3. Discussion
A phylogenetic analysis yielded a topology that was similar to that published by Bakalin et al. [
8]. Previously, we wrote [
8] (p. 63) the following: “Sect.
Stolonicaulon is highly distinctive from the other bulk of
Marsupella in a number of features, including … very rigid shoots, strongly distanced, scale-like leaves appressed to the stem and meso-xerophilous habitat that contradicts to the majority of other
Marsupella”. This phrase requires clarification. Indeed, all representatives of the section (as it is known today) are small plants, and most of them are wiry. However, their leaves may not be appressed to the stem and they are not highly distanced.
The basal branch to all other members of sect. Stolonicaulon is formed by Marsupella anastrophylloides, which definitely justifies its name based on its resemblance to small representatives of Anastrophyllum or Sphenolobopsis, as well as deeply incised bilobed leaves with narrow lobes. This species is the most dissimilar to the other representatives of the section. In this case, the genetic distance correlates well with morphology.
Another well-circumscribed taxon described here is Marsupella taiwanica, which is similar to Cephaloziella due to incised leaves and small regular underleaves and therefore strongly dissimilar to other Marsupella. However, the generative features of this taxon definitely possess the character of other representatives of sect. Stolonicaulon, including a long perigynium, which is partially exposed due to relatively short leaves.
Three other species are superficially (under a dissecting microscope) similar to each other morphologically (wiry shoots, distanced leaves, and a stem that is almost as wide as the left shoot). However, these species are not as closely related in the phylogenetic tree as could be suggested based on an initial look. The two species that are most closely related to each other are
Marsupella stoloniformis and
M. vermiformis. Their morphological differences have been discussed previously [
8]. These differences mainly involve short emarginate to entire leaves and somewhat hygrophilous ecology in
M. stoloniformis versus mostly bilobed, sharply pointed leaf lobes and somewhat xerophilic ecology in
M. vermiformis. The third species is similar to both aforementioned species and is described here as
M. praetermissa. The original specimen (treated here as holotype) was originally confused with
M. stoloniformis and appeared in the paper by Shaw et al. [
4] under the latter name. However, the comparison of this specimen with a type of
M. stoloniformis (KYO, Mizutani 2788!) revealed that it is not a conspecific of the type. Indeed,
M. praetermissa is characterized by a morphology that is similar to that of
M. stoloniformis (distanced, entire-to-shallowly emarginate leaves, and a comparatively thick stem); however, the leaves of this species narrowly spread from the stem, even under dry conditions, which clearly contradicts the always appressed leaves in the type and other known plants of
M. stoloniformis. The main distinguishing feature is regular, albeit small underleaves that are present in
M. praetermissa. The latter to a certain extent explains its relationships with
M. taiwanica in the phylogenetic tree (despite the superficial morphology, the two taxa are strongly dissimilar).
The occurrence of underleaves was not known in Marsupella prior to the present work. As currently reported, two described species, M. taiwanica and M. praetermissa, show regular underleaves. Moreover, it is worth mentioning that M. anastrophylloides rarely possess the ability to develop 1–2 subulate underleaves just above the ventral branch (although very irregular), whereas underleaves are completely absent in unbranched shoots. Therefore, in the taxon that is most genetically distanced from others (M. anastrophylloides), the presence of underleaves is rare. In contrast, underleaves are regularly noted in the two other taxa (M. taiwanica and M. praetermissa). In M. stoloniformis and M. vermiformis, underleaves are completely absent.
The stem cross section also possesses noticeable variation across sect. Stolonicaulon. Two earlier known taxa (M. stoloniformis and M. vermiformis) have no hyaloderm cells (the cells are thick-walled and not—or slightly—different in size from the cells inward in both). Marsupella taiwanica is an intermediate variant in this feature. Specifically, its outer cells in the stem cross section are larger than the inner cells and have distinctly thinner walls; however, these cell walls are much thicker than those that are observed in the two remaining taxa. Another extreme (in contrast to M. vermiformis and M. stoloniformis) is the pair M. praetermissa and M. anastrophylloides. These two taxa exhibit strongly different leaf morphologies but possess distinct hyalodermis of thin-walled cells that are strongly different from scleroderm cells inward.
Therefore, Marsupella sect. Stolonicaulon possesses wide variation in several morphological features:
(1) Leaf shape and comparative stem size (with the stem diameter). Leaves may be contiguous to highly distanced, ranging from deeply divided and appressed to the stem to narrowly spreading;
(2) Stem cross-section features. Although all species have thick-walled scleroderm cells and more or less thick-walled cells in the inner part, the outer cells of the stem can be either thick-walled or distinctly thin-walled and form a distinct hyaloderm (although they commonly only slightly differ in size from the inner cells);
(3) Underleaf presence. This feature makes the section unique among
Marsupella. Regular underleaves are present in
M. praetermissa and
M. taiwanica. Underleaves are also present in
M. anastrophylloides as a rare exception. Underleaves are generally a very unusual characteristic within Gymnomitriaceae. This family in the “old” sense did not include genera with underleaves at all [
6]. The only presumable ‘member’ of Gymnomitriaceae with regular underleaves (as long as leaf length!) is monotypic
Paramomitrion R.M. Schust., which has only been reported in sterile conditions from the only collection that was made by R.M. Schuster in Venezuelan Andes. It is questionable whether
Paramomitrion belongs to Gymnomitriaceae at all. In the original description of the genus and species, Schuster [
2] (p. 138) wrote the following: “I place it in the Gymnomitriaceae, next to
Eremonotus—but without any conviction that this is the final resting place”.
Eremonotus clearly belongs to Jungermanniaceae, whereas the molecular-phylogenetic affinity of
Paramomitrion is not known.
