Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport
Abstract
:1. Introduction
2. Results
2.1. Effect of Geraniol Treatment on Anthocyanin Accumulation in Grape Berry Skins of Field-Grown Grapevines
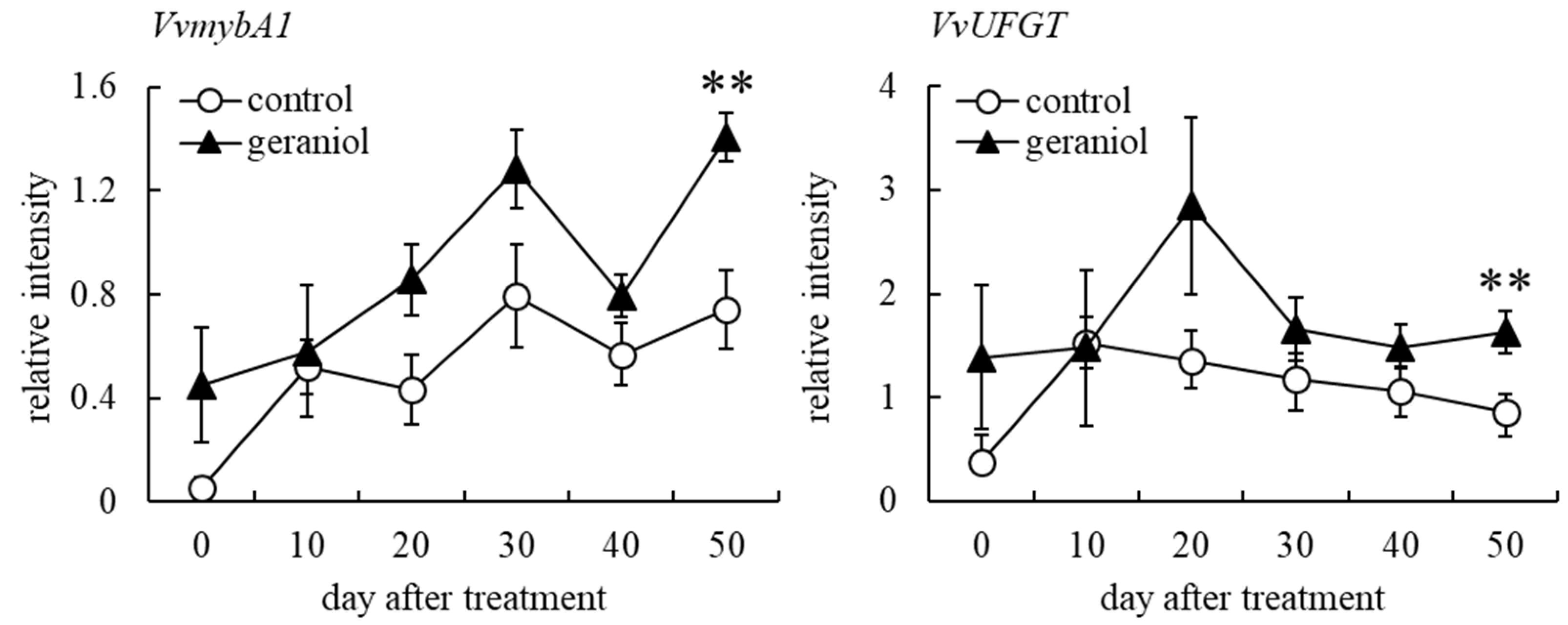
2.2. Effect of Geraniol Treatment on the Transcription of Anthocyanin-Biosynthesis-Related Genes in Grape Berry Skins of Field-Grown Grapevines
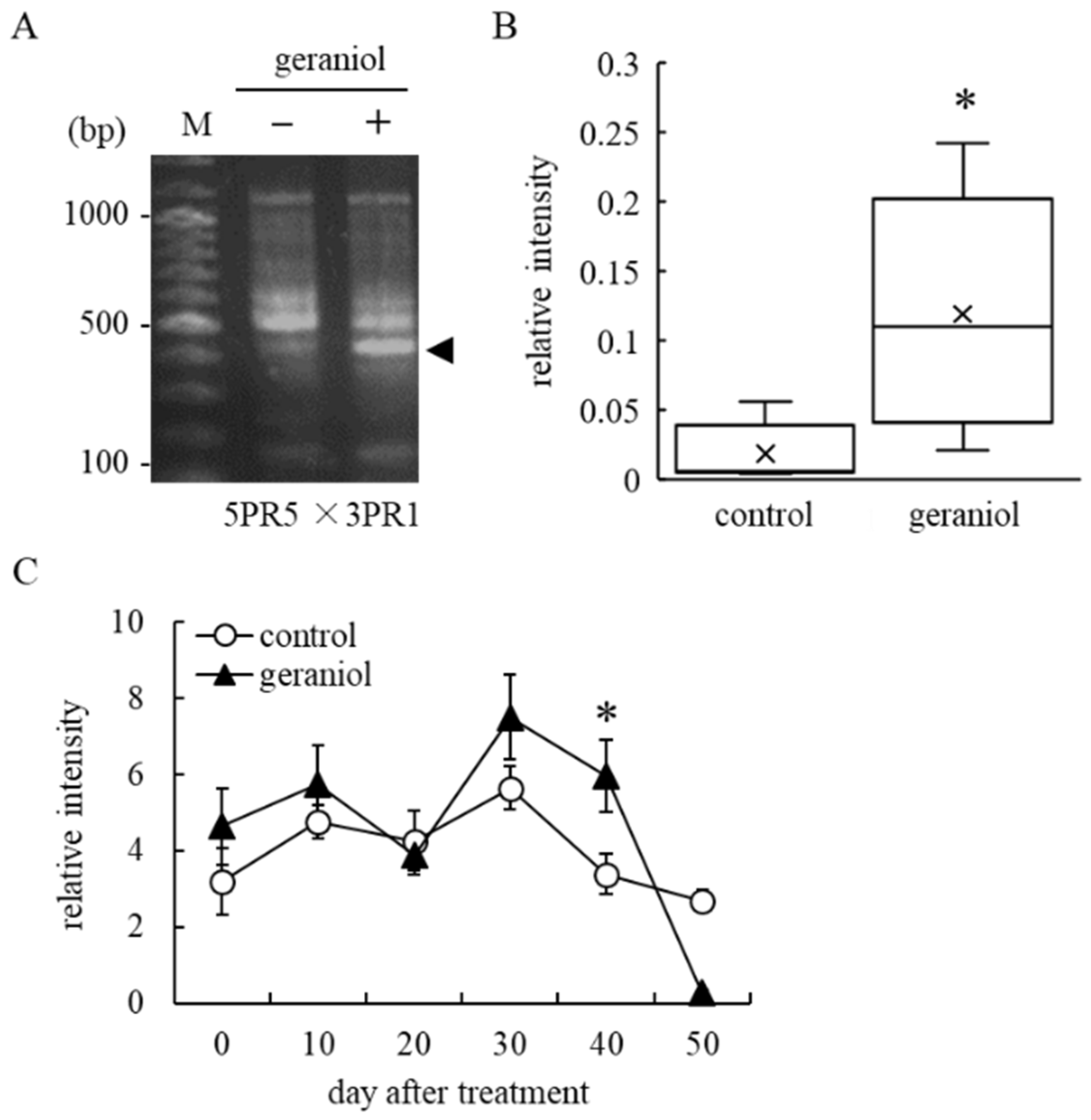
2.3. Effect of Geraniol Treatment on Anthocyanin Accumulation in Grape Cultured Cells
2.4. Identification of Upregulated Genes by Geraniol Treatment Using Reverse Transcription-Polymerase Chain Reaction (RT-PCR)-Based Differential Display
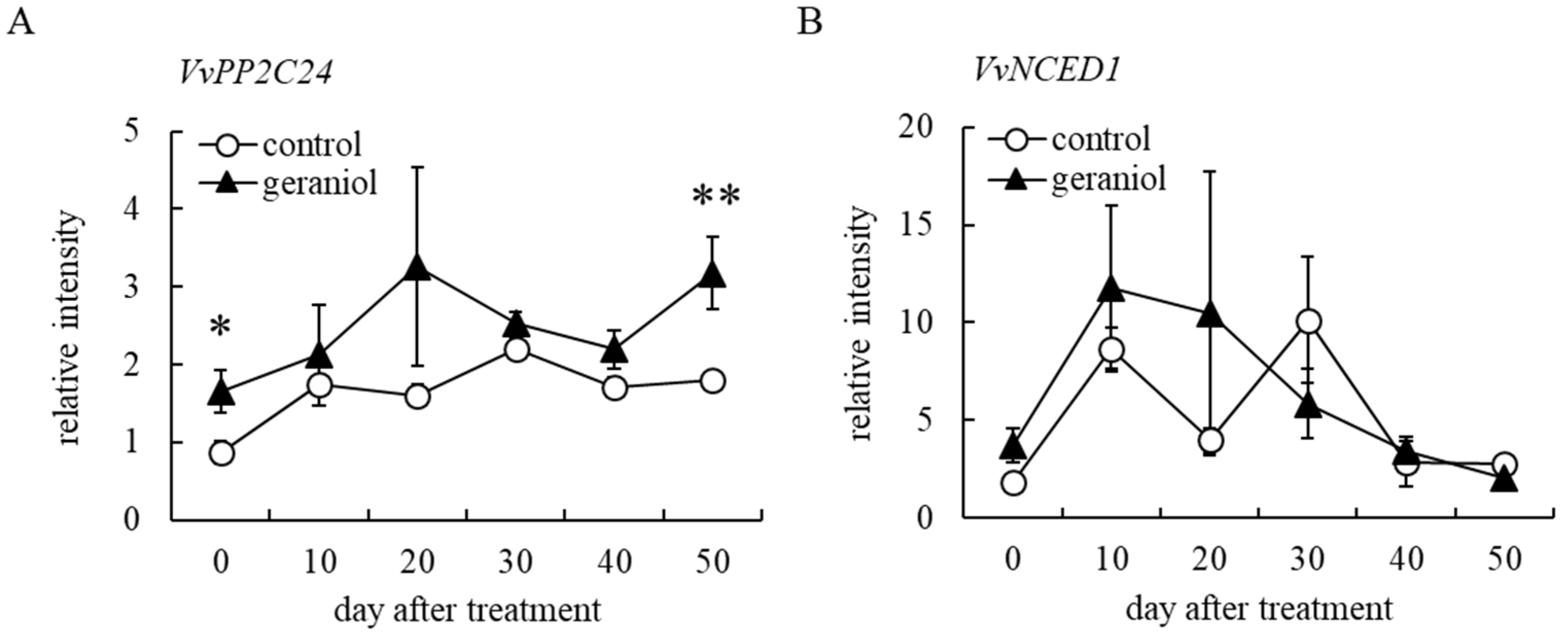
2.5. Geraniol Enhances Transcription of ABA-Responsible Gene, but Not ABA-Biosynthesis-Related Gene, in Grape Berry Skins of Field-Grown Grapevines
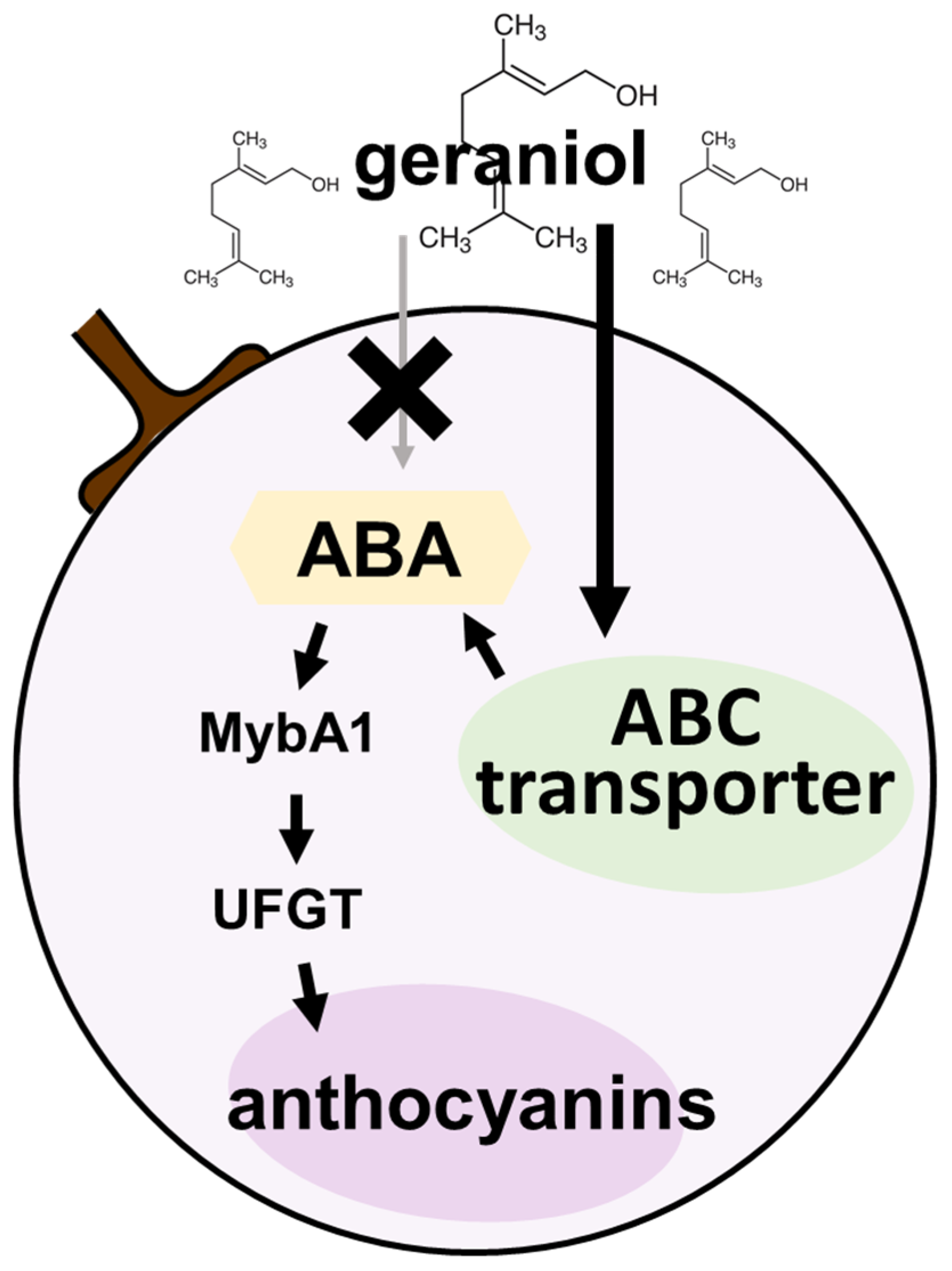
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Geraniol Treatment of Field-Grown Grape Bunches
4.3. Geraniol Treatment of VR Cells
4.4. Berry Characteristics
4.5. Total Anthocyanin Measurement
4.6. RNA Isolation
4.7. RT-PCR-Based Differential Display
4.8. Real-Time RT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castellarin, S.D.; Gaspero, D.G. Transcriptional control of anthocyanin biosynthetic genes in extreme phenotypes for berry pigmentation of naturally occurring grapevines. BMC Plant Biol. 2007, 30, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boss, P.K.; Davies, C.; Robinson, S.P. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Mol. Biol. 1996, 32, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.V.