Abstract
Plants in flammable ecosystems have different response strategies to fire, such as increasing germination after exposure to smoke and break of dormancy through heat shock. Peatlands are ecosystems that are more likely to be disturbed by fire with increasing temperatures, but it is not clear how fire affects spore germination of Sphagnum, the dominant plants in peatlands. Here, we hypothesize that Sphagnum spores respond positively to single and combined treatments of moderate heat and smoke (by increased germinability), while spore germinability decreases in response to high temperature. We exposed the Sphagnum spores of four selected species (S. angustifolium, S. fuscum, S. magellanicum and S. squarrosum) collected from peatlands in the Changbai Mountains to heat (40, 60 and 100 °C), on its own and combined with smoke-water treatments. Our results showed that a heat of 100 °C inhibited the spore germination or even killed spores of all species, while spore germination of three (Sphagnum angustifolium, S. fuscum and S. squarrosum) of the four species was promoted by 40 and 60 °C heat compared to the control (20 °C). Hollow species (S. angustifolium and S. squarrosum) showed a stronger positive responsive to heat than hummock species (S. fuscum and S. magellanicum). Sphagnum fuscum spores responded positively to the combined heat and smoke treatment while the other species did not. For the first time, we demonstrate the positive effects of heat on its own and in combination with smoke on spore germination in wetland mosses, which may be important for the establishment and persistence of peatmoss populations after fire.
1. Introduction
Many ecosystems depend on fire to maintain stability and their adaption to fire may determine their fate under the background of climate warming [1,2,3]. The survival and reproduction of plants can be directly affected by fire. Therefore, in the long-term evolution process, vascular plants in fire-prone ecosystems have formed a series of morphological and physiological fire-adapted traits. For example, pine species in North America can tolerate frequent lightning fires by forming a thick bark and delaying cone cracking [4]; The seeds of Australian Xanthorrhoea gracilis Endl. buried in soil can tolerate wildfires with a surface temperature of up to 300 °C and immediately germinate and flower [5]; germination of the epiphytic orchid, Oberonia ensiformis (J. E. Smith) Lindl, is strongly promoted by smoke, and its germination percentage is otherwise zero in the absence of smoke [6]. The fire adaptation strategy of plants makes the populations survive and develop continuously, and the fire-related renewal strategies, such as the response characteristics of propagules (vegetative or sexual) to fire factors, are particularly important for vegetation recovery and change of community structure after fire [7].
Fire-stimulated germination is found in many seed plants from fire-prone ecosystems [8,9]. Post-fire germination may be triggered by different mechanisms, with heat and smoke being the main fire-related germination cues [10]. Heat can improve germination by accelerating after-ripening in species with a water-permeable seed coat, or by rupturing the seed coat structure, allowing water uptake, in species with water impermeable seeds [11,12]. The smoke produced during fire can also stimulate germination and seedling growth [9,13,14,15]. The mechanism is that smoke contains active compounds (nitrogen oxides, glyceronitrile and butenolide), similar to the plant hormones gibberellin or cytokinin, which can promote embryo development and break seed dormancy [9,16,17,18].
In natural communities, smoke and heat often act together, but the way of action varies according to the location of seed distribution [19]. In general, heat mainly impacts the surface layer of the soil and the seeds remaining in the lower parts of the branches [19], the aerosol smoke mainly affects seeds remaining in the higher parts of the branches [20], while seeds deep in the soil are affected by the interaction of heat and aqueous smoke [9]. In addition, many studies show that smoke cannot stimulate germination of seeds that are physically dormant; therefore, smoke promotion in species with water-impermeable seeds is likely to occur only if seeds are released from physical dormancy by suitable environmental factors [12,21]. Thus, the response of the plant propagules (seeds and spores) may be very complex and is dependent on several factors including the interaction between fire-related cues such as heat and smoke, the depth at which seeds are buried, and a series of morphological and physiological traits of propagules [19,22].
Up to now, research on the impact of fire on vegetation succession and regeneration has mostly been concentrated on ecosystems around the Mediterranean region [8], Africa [23], Australia [24] and California in North America [1], but the effect of wildfires on non-fire prone ecosystems, such as peatlands, is poorly known [25]. Generally, peatlands are not prone to natural fires due to waterlogging. However, studies on charcoal from Canada [26] and Poland [27] have also shown that fire is an important disturbance in northern peatlands.