However, the molecular revision of the suborder Jungermanniineae [
4] has necessitated the transfer of
Nardia to Gymnomitriaceae (albeit within the isolated subfamily Nardioideae Váňa) among other things. Thus, Gymnomitriaceae became partly an ‘amphigastrious’ family. The present account has shown that distinct regular underleaves are also possible in the subfamily Gymnomitrioideae in addition to the atavistic underleaves, which were indicated for some species of
Gymnomitrion [
10]. Underleaves are occasionally present in
G. laceratum (Steph.) Horik. The presence of regular underleaves in
Marsupella is most likely a plesiomorphic trait, emphasizing its relationship to
Nardia. In this regard, two species of
Nardia have been described that are new to science within recent years (
Nardia minutifolia Furuki and
Nardia grollei Váňa et D.G. Long) and should be mentioned. Both are characterized by entire leaves that only slightly exceed the width of the stem, and both were not tested genetically. Such species may need to be tested to assess their relationship to
Marsupella sect.
Stolonicaulon. However, these species are most likely not related to the genus
Marsupella sect.
Stolonicaulon, which is characterized by cell sizes which are much smaller than that noted in both mentioned
Nardia species.
In addition to morphology, Marsupella sect. Stolonicaulon shows a peculiar distribution pattern that distinguishes it from other sections of the genus. All representatives of the section are distributed in the mountains in the tropical and subtropical regions of Pacific Asia to Melanesia (the northernmost occurrence of M. vermiformis is noted in the mountains of Jeju Island, where the vegetation of the lowlands is subtropical). One species, namely, Marsupella microphylla R.M. Schust., is known from the Neotropical Floristic Kingdom (Venezuela and Brazil), but its assignment to sect. Stolonicaulon can be questioned. This poorly understood species has not been studied using molecular genetic methods and its systematic position in Marsupella is unclear.
4. Taxonomic Treatment