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Castilla, I.; de Cortázar-Atauri, I.G.; Cook, B.I.; Lacombe, T.; Parker, A.; van Leeuwen, C.; Nicholas, K.A.; Wolkovich, E.M. Diversity buffers winegrowing regions from climate change losses. Proc. Natl. Acad. Sci. USA 2020, 117, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Brar, H.S.; Singh, Z.; Swinny, E.; Cameron, I. Girdling and grapevine leafroll associated viruses affect berry weight, colour development and accumulation of anthocyanins in ‘Crimson Seedless’ grapes during maturation and ripening. Plant Sci. 2008, 175, 885–897. [Google Scholar] [CrossRef]
- Matsuyama, S.; Tanzawa, F.; Kobayashi, H.; Suzuki, S.; Takata, R.; Saito, H. Leaf removal accelerated accumulation of delphinidin-based anthocyanins in ‘Muscat Bailey A’ (Vitis × labruscana (Bailey) and Vitis vinifera (Muscat Hamburg)) grape skin. J. Jpn. Soc. Hortic. Sci. 2014, 83, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Guidoni, S.; Allara, P.; Schubert, A. Effect of cluster thinning on berry skin anthocyanin composition of Vitis vinifera cv. Nebbiolo. Am. J. Enol. Vitic. 2002, 53, 224–226. [Google Scholar]
- Gutiérrez-Gamboa, G.; Zheng, W.; de Toda Martínez, F. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef]
- Yamazaki, M.; Ishida, A.; Suzuki, Y.; Aoki, Y.; Suzuki, S.; Enoki, S. Ethylene induced by sound stimulation enhances anthocyanin accumulation in grape berry skin through direct upregulation of UDP-glucose: Flavonoid 3-O-glucosyltransferase. Cells 2021, 10, 2799. [Google Scholar] [CrossRef]
- Pilati, S.; Bagagli, G.; Sonego, P.; Moretto, M.; Brazzale, D.; Castorina, G.; Simoni, L.; Tonelli, C.; Guella, G.; Engelen, K.; et al. Abscisic acid is a major regulator of grape berry ripening onset: New insights into ABA signaling network. Front. Plant Sci. 2017, 8, 1093. [Google Scholar] [CrossRef] [Green Version]
- Peppi, M.C.; Walker, M.A.; Fidelibus, M.W. Application of abscisic acid rapidly upregulated UFGT gene expression and improved color of grape berries. Vitis 2008, 47, 11–24. [Google Scholar]
