 a | 3.08 ± 0.10 a |
| Irrigation regime: | |||
| WWCtrl | 15.73 ± 0.30 b | 13.56 ± 1.34 a | 3.13 ± 0.04 a |
| WSCtrl | 16.00 ± 0.52 ab | 13.43 ± 1.09 a | 3.10 ± 0.08 a |
| Significance: | |||
| Treatments | 0.024 | 0.736 | 0.466 |
| Irrigation regime | 0.010 | 0.526 | 0.053 |
| Treat. × Irr. regime | 0.187 | 0.736 | 0.916 |
| 5 August 2021 | |||
| Treatments: | |||
| WWt | 16.86 ± 0.67 c | 12.43 ± 1.84 b | 3.21 ± 0.32 a |
| WSt | 17.20 ± 1.11 b | 14.26 ± 3.57 a | 3.13 ± 0.59 a |
| Irrigation regime: | |||
| WWCtrl | 16.06 ± 0.52 c | 10.36 ± 0.88 c | 3.17 ± 0.09 a |
| WSCtrl | 17.90 ± 1.71 a | 13.73 ± 0.23 a | 3.00 ± 0.07 a |
| Significance: | |||
| Treatments | 0.665 | 0.000 | 0.386 |
| Irrigation regime | 0.000 | 0.000 | 0.215 |
| Treat. × Irr. regime | 0.000 | 0.002 | 0.669 |
| 13 August 2021 | |||
| Treatments: | |||
| WWt | 19.30 ± 0.86 a | 5.36 ± 0.25 b | 3.47 ± 0.05 a |
| WSt | 19.98 ± 1.54 a | 5.93 ± 0.48 a | 3.47 ± 0.07 a |
| Irrigation regime: | |||
| WWCtrl | 18.22 ± 1.32 b | 5.76 ± 0.35 ab | 3.52 ± 0.08 a |
| WSCtrl | 20.09 ± 1.42 a | 5.56 ± 0.46 b | 3.50 ± 0.06 a |
| Significance: | |||
| Treatments | 0.268 | 0.854 | 0.092 |
| Irrigation regime | 0.005 | 0.049 | 0.709 |
| Treat. × Irr. regime | 0.178 | 0.000 | 0.602 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, E.; Fucile, M.; Mattii, G.B. Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress. Plants 2022, 11, 1735. https://doi.org/10.3390/plants11131735
Cataldo E, Fucile M, Mattii GB. Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress. Plants. 2022; 11(13):1735. https://doi.org/10.3390/plants11131735
Chicago/Turabian StyleCataldo, Eleonora, Maddalena Fucile, and Giovan Battista Mattii. 2022. "Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress" Plants 11, no. 13: 1735. https://doi.org/10.3390/plants11131735
APA StyleCataldo, E., Fucile, M., & Mattii, G. B. (2022). Leaf Eco-Physiological Profile and Berries Technological Traits on Potted Vitis vinifera L. cv Pinot Noir Subordinated to Zeolite Treatments under Drought Stress. Plants, 11(13), 1735. https://doi.org/10.3390/plants11131735