Marsupella anastrophylloides Bakalin, Vilnet et Maltseva sp. nov.
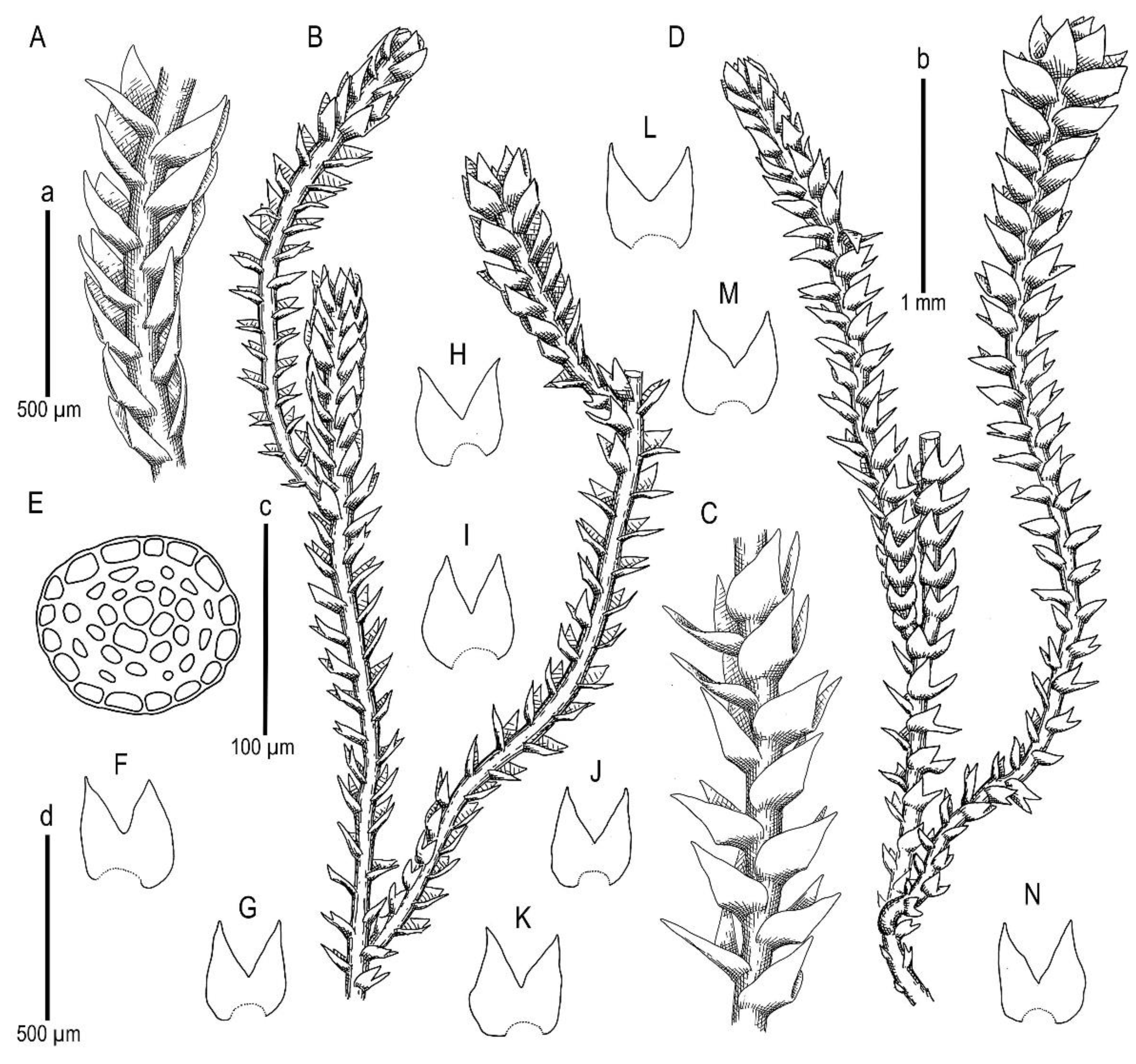
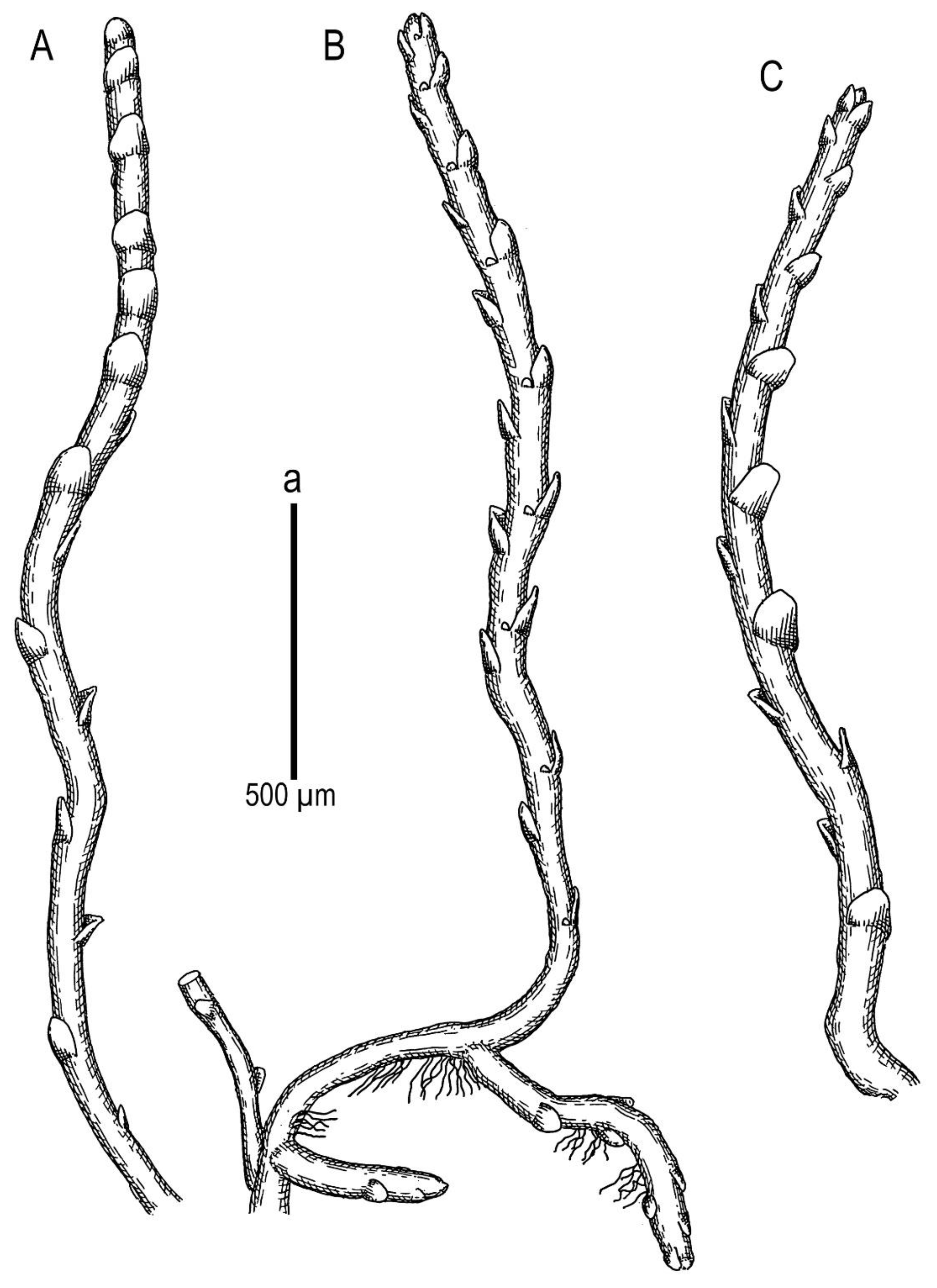
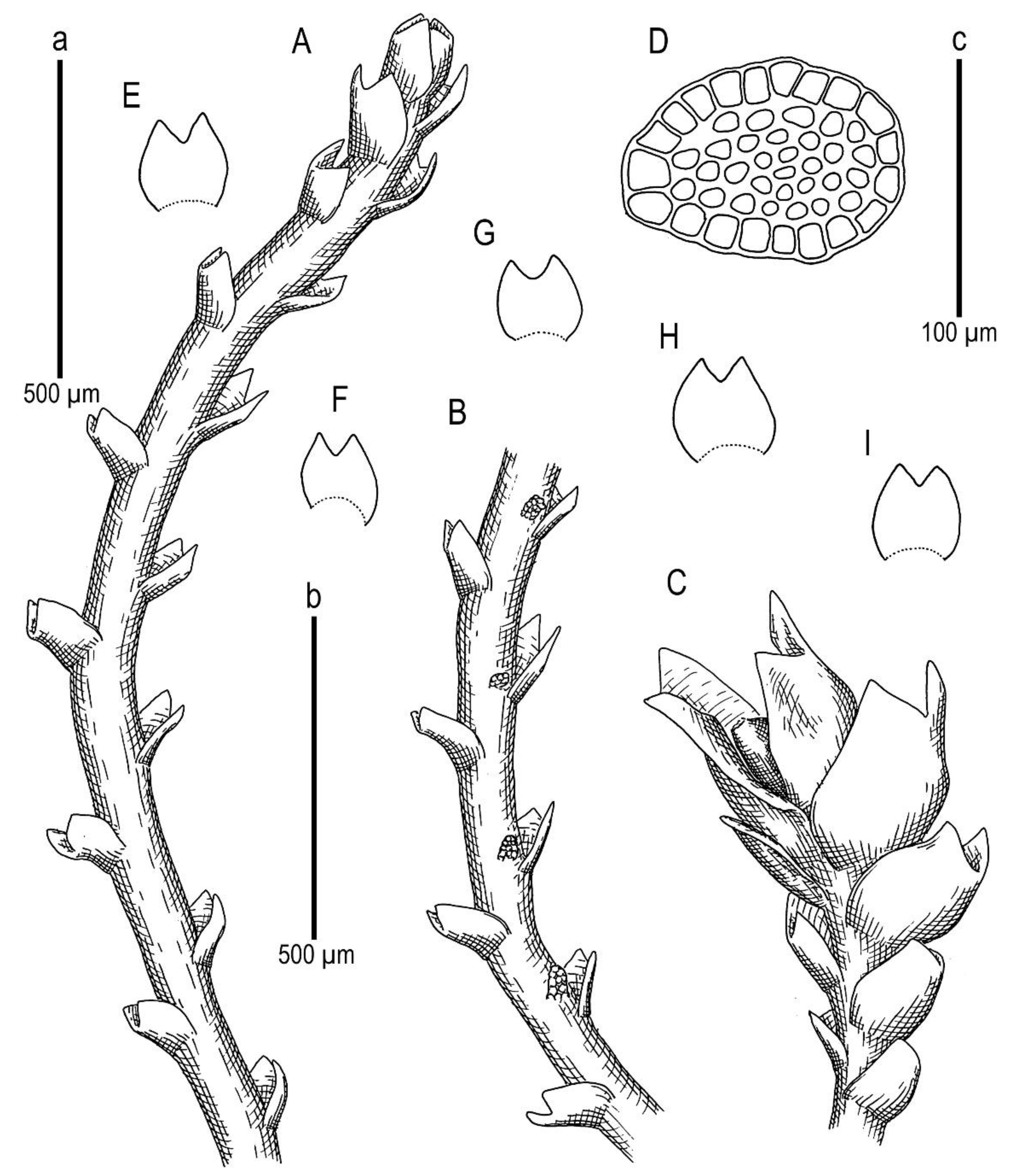
Description. Plants scattered in the mat of Kurzia makinoana, copper and pinkish brownish to purplish, somewhat rigid and very fragile when dry, prostrate to loosely ascending, 300–400 µm wide and 3–15 mm long, rhizomatous base absent. Rhizoids virtually absent. Stem brownish to purplish, branching sparse, ventral, producing normal shoots, ventral merophyte 1–2 cells wide, free of leaves line in dorsal side 0–1 cells wide; cross section orbicular, 7–8 cells high, outer cells hyalodermatic, thin-walled, with small concave trigones, 7–8 µm in diameter to oblong 8–10 × 6–7 µm, inward with distinct scleroderma composed by very thick-walled cells with moderately sized, concave trigones, and then passing into inner cells with thickened walls (although by far not as thick as outer), 10–12 µm in diameter. Leaves contiguous to somewhat distant (never strongly so), transversely inserted and oriented and obliquely spreading, ovate in outline, divided to 3/5–2/3 of leaf length into 2 slightly diverging lobes by V- to gamma-shaped sinus, lobes acute, terminating by 1–2 uniseriate cells, 240–270 × 170–250 µm, widest below sinus. Underleaves absent, rarely occurring as short subulate remnants between 1 or 2 pairs of leaves above origin of ventral branch. Cells in the middle of undivided part of leaf lamina subisodiametric, 8–10 µm in diameter to oblong, 10–20 × 7–13 µm, prominently thick-walled with moderately sized concave trigones, middle lamellae well visible in the trigones, pink to purplish in color, other parts of cell walls pinkish brownish. Cells in lobe middle subisodiametric, 7–10 µm in diameter, apical cells oblong, 8–15 × 6–10 µm, very thick-walled, with visible middle lamella in the trigones those are moderate in size, concave; cuticle virtually smooth throughout, although sometimes indistinctly verrucose along leaf margin. Generative structures unknown.