- Gagné, S.; Estève, K.; Deytieux, C.; Saucier, C.; Gény, L. Influence of abscisic acid in triggering “véraison” in grape berry skins of Vitis vinifera L. cv. Cabernet Sauvignon. J. Int. Sci. Vigne Vin 2006, 40, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Enoki, S.; Hattori, T.; Ishiai, S.; Tanaka, S.; Mikami, M.; Arita, K.; Nagasaka, S.; Suzuki, S. Vanillylacetone up-regulates expression of genes leading to anthocyanin accumulation by inducing endogenous abscisic acid in grape cell cultures. J. Plant Physiol. 2017, 219, 22–27. [Google Scholar] [CrossRef]
- Moriyama, A.; Nojiri, M.; Watanabe, G.; Enoki, S.; Suzuki, S. Exogenous allantoin improves anthocyanin accumulation in grape berry skin at early stage of ripening. J. Plant Physiol. 2020, 253, 153253. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelberth, J.; Alborn, H.T.; Schmelz, E.A.; Tumlinson, J.H. Airborne signals prime plants against insect herbivore attack. Proc. Natl. Acad. Sci. USA 2004, 101, 1781–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazazzara, V.; Vicelli, B.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Trichoderma spp. volatile organic compounds protect grapevine plants by activating defense-related processes against downy mildew. Physiol. Plant. 2021, 172, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Lazazzara, V.; Bueschl, C.; Parich, A.; Pertot, I.; Schuhmacher, R.; Perazzolli, M. Downy mildew symptoms on grapevines can be reduced by volatile organic compounds of resistant genotypes. Sci. Rep. 2018, 8, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological properties of geraniol—A review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, S.; Goto-Yamamoto, N.; Hirokawa, H. Retrotransposon-induced mutations in grape skin color. Science 2004, 304, 982. