The Utility of Ground Bryophytes in the Assessment of Soil Condition in Heavy Metal-Polluted Grasslands
Abstract
:1. Introduction
2. Results
2.1. Soil Properties of Heavy Metal-Polluted Sites
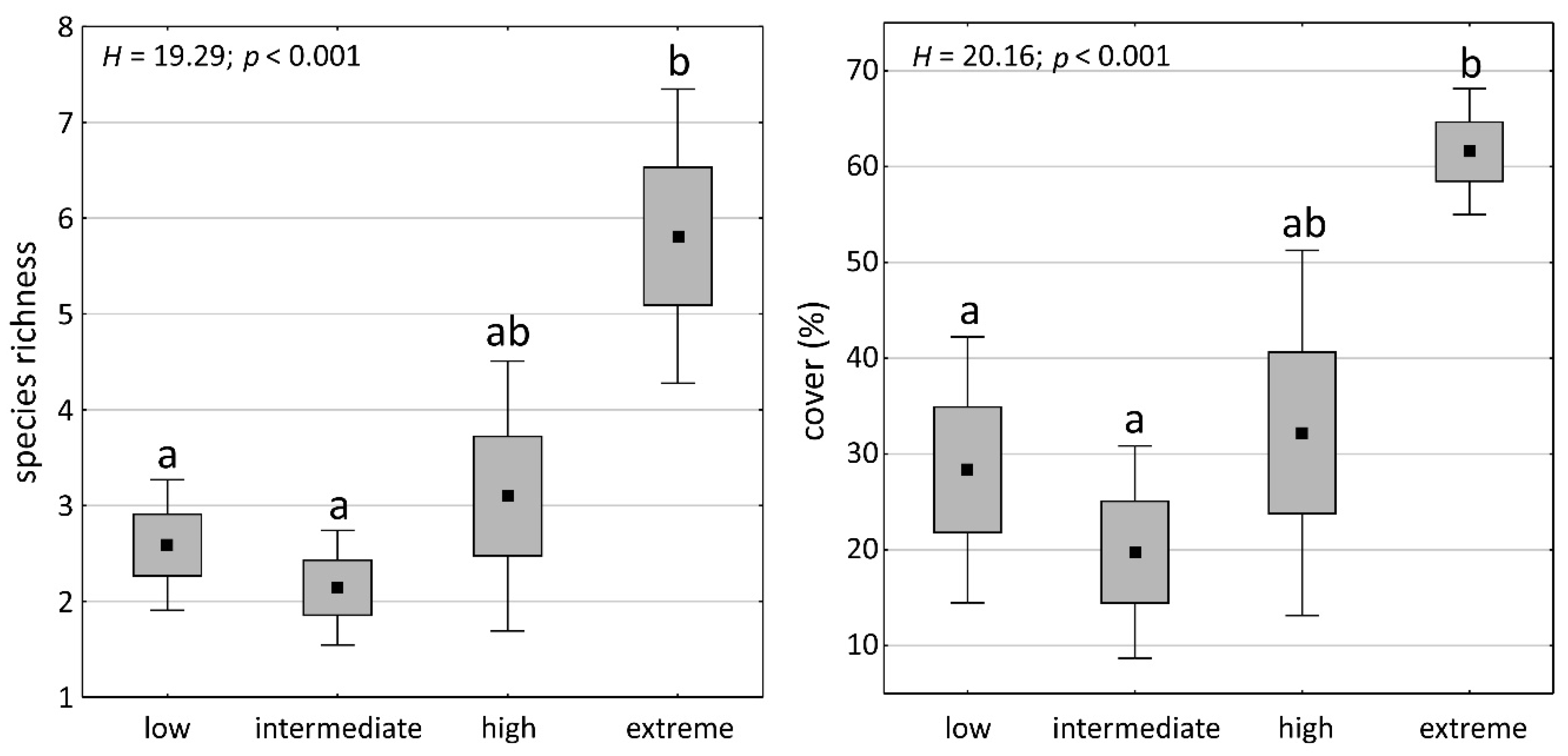
2.2. Bryophyte Species Richness and Cover in Soil Condition Classes
2.3. Factors Affecting Bryophyte Species Richness and Cover
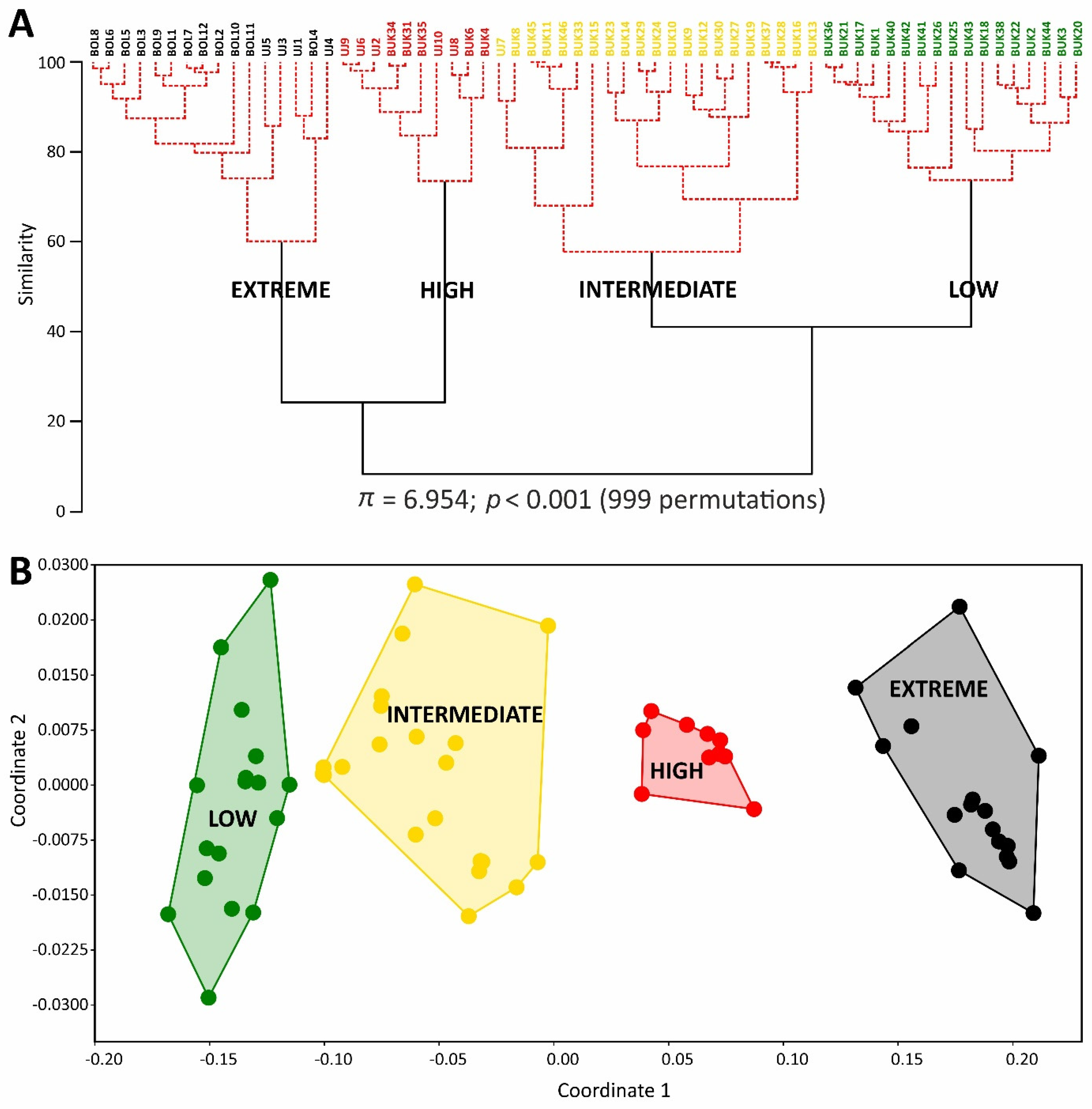
2.4. Bryophyte Species Composition
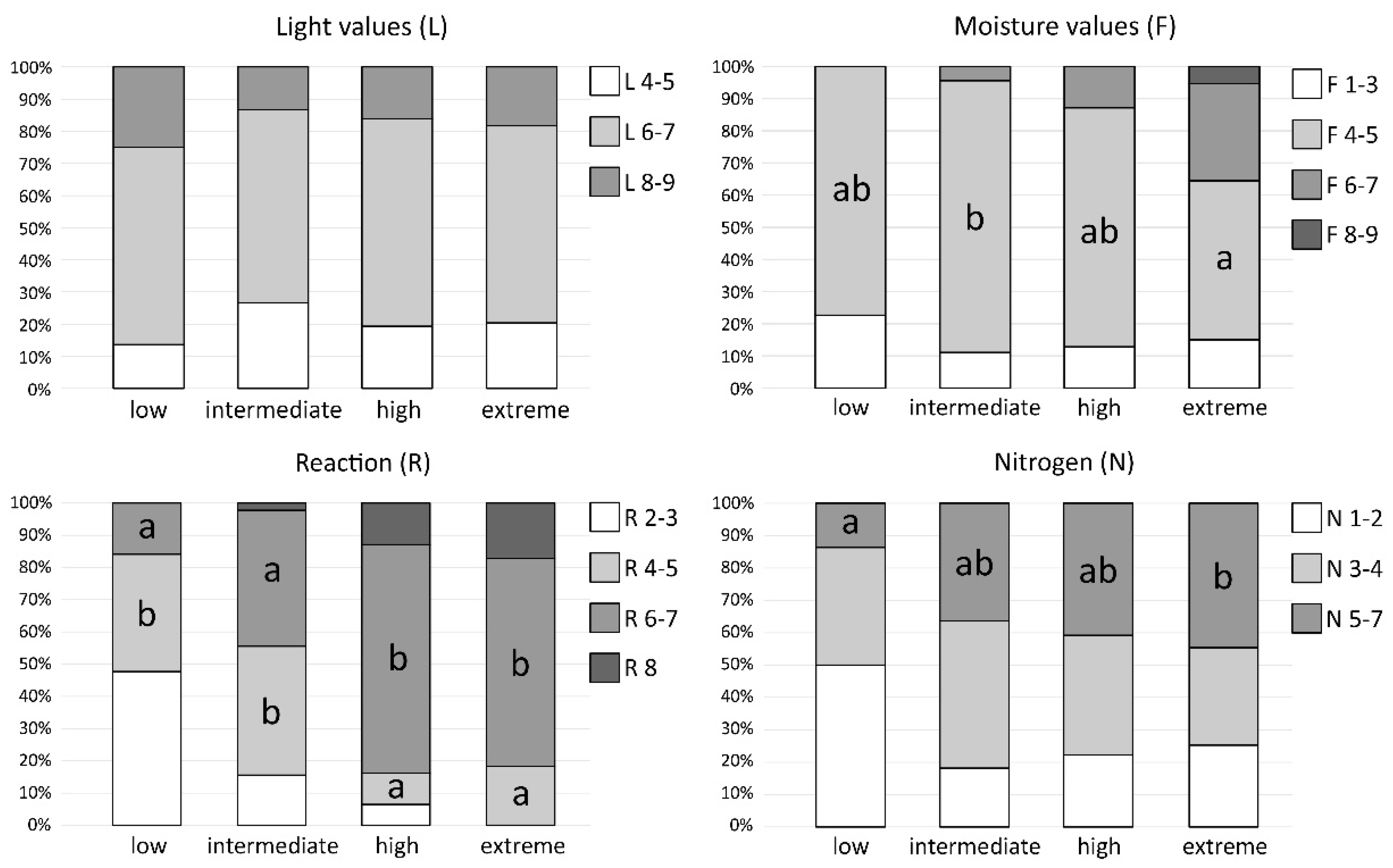
2.5. Ecological Preferences of Bryophytes
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Field Studies, Sampling, and Bryophyte Identification
4.3. Soil Chemical Analysis
4.4. Calculations and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crum, H. Structural Diversity of Bryophytes; University of Michigan Herbarium: Ann Arbor, MI, USA, 2001. [Google Scholar]
- Bramley-Alves, J.; King, D.H.; Robinson, S.A.; Miller, R.E. Dominating the Antarctic environment: Bryophytes in a time of change. In Photosynthesis in Bryophytes and Early Land Plants; Hanson, D.T., Rice, S.K., Eds.; Springer: Berlin, Germany, 2014; pp. 309–324. [Google Scholar]
- Klaus, V.H.; Müller, J. The role of bryophytes in Central European grasslands. In Grasslands Biodiversity and Conservation in a Changing World; Mariotte, P., Kardol, P., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2014; pp. 251–278. [Google Scholar]
- Cuny, D.; Denayer, F.O.; de Foucault, B.; Schumacker, R.; Colein, P.; van Haluwyn, C. Patterns of metal soil contamination and changes in terrestrial cryptogamic communities. Environ. Pollut. 2014, 129, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Rola, K.; Osyczka, P. Cryptogamic communities as a useful bioindication tool for estimating the degree of soil pollution with heavy metals. Ecol. Indic. 2018, 88, 454–464. [Google Scholar] [CrossRef]
- Tyler, G. Bryophytes and heavy metals—A literature review. Bot. J. Linn. Soc. 1990, 104, 231–253. [Google Scholar] [CrossRef]
- Proctor, M.C.F. Physiological ecology: Water relations, light and temperature responses, carbon balance. In Bryophyte Ecology; Smith, A.J.E., Ed.; Chapman and Hall: London, UK, 1982; pp. 45–57. [Google Scholar]