Holotype: Vietnam, Hà Giang Province, Vi Xuyên District, Cao Bo Commune, Tay Con Linh Range, Tay Con Linh Nature Reserve (22°47′53.0″ N 104°48′33.5″ E), 2296 m a.s.l., south subtropical mountain evergreen forest with large conglomerate cliffy massif, open mesic cliff; 22 March 2020, V.A. Bakalin & K.G. Klimova, V-15-6-20 (VBGI).
Etymology: Its name was given based on morphological similarity to small Anastrophyllum.
Ecology: The species was only collected once, therefore, its ecology is incompletely known. It was gathered in a southern subtropical mountain forest over open mesic cliffs as an admixture in the mat of Kurzia makinoana. The plants were creeping over Kurzia. The same patch contains a sparse admixture of the xeromorphic modification of Anastrophyllum bidens.
Comment: Due to its prominent external characteristics, including contiguous obliquely spreading and deeply divided leaves, the species could be confused with all other known Marsupella. However, in the sterile state (as it was only collected), the species may be mistaken for depauperate plants of Anastrophyllum bidens, from which it differs based on a distinct tint of copper or pinkish pigmentation and distinctly smaller leaf cells (7–13 µm wide, versus 15–20 µm wide in the midleaf) with thick walls, concave trigones (versus trigones large, prominently bulging), and fragile shoots (versus shoots more elastic). Disregarding the copper and pinkish pigmentation, the species somewhat resembles Sphenolobopsis pearsonii, but the leaf cuticle is virtually smooth and regular underleaves are absent in Marsupella anastrophylloides. The main difference from the latter potentially occurs in generative structures, which are unfortunately unknown.
Given the strong molecular dissimilarity to other taxa and no distantly similar regional taxa, we hypothesized that the species has undergone a long period of isolation and speciation. If so, the expectation of its current distribution is very speculative since the ‘old-existing’ taxon might have a wide original area and be preserved in several restricted areas as a geographic relict.