Peat mosses, namely the genus of Sphagnum are the dominant and most important carbon sequestering plants in northern peatlands [28]. Many Sphagnum species regularly produce spores every year, and most spores will be buried in the stratum, with the accumulation of peat, to form a spore bank [29,30,31]. Since the surface vegetation and/or soil has good thermal buffering during wildfires, spores in deep peat layers can avoid direct damage [29,32]. Mechanisms of bryophyte regeneration and colonization by gametophytes following natural disturbances, particularly fire, have been documented in bogs [33,34,35] and in rich fens [36]. However, it is not clear whether peatland bryophyte (especially Sphagnum) spores will respond to fire and its related cues.
Recently, the role of fire for potential regeneration of peatland bryophytes from spores has been addressed in some studies [32,37], and especially the promotive effect of smoke on spore germination has been demonstrated [37]. We, however, still lack a comprehensive understanding in how heat alone or its combination with smoke affect the germination of bryophyte spores. In this study, our aim was to test the following hypotheses: (1) Heat will improve the germination of Sphagnum spores; (2) the positive effects of heat on spore germination may be more pronounced when interacting with smoke; and (3) since spores of hollow species (S. angustifolium and S. squarrosum) are more susceptible to fire than those of hummock species (S. fuscum and S. magellanicum) during dry periods, the spores of hollow species may show a more accentuated response than hummock species to heat and smoke.
2. Results
2.1. Effects of Heat on Spore Germination
2.1.1. Germination Percentage (GP)
There were significant differences in spore germination among the four Sphagnum species (p < 0.001, Table 1). The GP of S. fuscum and S. squarrosum was lower, 24.6 ± 2.4% and 40.3 ± 2.0% (Figure 1b and Figure 2b,d), while the GP of S. angustifolium and S. magellanicum was relatively high, 56.2 ± 6.1% and 66.0 ± 4.5%, respectively (Figure 1b and Figure 2a,c). Heat had a significant effect on spore germination, showing a unimodal response to increasing temperature (p < 0.001) (Table 1; Figure 1b). There were interspecific differences in the germination behavior (p < 0.001) (Table 1; Figure 1b). The spore GPs of S. angustifolium, S. fuscum and S. squarrosum increased with moderate heat temperature (40 °C and 60 °C) (p = 0.017 for S. angustifolium; p = 0.031 for S. fuscum; p = 0.002 for S. squarrosum) and reached a maximum when treated with 60 °C (10 min), 38.5 ± 2.0%, 78.8 ± 3.5% and 67.9 ± 3.1%, respectively (Figure 1b and Figure 2a,b,d). However, there was no difference in spore germination of S. magellanicum between heat of 40 °C and 60 °C and the control (p = 0.10, Table 2; Figure 1b and Figure 2c). Heat at 100 °C significantly inhibited spore germination of all species (p < 0.001) (Figure 1b and Figure 2).

Table 1.
Two-way analysis of variance (ANOVA) for the effect of species and heat temperature on germination percentage (GP) and spore viability (V, dyeing percentage) of Sphagnum species.
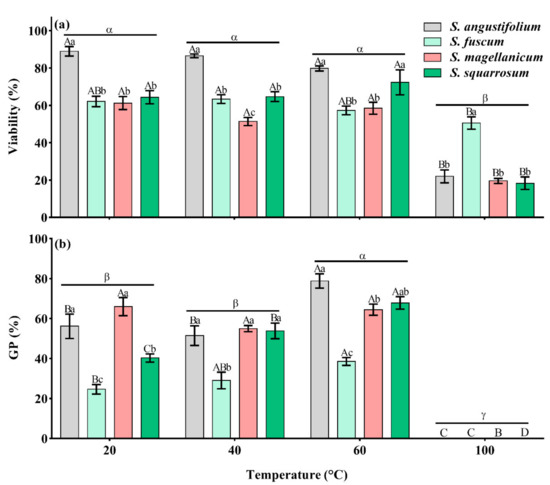
Figure 1.
Effects of different heat temperatures on spore (a) viability (dyeing percentage) (b) germination percentage (GP) and in four Sphagnum species. Error bars represent SEM (n = 3). Lowercase letters (a, b and c) represent significant difference among species at the same heat temperature and uppercase letters (A, B, C and D) represent significant difference among heat temperatures in the same species (also in others). “α”, “β” and “γ” represent significant difference among the total germination percentage (21d) and viability after heat treatment.
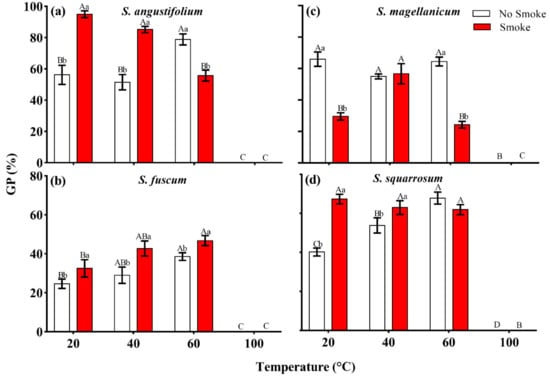
Figure 2.