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Chuang, L.F.; Doi, R.H.; Chuang, R.Y. Identification of opioid-regulated genes in human lymphocytic cells by differential display: Up-regulation of Krüppel-like factor 7 by morphine. Exp. Cell Res. 2003, 291, 340–351. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.; Lee, M.; Kim, Y.; Assmann, S.; Martinoia, E.; Lee, Y. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 2355–2360. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, M.; Pagliarani, C.; Novák, O.; Ferrandino, A.; Cardinale, F.; Visentin, I.; Schubert, A. Exogenous strigolactone interacts with abscisic acid-mediated accumulation of anthocyanins in grapevine berries. J. Exp. Bot. 2018, 69, 2391–2401. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.L.; Dai, L.L.; Ma, N.; Zhang, C.L. Transcriptome and physiological analyses reveal that AM1 as an ABA-mimicking ligand improves drought resistance in Brassica napus. Plant Growth Regul. 2018, 85, 73–90. [Google Scholar] [CrossRef]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Antoni, R.; Rodriguez, L.; Gonzalez-Guzman, M.; Pizzio, G.A.; Rodriguez, P.L. News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr. Opin. Plant Biol. 2011, 14, 547–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.H.; Yang, B.H.; Wang, X.W.; Li, J.N.; Li, S.; Yang, X.; Ren, R.H.; Fang, Y.L.; Xu, T.F.; Zhang, Z.W.; et al. ABA signaling plays a key role in regulated deficit irrigation-driven anthocyanins accumulation in ‘Cabernet Sauvignon’ grape berries. Environ. Exp. Bot. 2021, 181, 104290. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Shi, X.; Wang, Z.; Ji, X.; Wang, Z.; Wang, B.; Zheng, X.; Wang, H. Evolution and expression of NCED family genes in Vitis vinifera. Chin. Bull. Bot. 2019, 54, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Lee, I.S.; Kim, K.H.; Park, J.; Kim, Y.; Choi, J.H.; Choi, J.S.; Jang, H.J. Activation of intestinal olfactory receptor