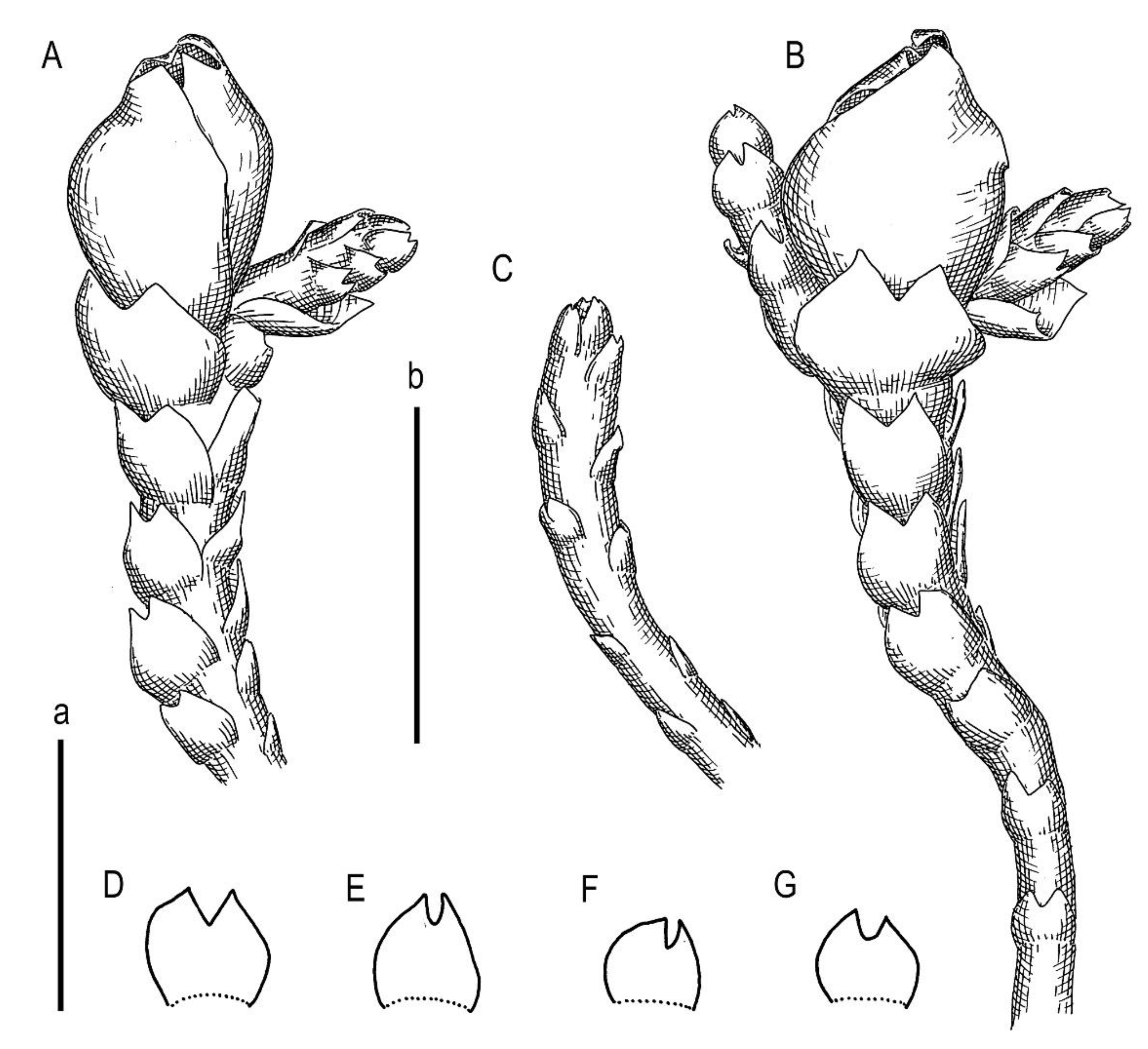
Marsupella praetermissa Bakalin et Vilnet sp. nov.
Description. Plants brownish to brown, wiry, rigid, forming loose patches with Riccardia, Sphenolobopsis pearsonii and Gymnomitrion (Apomarsupella group), 120–150 (170) µm wide and 3–8 mm long. Rhizomatous base is ill-developed. Rhizoids colorless to purplish at the branch base, above only as exception and never in upper half of the well-developed shoot. Stem sparsely ventrally branched in rhizomatous base, cells in dorsal surface subquadrate to 4–6-gonal, ventral merophyte 2 cells wide, dorsal row free of leaves 0–1 cell wide; cross section transversely ellipsoidal to rotundate, 80–110 µm in diameter to 90 × 100–120 µm, outer cells thin-walled (hyalodermatic), 7–8 µm in diameter with small concave trigones, inward suddenly become thick-walled, with visible middle lamella, 6–9 µm in diameter, with large triangular trigones, cell walls of medullary cells slightly thinner, but still very thick. Leaves transversely inserted and oriented, obliquely spreading, somewhat concave-canaliculate, sheathing the stem, leaf apex entire to emarginate, well developed leaves longer than wide, 100–170 × 90–150 µm. Underleaves vestigial, although highly regular, spathulate to ovate, (1–)2 cells wide and 2–3 cells long, rarely subulate 1 cell wide and 3 cells long. Midleaf cells subisodiametric to oblong, 6–8 µm in diameter to 7–10 × 6–8 µm, thin-walled or with thickened walls, trigones moderate in size, concave; cells along leaf margin slightly larger and with thicker and deeper colored walls than just inward, 8–10 µm in diameter or oblong (elongate along margin), 8–10 × 6–8 µm; cuticle virtually smooth throughout. Generative structures unknown.
Holotype: China, Yunnan Province, Gongshan County, Cikai Xheng, east slope of Gaoligong Shan, Nu Jiang (Salween) catchment, Yipisaka Lake at head of Pula He valley, 2.2 km SSE of tunnel (27°45′12.4″ N 98°27′36.4″ E), 3455 m a.s.l., granitic cliff on alpine lake shore; on small boulder under shady dripping cliff, 12 August 2006, D.G. Long, Long 35,742 (E, duplicate in VBGI).