- Roberts, A.W.; Roberts, E.M.; Haigler, C.H. Moss cell walls: Structure and biosynthesis. Front. Plant Sci. 2012, 3, 166. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, H.G.; Dirnbock, T.; Hulber, K.; Mirtl, M. Assessing airborne pollution effects on bryophytes—Lessons learned through long-term integrated monitoring in Austria. Environ. Pollut. 2007, 147, 696–705. [Google Scholar] [CrossRef]
- Salemaa, M.; Vanha-Majamaa, I.; Derome, J. Understorey vegetation along a heavy-metal pollution gradient in SW Finland. Environ. Pollut. 2001, 112, 339–350. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley: Chichester, UK, 2001. [Google Scholar]
- Jules, E.S.; Shaw, A.J. Adaptation to metal-contaminated soils in populations of the moss, Ceratodon purpureus: Vegetative growth and reproductive expression. Am. J. Bot. 1994, 81, 791–797. [Google Scholar] [CrossRef]
- Denayer, F.O.; van Haluwyn, C.; de Foucault, B.; Schumacker, R.; Colein, P. Use of bryological communities as a diagnostic tool of heavy metal soil contamination (Cd, Pb, Zn) in northern France. Plant Ecol. 1999, 140, 191–201. [Google Scholar] [CrossRef]
- Zvereva, E.; Kozlov, M. Impacts of industrial polluters on bryophytes: A meta-analysis of observational studies. Water Air Soil Pollut. 2011, 218, 573–586. [Google Scholar] [CrossRef]
- Bergamini, A.; Pauli, D.; Peintinger, M.; Schmid, B. Relationships between productivity, number of shoots and number of species in bryophytes and vascular plants. J. Ecol. 2001, 89, 920–929. [Google Scholar] [CrossRef]
- Rola, K.; Osyczka, P. Cryptogamic community structure as a bioindicator of soil condition along a pollution gradient. Environ. Monit. Assess. 2014, 186, 5897–5910. [Google Scholar] [CrossRef] [PubMed]
- Kassen, R. The experimental evolution of specialists, generalists, and the maintenance of diversity. J. Evol. Biol. 2002, 15, 173–190. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Nash, T.H.; Nash, E.H. Sensitivity of mosses to sulfur dioxide. Oecologia 1974, 17, 257–263. [Google Scholar] [CrossRef]
- Plášek, V.; Nowak, A.; Nobis, M.; Kusza, G.; Kochanowska, K. Effect of 30 years of road traffic abandonment on epiphytic moss diversity. Environ. Monit. Assess. 2014, 186, 8943–8959. [Google Scholar] [CrossRef]