stimulates glucagon-like peptide-1 secretion in enteroendocrine cells and attenuates hyperglycemia in type 2 diabetic mice. Sci. Rep. 2017, 7, 13978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, M.J.R.; Houghton, P.J.; Barlow, D.J.; Pocock, V.J.; Milligan, S.R. Assessment of estrogenic activity in some common essential oil constituents. J. Pharm. Pharmacol. 2002, 5, 1521–1528. [Google Scholar] [CrossRef]
- Gilpin, S.; Hui, X.; Maibach, H. In vitro human skin penetration of geraniol and citronellol. Dermatitis 2010, 21, 41–48. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Bradaia, A.; Fischer, B.; Coelho, D.; Schöller-Guinard, M.; Gosse, F.; Raul, F. Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells. J. Pharmacol. Exp. Ther. 2002, 303, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Cormier, F.; Do, C.B.; Moresoli, C.; Archambault, J.; Chavarie, C.; Chaouki, F.; Pépin, M.F. Anthocyanin release from grape (Vitis vinifera L.) cell suspension. Biotechnol. Lett. 1992, 14, 1029–1034. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Jones, G.V.; Edwards, E.J.; Bonada, M.; Sadras, V.O.; Krstic, M.P.; Herderich, M.J. Climate change and its consequences for viticulture. In Woodhead Publishing Series in Food Science, Technology and Nutrition, Managing Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2022; pp. 727–778. [Google Scholar]
- Portu, J.; Gonzalez-Arenzana, L.; Hermosín-Gutiérrez, I.; Santamaría, P.; Garde-Cerdan, T. Phenylalanine and urea foliar applications to grapevine: Effect on wine phenolic content. Food Chem. 2015, 180, 55–63. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Chen, Y.; Enoki, S.; Igarashi, D.; Suzuki, S. Exogenous isoleucine and phenylalanine interact with abscisic acid-mediated anthocyanin accumulation in grape. Folia Hortic. 2019, 31, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef]