Interactive effect between smoke–water and heat on spore germination percentage (GP) in four Sphagnum species ((a), Sphagnum angustifolium; (b), S. fuscum; (c), S. magellanicum; (d), S. squarrosum). Error bars represent SEM (n = 3). Lowercase letters (a, b and c) represent significant difference among smoke–water treatments at the same heat-shock temperature and uppercase letters (A, B, C and D) represent significant difference among heat temperatures at the same smoke–water treatment.

Table 2.
One-way analysis of variance (ANOVA) for the effect of heat singly and combined with smoke on spore germination percentage (GP), germination speed (GS) and treatment effect index (TEI) of four Sphagnum species.
2.1.2. Germination Speed (GS) and Heat Treatment Effect Index (TEI)
After heat treatments (40 °C and 60 °C), there were no differences in GS in S. angustifolium, S. magellanicum and S. squarrosum compared with the controls (p > 0.05, Table 2; Figure 3a,c,d), while the GS of S. fuscum clearly decreased (p = 0.031, Table 2; Figure 3b).
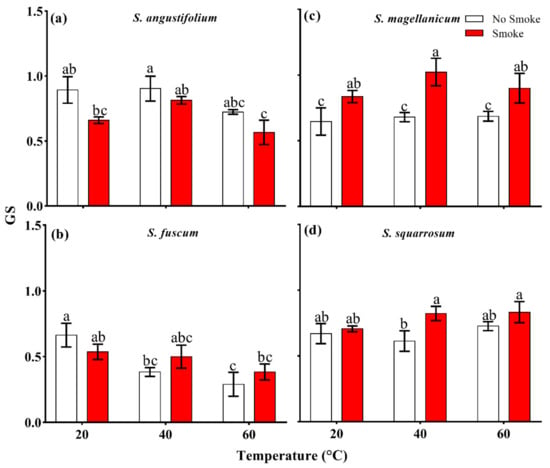
Figure 3.
Interactive effect between smoke–water and heat on spore germination speed (GS) in four Sphagnum species. ((a), Sphagnum angustifolium; (b), S. fuscum; (c), S. magellanicum; (d), S. squarrosum). Error bars represent SEM (n = 3). Different lowercase letters represent significant differences (p < 0.05) between the treatments (smoke–water and heat treatments singly and combined).
The heat treatment effect index (TEI) of S. angustifolium at 60 °C was higher than that of the control group (p = 0.028; Table 2; Figure 4a), while that at 40 °C did not differ. The TEI of S. squarrosum was 0.25 ± 0.05 (40 °C) and 0.40 ± 0.03 (60 °C), both greater than that of the control group (p = 0.049 for 40 °C; p < 0.001 for 60 °C) (Table 2; Figure 4d). Compared to the control group, the TEI of S. fuscum and S. magellanicum did not change after heat treatment at 40 °C and 60 °C (p = 0.260 for S. fuscum; p = 0.056 for S. magellanicum) (Table 2; Figure 4b,c).
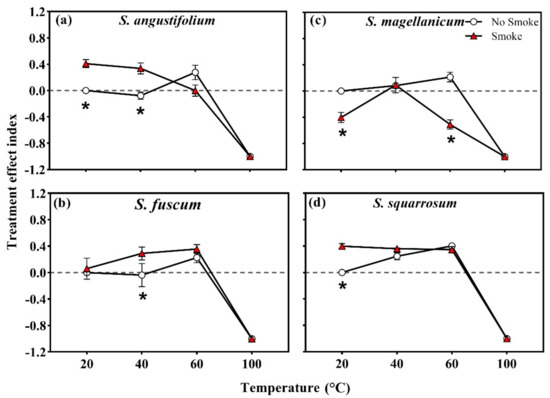
Figure 4.
Treatment effect index (TEI) of four Sphagnum species ((a), Sphagnum angustifolium; (b), S. fuscum; (c), S. magellanicum; (d), S. squarrosum) after single and combined treatment of heat temperature and smoke–water. Error bars represent SEM (n = 3). Different lowercase letters represent significant differences (p < 0.05) between heat treatments. Asterisks represent significant differences (p < 0.05) among smoke–water treatments at the same heat-shock temperature.