Etymology: the name ‘praetermissa’ is translated as ‘overlooked’ from Latin due to its superficial similarity to M. stoloniformis.
Ecology: The species is known from the single specimen that was collected from granitic cliffs on the alpine lake shore (a small boulder under a shady dripping cliff) at an elevation of 3455 m a.s.l. (
Figure 4, left corner). This is a primarily forestless landscape on gentle NE-facing slopes and a complex of lakes. The nearest forests are approximately 3 km downstream at the S-facing slopes at elevations of 3200–3300 m a.s.l.; however, scattered trees are present near the collecting locality. The associates within the specimens included
Riccardia sp.,
Gymnomitrion sp. (belonging to the morphological group ‘
Apomarsupella’), and
Sphenolobopsis pearsonii (Spruce) R.M. Schust.
Comment: The distribution of the taxon could hardly be expected given the availability of only one record. Although the collecting locality is formally located in the Salween (Nu Jiang) River catchment, the collecting locality is only 500 m eastward from the pass to the Irrawaddy (Ayeyarwady) River catchment, the largest Myanmar River. Therefore, in a broad context, this is an area where several main river upper courses meet one another (Salween, Irrawaddy, Brahmaputra, and Mekong). Although strong differences in the species content between closely situated sites have been known here for over a century [
11,
12], the actual distribution of the taxon may be not very narrow, and additional records, including those from remote areas (around Sino-Himalaya), are highly possible.
Superficially, when assessing a small sample under the dissection microscope, the species is morphologically very similar to
M. stoloniformis, which may explain the original misidentification of the specimen. The specimen was subjected to sequencing by Shaw et al. [
4]. In the various topologies we attempted to combine, this specimen occupied an isolated position from the accession of
M. stoloniformis from Vietnam that required further morphological investigation. Shortly thereafter, it was noted that even in dry conditions, the superficial morphology of the taxon was different from that of
M. stoloniformis (both type in KYO and our vouchers from Vietnam). The leaves of
M. stoloniformis are always (even when wet) appressed to the stem, such that the stem diameter is almost the same as the plant width. However, as described here,
M. praetermissa has leaves that narrowly spread from the stem, even when the plants are dry. Another very distinctive feature that is evident under the dissecting microscope is the presence of small but regular underleaves—a feature that is unknown in
M. stoloniformis. The third difference helps to distinguish the species from
M. stoloniformis and is evident in the stem cross section in the microscope slide.
Marsupella praetermissa has distinctly hyalodermatic outer cells with very thin walls, while the outer cells of
M. stoloniformis are distinctly thick and hardly different from medullary cells.
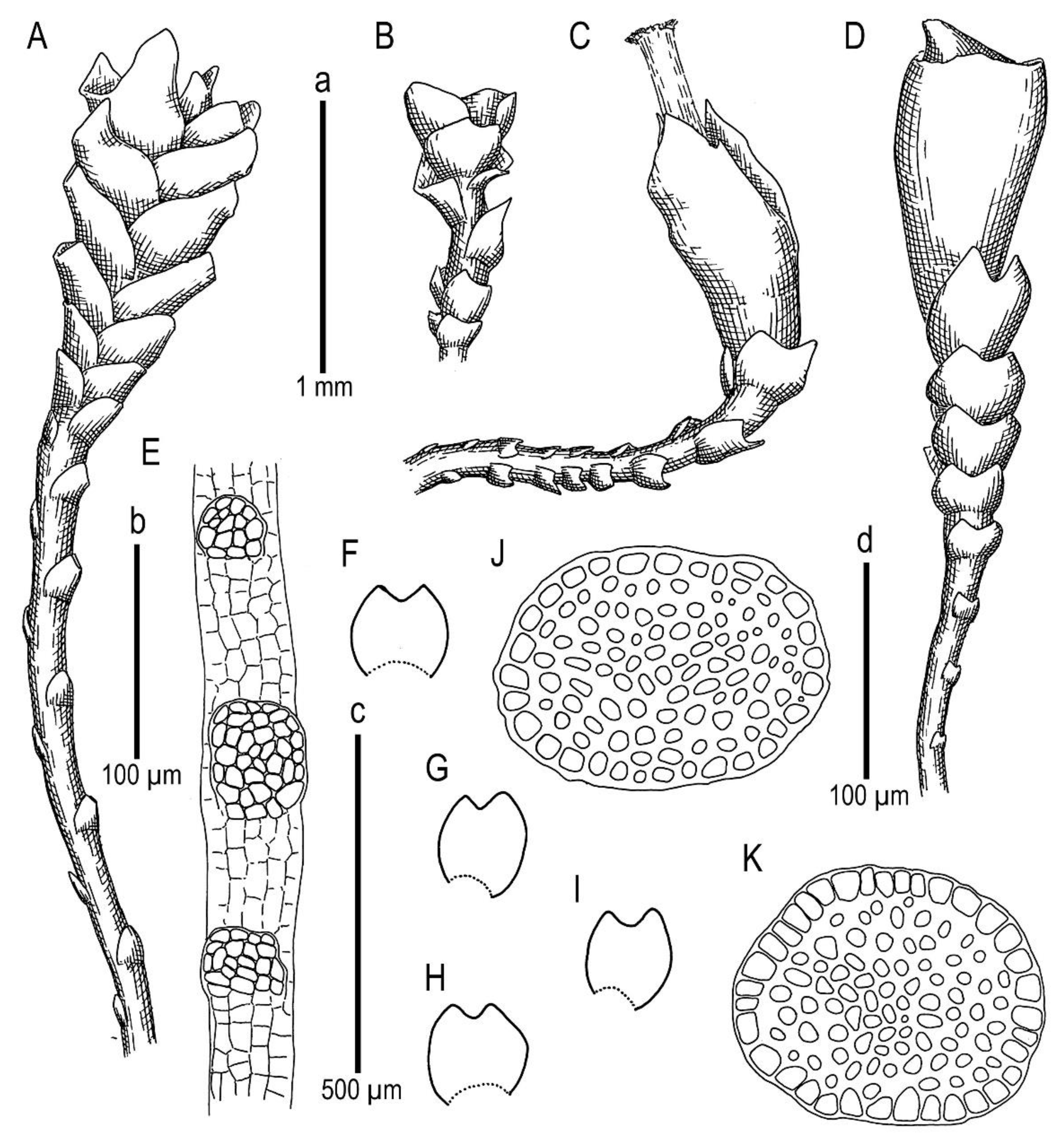
Marsupella taiwanica Mamontov, Vilnet et Schäf.-Verw. sp. nov.