- Botondi, R.; Lodola, L.; Mencarelli, F. Postharvest ethylene treatment affects berry dehydration, polyphenol and anthocyanin content by increasing the activity of cell wall enzymes in Aleatico wine grape. Eur. Food Res. Technol. 2011, 232, 679–685. [Google Scholar] [CrossRef]
- Luan, F.; Wüst, M. Differential incorporation of 1-deoxy-d-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry 2002, 60, 451–459. [Google Scholar] [CrossRef]
- May, B.; Lange, B.M.; Wüst, M. Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: Evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 2013, 95, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, T.; Kato, S.; Ishida, K.; Kodama, T.; Minoda, Y. Production of anthocyanins by Vitis cells in suspension culture. Agric. Biol. Chem. 1983, 47, 2185–2191. [Google Scholar] [CrossRef]
- Bakker, J.; Preston, N.W.; Timberlake, C.F. The determination of anthocyanins in aging red wines: Comparison of HPLC and spectral methods. Am. J. Enol. Vitic. 1986, 37, 121–126. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikami, N.; Konya, M.; Enoki, S.; Suzuki, S. Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport. Plants 2022, 11, 1694. https://doi.org/10.3390/plants11131694
Mikami N, Konya M, Enoki S, Suzuki S. Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport. Plants. 2022; 11(13):1694. https://doi.org/10.3390/plants11131694
Chicago/Turabian StyleMikami, Norika, Mayu Konya, Shinichi Enoki, and Shunji Suzuki. 2022. "Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport" Plants 11, no. 13: 1694. https://doi.org/10.3390/plants11131694
APA StyleMikami, N., Konya, M., Enoki, S., & Suzuki, S. (2022). Geraniol as a Potential Stimulant for Improving Anthocyanin Accumulation in Grape Berry Skin through ABA Membrane Transport. Plants, 11(13), 1694. https://doi.org/10.3390/plants11131694