- Taoda, H. Mapping of atmospheric pollution in Tokyo based upon epiphytic bryophytes. Jpn. J. Ecol. 1972, 22, 125–133. [Google Scholar]
- Burton, M.A.S. Terrestrial and aquatic bryophytes as monitors of environmental contaminants in urban and industrial habitats. Bot. J. Linn. Soc. 1990, 104, 267–280. [Google Scholar] [CrossRef]
- Holyoak, D.T.; Lockhart, N. A survey of bryophytes and metallophyte vegetation of metalliferous mine spoil in Ireland. J. Min. Herit. Trust Irel. 2011, 11, 3–16. [Google Scholar]
- Becker, T.; Brändel, M. Vegetation-environment relationships in a heavy metal-dry grassland complex. Folia Geobot. 2007, 42, 11–28. [Google Scholar] [CrossRef]
- Kannukene, L. Bryophytes in the forest ecosystem influenced by cement dust. In Dust Pollution and Forest Ecosystems. A Study of Conifers in an Alkalized Environment; Mandre, M., Ed.; Institute of Ecology: Tallinn, Estonia, 1995; pp. 141–147. [Google Scholar]
- Paal, J.; Degtjarenko, P. Impact of alkaline cement-dust pollution on boreal Pinus sylvestris forest communities: A study at the bryophyte synusiae level. Ann. Bot. Fenn. 2015, 52, 120–134. [Google Scholar] [CrossRef]
- Cooke, J.A. Mining. In Ecosystems of Disturbed Ground, Ecosystems of the World; Walker, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 16, pp. 365–384. [Google Scholar]
- Simon, E. Heavy metals in soils, vegetation development and heavy metal tolerance in plant populations from metalliferous areas. New Phytol. 1978, 81, 175–188. [Google Scholar] [CrossRef]
- Ingerpuu, L.; Liira, J.; Pärtel, M. Vascular plants facilitated bryophytes in a grassland experiment. Plant Ecol. 2005, 180, 69–75. [Google Scholar] [CrossRef]
- Löbel, S.; Dengler, J.; Hobohm, C. Species richness of vascular plants, bryophytes and lichens in dry grasslands: The effects of environment landscape structure and competition. Folia Geobot. 2006, 41, 377–393. [Google Scholar] [CrossRef]
- Grace, J.P. The factors controlling species density in herbaceous plant communities: An assessment. Perspect. Plant Ecol. 1999, 2, 1–28. [Google Scholar] [CrossRef]
- During, H.J.; Lloret, F. The species-pool hypothesis from a bryological perspective. Folia Geobot. 2001, 36, 63–70. [Google Scholar] [CrossRef]