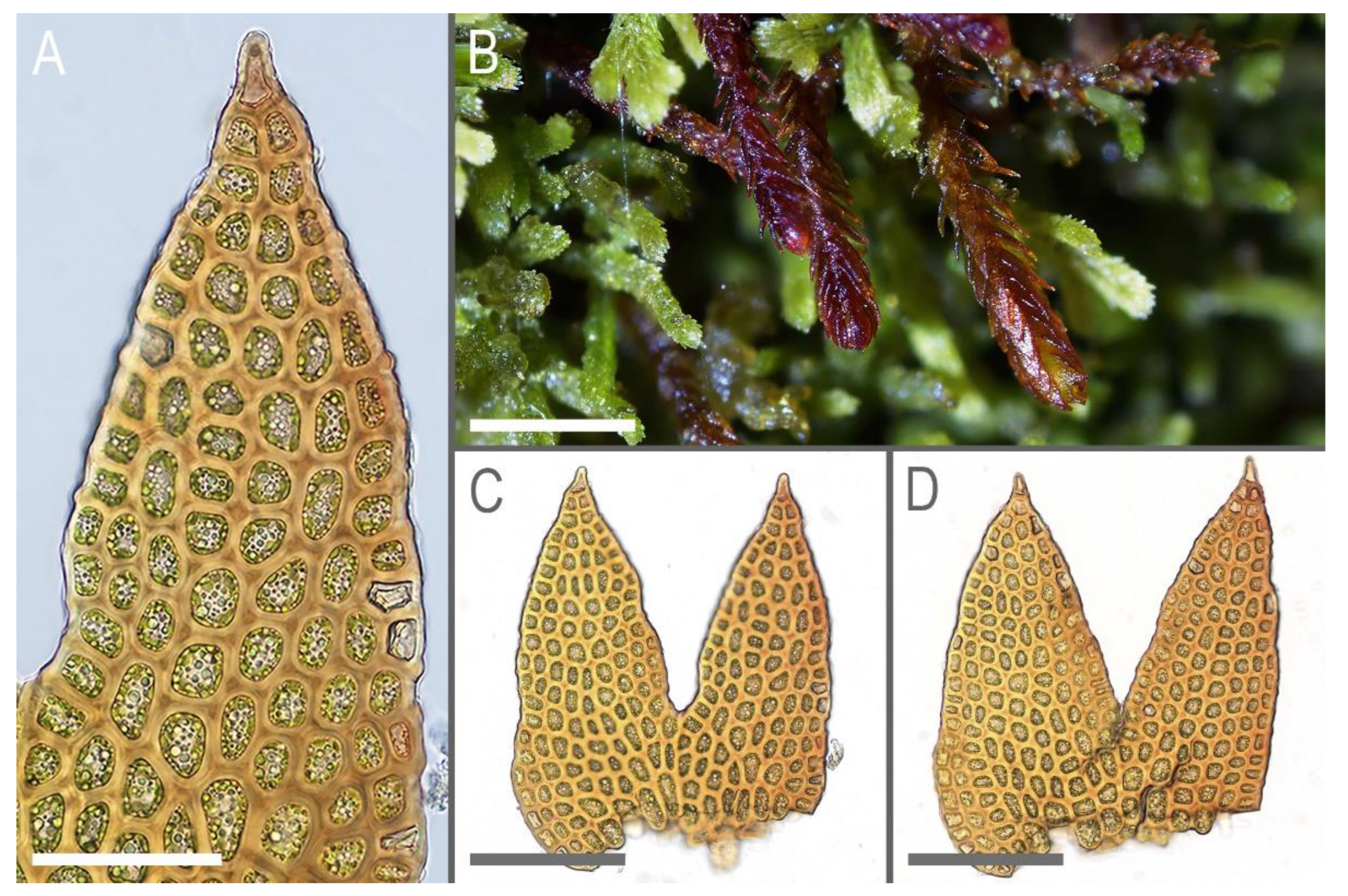
Description. Plants in loose mats, small, Cephaloziella-like, more or less rigid, brown to blackish brown (when dry), sterile shoots 200–250 µm wide, up to 4 mm long; gynoecial sectors 500–650 µm wide. Rhizoids absent or very few, colorless to grayish. Stem rarely produces ventral branches originated from axils of underleaves, almost always with 1–2 subfloral ventral innovations; stem cross section usually slightly transversely elliptic, differentiated into outer and medullar cells. Outer layer cells 7–14 µm along margin, with brownish, thin, or slightly unequally thickened walls, with small to moderate concave trigones. Medullar cells rounded to elliptical, 7–15 µm in diameter, with concave to convex and often confluent trigones, walls thick, yellowish colored, sometimes with visible median lamella. Leaves remote, transversely inserted, obliquely spreading and subtransversely oriented, widely ovate, 120–200 µm wide, 110–165 µm long, 1.01–1.23 times wider than long, margins plane, with “keel” line nearly straight, divided to 0.28–0.35(0.39) of the leaf length, sinus acute-angled to rectangular, lobes subequal, triangular, 6–8 cells wide at base, acute to apiculate and sometimes hooked at apex, ending by 1–2 superimposed cells. Cells in the leaf middle and leaf lobes oblong to subisodiametric, 5–6-angled, 7–18 × 10–17(22) µm, walls thin to equally thickened, trigones small, indistinct, concave-triangular, cuticle smooth, marginal cells rectangular to 5-angled. Underleaves usually distinct, located near the base of leaf, small, subulate or spatuliform, consisting of 3–8 cells. Dioecious. Male plants not seen. Unfertilized perianths with subfloral innovations below, subgynoecial leaves in 2–3 pairs, larger than sterile leaves, 270–430 µm wide, 250–370 µm long, with subequal to strongly unequal lobes, outer margins in upper part and sinus margins sometimes narrowly reflexed. Unfertilized perianth onion-shaped, shorter than the bracts, ca 140–240 × 270–300 µm. Perigynium well developed, 0.4–0.66 of the length of the unfertilized perianth, bracts sheathing the perianth, with lobe apices decurved from perianth. Otherwise unknown.