2.1.3. Spore Viability
From the methylene blue dyeing of spores, there was a significant difference in the initial spore viability among the four Sphagnum species (p < 0.001) (Table 1; Figure 1a). The initial viability of S. angustifolium was the highest (88.9 ± 2.6%), and the viability of S. magellanicum was the lowest (61.3 ± 3.5%) (Figure 1a). After heat treatment, the viability of all tested species decreased significantly (p < 0.001 for S. angustifolium, S. magellanicum and S. squarrosum; p = 0.027 for S. fuscum) (Table 1; Figure 1a) mainly because of the strong negative effect of 100 °C; There was no significant difference in spore viability between treatments at 40 °C, 60 °C and the control group (Figure 1a).
2.2. Effects of Heat + Smoke on Spore Germination
2.2.1. Germination Percentage
The germination was significantly affected by the combined treatment of heat and smoke-water in all four tested species (p < 0.01, Table 2; Figure 2). Compared to the control group (20 °C + no smoke), the GP of S. angustifolium increased significantly under the combined treatment of 40 °C + smoke (p < 0.001, Table 2; Figure 2a). Except 100 °C + smoke treatment, spore germination of S. fuscum increased in all combined treatments (p = 0.007) and reached the highest value (46.7 ± 2.6%) at 60 °C + smoke (Table 2; Figure 2b). In S. magellanicum, GP was highest in the control group (66.0 ± 4.5%) and, besides from 40 °C + smoke, all other smoke treatments decreased its germination (p < 0.001) (Table 2; Figure 2c). Compared to the control group, both 40 °C + smoke and 60 °C + smoke clearly promoted spore germination in S. squarrosum (p < 0.001, Table 2; Figure 2d).
2.2.2. Germination Speed and Heat Treatment Effect Index
The GS in S. angustifolium and S. fuscum decelerated under the combined treatment of 60 °C + smoke (p < 0.05 for both, Figure 3a,b). In S. magellanicum, the combined treatments of 40 °C + smoke and 60 °C + smoke obviously accelerated the GS (p < 0.05, Figure 3c).
In terms of the treatment effect index (TEI), S. angustifolium, S. fuscum and S. squarrosum showed positive values in the combined treatments of 40 °C + smoke and/or 60 °C + smoke (p < 0.05 for all, Table 2; Figure 4a,b,d). In S. magellanicum, the TEI was negative in the combined treatment of 60 °C + smoke (p = 0.001, Table 2; Figure 4c).
3. Discussion
3.1. Heat and Spore Germination
The results show that Sphagnum spores can tolerate temperatures of up to 60 °C, and that temperatures of 40 °C or 60 °C actually stimulate spore germination, which verifies our first hypothesis. Generally, seeds with a thick and water-impermeable coat tend to respond positively to heat shock [12,38]. The mechanism is that high temperatures stimulate germination by rupturing the seed coat, allowing water uptake, in species with otherwise water-impermeable seeds. The dormancy-breaking temperatures of these seeds are generally relatively high [11], with temperature ranges of 80–100 °C [39]. Williams et al. [24] found that a short-term high temperature treatment at 80–100 °C can break the seed dormancy of several plant species, thereby stimulating seed germination. Zhang et al. [40] found that Dodonaea viscosa seeds have an obvious heat response at temperature above 40 °C, and that germination reaches its maximum after treatment at 80 °C (for 10 min). In contrast, the temperature threshold (40 °C and 60 °C) that can stimulate Sphagnum spore germination in this study is relatively low. This indicates that high temperatures do not play a mechanical role in rupturing the spore wall, like in seeds, but is more likely to break dormancy by affecting the physiological process of spores [41].
3.2. Effect of Smoke on Spore Germination
Keely et al. [42] and Baldwin et al. [43] provided evidence that trace gases in smoke such as nitrogen dioxide (NO2) and potentially nitric oxide (NO) are responsible for induced germination and dormancy release of annuals in chaparral. Although NO is recognized as a plant signaling compound [44] and a promoter of seed germination [43], the proposed role of NOx as the dormancy-breaking cue in plant-derived smoke (PDS) has been challenged by several studies. Preston et al. [45] found that smoke-responsive plant species did not respond to NOx generated from solutions of sodium nitroprusside, and the stimulatory effect of smoke could not be inhibited with a specific nitrogen-oxide scavenger [46]. Flematti et al. [16], in 2004, isolated the predominant chemical compound in smoke-water and identified as karrikinolide (KAR1: 3-methyl-2H-furo[2,3-c]pyran-2-one), and it was considered as the most recognized active component that promotes seed germination [9]. Although there is no unified understanding of the substances in smoke–water, it is still believed that the KAR1 and NOx in PDS and its solution may promote the germination of many plant seeds at the same time or different seeds respectively. In this study, although we did not report whether the single effect of smoke–water can promote spore germination, from the more significant results of heat treatment (20 °C, 40 °C and 60 °C) + smoke on spore germination of three Sphagnum species (S. angustifolium, S. fuscum and S. squarrosum) compared with the control group and corresponding heat treatment, we can get that smoke–water does have a certain promotive effect on spore germination (Figure 2). In addition, our previous study also clearly proved that smoke–water can strongly promote the germination of ten bryophyte spores [37]. In a certain sense, smoke and/or heat + smoke responsiveness of spores could not only be useful to remind that basic features of dormancy-breaking treatments, but also fulfill the knowledge gap in the bryophyte dormancy and reveal the not yet known mechanisms to initiate dormancy-breaking of spores [47].