- Bu, Z.J.; Chen, X.; Jiang, L.H.; Li, H.K.; Zhao, H.Y. Research advances on interactions among bryophytes. Chin. J. Appl. Ecol. 2009, 20, 460–466. [Google Scholar]
- Shacklette, H.T. Copper Mosses as Indicators of Metal Concentration; Geological Survey Bulletin 1198G, United States Government Printing Office: Washington, DC, USA, 1967. [Google Scholar]
- Crundwell, A.C. Ditrichum plumbicola, a new species from lead-mine waste. J. Bryol. 1976, 9, 167–169. [Google Scholar] [CrossRef]
- Stebel, A.; Ochyra, R.; Godzik, B.; Bednarek-Ochyra, H. Bryophytes of the Olkusz Ore-Bearing Region (Southern Poland); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2015. [Google Scholar]
- Tsikritzis, L.I.; Ganatsios, S.S.; Duliu, O.G.; Sawidis, T.D. Heavy metals distribution in some lichens, mosses, and trees in the vicinity of lignite power plants from West Macedonia, Greece. J. Trace Microprobe Tech. 2002, 20, 395–413. [Google Scholar] [CrossRef]
- Becker, T.; Dierschke, H. Vegetation response to high concentrations of heavy metals in the Harz Mountains, Germany. Phytocoenologia 2008, 38, 255–265. [Google Scholar] [CrossRef]
- Rola, K.; Osyczka, P. Data on cryptogamic biota in relation to heavy metal concentrations in soil. Data Brief 2018, 19, 1110–1119. [Google Scholar] [CrossRef]
- Zechmeister, H.G.; Grodzińska, K.; Szarek-Łukaszewska, G. Bryophytes. In Bioindicators and Biomonitors: Principles, Concepts and Applications; Markert, B.A., Breure, A.M., Zechmeister, H.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 329–375. [Google Scholar]
- Holyoak, D.T. Bryophytes and Metalophyte Vegetation on Metalliferous Mine-Waste in Ireland: Report to the National Parks and Wildlife Service of a Survey in 2008. Unpublished Report of the National Parks and Wildlife Service. 2008. Available online: https://www.npws.ie/sites/default/files/publications/pdf/Holyoak_2008_Metalliferous_mine_survey.pdf (accessed on 7 April 2020).
- Baumbach, H. Metallophytes and metallicolous vegetation: Evolutionary aspects, taxonomic changes and conservational status in Central Europe. In Perspectives on Nature Conservation—Patterns, Pressures and Prospects; Tiefenbacher, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 93–118. [Google Scholar]