Holotype: China, Taiwan Province, Nantou Co., Taroko National Park, roadside along Highway 8 between High Experimental Station/Visitor Center and Mt. Hehuan North & West Peak Trail (24°09.79′ N, 121°17.3′ E), 2970–3000 m a.s.l., on soil that is exposed to sunlight, 17 October 2016, A. Schäfer-Verwimp 37663 (MHA, duplicates in PRC (sub
Cephaloziella divaricata), TAIE, JE, CAS, FR, TAIM, VBGI, KPABG). The specimen was mentioned as
Cephaloziella divaricata as new to Taiwan by Schäfer-Verwimp et al. [
13].
Other specimens that were examined (paratypes): China, Taiwan Province, Chiayi Co., Yushan National Park, along the 2.2 km trail from Paiyun Lodge to Yushan West Peak (23°27′59.1″ N 120°56′50.0″ E), 3415 m a.s.l., Abies kawakamii forest and Yushania bamboo understory, in a rock crevice that was exposed to sunlight, 25 October 2018, A. Schäfer-Verwimp 39136 (MHA, TAIE, JE, VBGI, KPABG, CAS, FR).
Ecology: Acidophilic meso-xerophyte. This is a rare and poorly known species, and the data on its ecology may be incomplete. Marsupella taiwanica occupies well-exposed habitats, including bare soil on roadsides and rock fissures in high mountains where it forms blackish-brown pure patches.
Comment:
Marsupella taiwanica is most similar to several
Cephaloziella species, especially
C. divaricata (Sm.) Schiffn.;
C. grimsulana (J.B. Jack ex Gottsche & Rabenh.) Lacout.; and
C. varians (Gottsche) Steph., due to (1) small shoots with brownish coloration forming blackish brown patches; (2) remote and transversely inserted, erect spreading leaves; (3) the presence of regular underleaves. All the mentioned
Cephaloziella species occasionally occur in sterile conditions. However, in patches of
Marsupella taiwanica, fertile plants are also occasionally absent. These circumstances make it difficult to distinguish
M. taiwanica from the aforementioned
Cephaloziella species. Under sterile conditions,
M. taiwanica can be distinguished from these species based on the following indirect features. In
Cephaloziella, the sterile leaves are generally more deeply bilobed for 0.5–0.8 their length, especially near the shoot apex [
14] (p. 36, Figure 498: 10). However, in
M. taiwanica, the sterile leaves have a shallower sinus, usually ca. 0.3 of the leaf length. In
Cephaloziella, the underleaves are mostly located on stems between the bases of opposite leaves cf. [
14,
15,
16], whereas the underleaves are usually located very close to the base of one of the leaves in a leaf pair in
M. taiwanica. The fertile (gynoecial) shoots of
M. taiwanica easily differ from
Cephaloziella based on the shape of the perianths (hidden within bracts, abruptly narrowed to the mouth), the presence of distinct perigynium, and the shape of the bracts (lobes acute to obtuse or rounded at the apex with shallow sinus and entire margins). The present species differs from all
Marsupella based on its
Cephaloziella-like habit. Another feature is the presence of regular underleaves shared within
Marsupella with
M. praetermissa that are strongly dissimilar in other morphological features.
Identification Key to the Marsupella Sect. Stolonicaulon
1. Plants wiry, with strongly distanced appressed to the stem or narrowly spreading leaves, the stem diameter almost as large as the leaved shoot, leaves entire or shortly (less 1/5 in sterile shoots) divided … 2
1. Plants with contiguous to distanced, obliquely spreading, distinctly incised leaves 1/3–2/3 of the leaf length … 4
2. Plants with small, but regular underleaves, leaves narrowly spreading, stem cross section with hyalodermis … M. praetermissa
2. Plants free of underleaves, leaves appressed to the stem, stem cross section without hyalodermis … 3
3. Leaves entire to emarginate, if divided lobes rounded …
M. stoloniformis (
Figure 9 and
Figure 10)
3. Leaves always divided by semi-crescentic sinus with lobes acute …
M. vermiformis (
Figure 11)
4. Plants similar to small Anastrophyllum, leaves contiguous, divided for 2/3 of the length, distinct hyalodermis present … M. anastrophylloides
4. Plants similar to Cephaloziella, leaves distant, divided to 1/3 of the leaf length, no distinct hyalodermis present (stem outer cells larger than inner, but merely thick-walled) … 5
5. Regular underleaves present [Taiwan] … M. taiwanica
5. Plants underleaf free [Venezuela, Brazil] … M. microphylla*
*—The taxon is known from a very few specimens; the concept of this taxon is derived from literature sources and needs critical testing.