- Rola, K.; Osyczka, P.; Nobis, M.; Drozd, P. How do soil factors determine vegetation structure and species richness in post-smelting dumps? Ecol. Eng. 2015, 75, 332–342. [Google Scholar] [CrossRef]
- Dierssen, K. Distribution: Ecological amplitude and phytosociological characterization of European bryophytes. Bryophyt. Bibl. 2001, 56, 1–289. [Google Scholar]
- Dąbrowski, J.; Seniczak, S.; Dąbrowska, B.; Hermann, J.; Lipnicki, L. The arboreal mites (Acari) and epiphytes of young Scots pine forests, in the region polluted by a cement and lime factory ‘Kujawy’ at Bielawy. Akad. Tech.-Rol. Im. Jana I Jędrzeja Śniadeckich Bydg. Zesz. Nauk. (Ochr. Sr. 1) 1997, 208, 71–82. [Google Scholar]
- Folkeson, L. Depauperation of the moss and lichen vegetation in a forest polluted by copper and zinc. In Air Pollution and Stability of Coniferous Forest Ecosystems; Klimo, E., Saly, R., Eds.; University of Agriculture: Brno, Czech Republic, 1985; pp. 297–307. [Google Scholar]
- Gignac, L.D. Distribution of bryophytes on peatlands contaminated by metals in the vicinity of Sudbury, Ontario, Canada. Cryptogam. Bryol. 1987, 8, 339–351. [Google Scholar]
- Ruokolainen, L.; Salo, K. The succession of boreal forest vegetation during ten years after slash-burning in Koli National Park, eastern Finland. Ann. Bot. Fenn. 2006, 43, 363–378. [Google Scholar]
- Lepp, N.W.; Salmon, D. A field study of the ecotoxicology of copper to bryophytes. Environ. Pollut. 1999, 106, 153–156. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L. Industrial barrens: Extreme habitats created by non-ferrous metallurgy. Rev. Environ. Sci. Bio/Technol. 2007, 6, 231–259. [Google Scholar] [CrossRef]
- Glime, J.M. Adaptive strategies: Life cycles. In Bryophyte Ecology, Vol 1., Physiological Ecology; Glime, J.M., Ed.; Michigan Technological University and the International Association of Bryologists: Houghton, MI, USA, 2017; pp. 446–461. [Google Scholar]
- Fabure, J.; Meyer, C.; Denayer, F.; Gaundry, A.; Gilbert, D.; Bernard, N. Accumulation capacities of particulate matter in an acrocarpous and pleurocarpous moss exposed at three differently polluted sites (industrial, urban and rural). Water Air Soil Pollut. 2010, 212, 205–217. [Google Scholar] [CrossRef]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. BRYOATT: Attributes of British and Irish Mosses, Liverworts and Hornworts; Centre for Ecology and Hydrology: Wallingford, UK, 2007. [Google Scholar]
- Blanár, D.; Guttová, A.; Mihál, I.; Plášek, V.; Hauer, T.; Palice, Z.; Ujházy, K. Effect of magnesite dust pollution on biodiversity and species composition of oak-hornbeam woodlands in the Western Carpathians. Biologia 2019, 74, 1591–1611. [Google Scholar] [CrossRef]
- van Haluwyn, C.; van Herk, C.M. Bioindication: The community approach. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 2002; pp. 39–64. [Google Scholar]
- Degtjarenko, P.; Marmor, L.; Randlane, T. Changes in bryophyte and lichen communities on Scots pines along an alkaline dust pollution gradient. Environ. Sci. Pollut. Res. 2016, 23, 17413–17425. [Google Scholar] [CrossRef]
- Chrastný, V.; Vaněk, A.; Teper, L.; Cabala, J.; Procházka, J.; Pechar, L.; Drahota, P.; Penížek, V.; Komárek, M.; Novák, M. Geochemical position of Pb, Zn and Cd in soils near the Olkusz mine/smelter, South Poland: Effects of land use, type of contamination and distance from pollution source. Environ. Monit. Assess. 2012, 184, 2517–2536. [Google Scholar] [CrossRef] [PubMed]
- Gruszecka, A.M.; Wdowin, M. Characteristics and distribution of analyzed metals in soil profiles in the vicinity of a postflotation waste site in the Bukowno region, Poland. Environ. Monit. Assess. 2013, 185, 8157–8168. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Woch, M.W.; Kapusta, P. Inconspicuous waste heaps left by historical Zn-Pb mining are hot spots of soil contamination. Geoderma 2014, 235–236, 1–8. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Rożek, K.; Rola, K.; Błaszkowski, J.; Leski, T.; Zubek, S. How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil? Forest Ecol. Manag. 2020, 465, 118091. [Google Scholar] [CrossRef]
- Hill, M.O.; Bell, N.; Bruggeman-Nannenga, M.A.; Brugués, M.; Cano, M.J.; Enroth, J.; Flatberg, K.I.; Frahm, J.P.; Gallego, M.T.; Garilleti, R.; et al. An annotated checklist of the mosses of Europe and Macaronesia. J. Bryol. 2006, 28, 198–267. [Google Scholar] [CrossRef]
- Varol, M. Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 2011, 195, 355–364. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements of Soils and Plants; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar]
- Clarke, K.R.; Somerfield, P.J.; Gorley, R.N. Testing of null hypotheses in exploratory community analyses: Similarity profiles and biota-environment linkage. J. Exp. Mar. Biol. Ecol. 2008, 366, 56–69. [Google Scholar] [CrossRef]
- Taguchi, Y.H.; Oono, Y. Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 2005, 21, 730–740. [Google Scholar] [CrossRef]
- Cattell, R.B. The scree plot test for the number of factors. Multivar. Behav. Res. 1966, 1, 140–161. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Brower, J.C.; Kile, K.M. Seriation of an original data matrix as applied to palaeoecology. Lethaia 1988, 21, 79–93. [Google Scholar] [CrossRef]
- Ellenberg, H.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa, Scripta Geobotanica, 2nd ed.; Verlag Erich Goltze KG: Göttingen, Germany, 1991. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2016. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]




| Factor No. | Variables (Factor Loadings) | Variance Explained (%) |
|---|---|---|
| Factor 1 | Vascular plant cover (−0.91) | 53.81 |
| Factor 2 | C organic (0.97), N total (0.96) | 21.85 |
| Factor 3 | Pb (0.94), PLI (0.83), Cd (0.72), Zn (0.70) | 7.99 |
| Factor 4 | pH (0.92) | 7.02 |
| Bryophyte Species Richness | ||||
|---|---|---|---|---|
| N = 62 | Standardized β Coefficient | SE for β Coefficient | t | p |
| Constant | 16.466 | <0.001 | ||
| Factor 1 | 0.486 | 0.100 | 4.849 | <0.001 |
| Factor 3 | 0.450 | 0.100 | 4.509 | <0.001 |
| Factor 4 | 0.261 | 0.100 | 2.606 | 0.012 |
| Bryophyte Cover | ||||
| N = 64 | Standardized β Coefficient | SE for β Coefficient | t | p |
| Constant | 13.230 | <0.001 | ||
| Factor 1 | 0.625 | 0.094 | 6.656 | <0.001 |
| Factor 3 | 0.220 | 0.094 | 2.340 | 0.023 |
| Factor 4 | 0.179 | 0.094 | 1.905 | 0.062 |
| Soil Condition Class | Low | Intermediate | High | Extreme |
|---|---|---|---|---|
| Low | 29.63 | 7.46 | 9.85 | |
| Intermediate | 0.003 | 8.77 | 10.62 | |
| High | 0.001 | 0.001 | 21.42 | |
| Extreme | 0.001 | 0.001 | 0.004 |
| GROUP A—species sensitive to heavy metal pollution, growing on acidic soil with a relatively low content of organic carbon and total nitrogen | GROUP B—nonspecific species tolerant to elevated heavy metal concentration in soil, but inhabiting sites with a wide spectrum of heavy metal concentrations in soil | GROUP C—species preferring heavy metal-polluted soils with high organic carbon and total nitrogen contents and slightly alkaline pH |
| Frequent | Frequent | Frequent |
| Cephaloziella divaricata | Ceratodon purpureus | Tortella tortuosa |
| Polytrichum piliferum | Bryum caespiticium | Amblystegium serpens |
| Pohlia nutans | Campylium chrysophyllum | |
| Barbula unguiculata | Weissia controversa | |
| Bryum pseudotriquetrum | ||
| Brachythecium salebrosum | ||
| Calliergonella cuspidata | ||
| Additional | Additional | Additional |
| Polytrichum juniperinum | Bryum argenteum | Schistidium crassipilum |
| Brachythecium velutinum | Hypnum cupressiforme | Plagiomnium affine |
| Cirriphyllum piliferum | Brachythecium albicans | Plagiomnium undulatum |
| Rhizomnium punctatum | ||
| Bryum pallescens | ||
| Eurhynchium hians | ||
| Plagiomnium cuspidatum | ||
| Sanionia uncinate | ||
| Abietinella abietina | ||
| Brachythecium rutabulum | ||
| Homalothecium lutescens | ||
| Thuidium recognitum | ||
| Eurhynchium praelongum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rola, K.; Plášek, V. The Utility of Ground Bryophytes in the Assessment of Soil Condition in Heavy Metal-Polluted Grasslands. Plants 2022, 11, 2091. https://doi.org/10.3390/plants11162091
Rola K, Plášek V. The Utility of Ground Bryophytes in the Assessment of Soil Condition in Heavy Metal-Polluted Grasslands. Plants. 2022; 11(16):2091. https://doi.org/10.3390/plants11162091
Chicago/Turabian StyleRola, Kaja, and Vítězslav Plášek. 2022. "The Utility of Ground Bryophytes in the Assessment of Soil Condition in Heavy Metal-Polluted Grasslands" Plants 11, no. 16: 2091. https://doi.org/10.3390/plants11162091
APA StyleRola, K., & Plášek, V. (2022). The Utility of Ground Bryophytes in the Assessment of Soil Condition in Heavy Metal-Polluted Grasslands. Plants, 11(16), 2091. https://doi.org/10.3390/plants11162091