3.3. Spore Germination in Relation to Heat and Smoke–Water
Under natural conditions, smoke and heat production during combustion are basically synchronous. Therefore, the effect of fire on propagule germination is considered to be the result of the interaction between heat and smoke [10]. In this experiment, germination of S. fuscum after heat treatment (40 °C and 60 °C) + smoke–water was higher than after the single treatment of heat (Table 2; Figure 2). This is consistent with our second hypothesis and do agree with previous findings in seed germination of some species. For example, Tieu et al. [22] studied the germination of seven naturally distributed plant seeds in South Australia under the combined treatment of heat and smoke and found that germination of Sowerbaea laxiflora seeds did not change when exposed to heat, while it increased when treated with heat + smoke. Zirondi et al. [38] found that after exposure to 100 °C + smoke-water, the germination of almost all tested legume species with impermeable seed coats increased significantly compared to the control, and single treatments by smoke and heat. However, combined treatments were not more effective than single heat or smoke-water treatment for stimulating germination of spores in the three other species (S. angustifolium, S. magellanicum and S. squarrosum). This is similar to the results of studies by Abella et al. [48] and Figueroa et al. [49] who found that there was no more effective stimulation of seed germination after the combined treatments of smoke and heat than after single smoke or heat treatments.
3.4. Fire-Responsive Germination and Species Habitat Preference
Plant propagule (seed and spore) dormancy and germination characteristics may vary depending on habitats and species’ life history strategies [50,51,52]. A previous study showed that species indicating disturbance and those preferring soils more or less rich in nitrogen respond more strongly to fire related cues [51]. Naturally, reflecting microtopographical differences, hollow species are more vulnerable to fire than hummock species in dry periods [53,54]. In this study, the tested hollow species (S. angustifolium and S. squarrosum) showed a strong positive response to smoke-water. However, the response of hummock species to smoke was inconsistent. S. fuscum showed a positive response but not as strong as the hollow species, S. magellanicum showed a negative response (Figure 1). Moderate heat treatment (40 °C and/or 60 °C) had an effect on all four tested species, but the stimulation of germination was higher in hollow than in hummock species. In addition, the combined heat (40 and/or 60 °C) + smoke treatment significantly stimulated spore germination in the two hollow species, while the effect on the hummock species was more variable (Figure 2). This result supports our third hypothesis, that the spores of hollow species are more adapted to fire than the hummock species. The difference in response of different species to heat and smoke may be an important mechanism for the co-existence of multiple species in these northerly peatlands.
3.5. Fire Disturbance and Establishment of Sphagnum Spores
Bryophytes are generally considered as pioneer species in the process of post fire secondary succession, but their specific re-establishment mechanism is not clear [35]. Some scholars believe that the spore bank is important for the re-establishment of Sphagnum populations after catastrophic disturbance [31,55,56]. In this study, we found that low-intensity heat and smoke–water, on its own and in combination, can stimulate germination of Sphagnum spores. In Hani peatland of the Changbai Mountains, the temperature can easily reach 40 °C or even higher [32], which is beneficial for the germination and establishment of Sphagnum spores. During a fire, the surface temperature of a peatland will exceed 100 °C, but at a depth of only 1–5 cm, the surface moss layer and peat acts as effective insulators to moderate the temperature increase, and buried spores can retain their viability [32]. In addition, our experiment also indicates that Sphagnum spores have a certain tolerance to high temperature (40–60 °C). Thus, adaptable heat temperatures in deep peat layers may be one important reason and condition for the activation of the persistent spore bank of Sphagnum mosses, for re-establishment after a fire.
Combustion is a complex process. In addition to generating a large amount of heat energy, it will also cause changes in many habitat conditions, such as soil structure, nutrient status, light conditions, water level and microtopography [25,57,58]. Therefore, in the future research, we should comprehensively consider the various effects of fire to reveal the mechanisms of fire affecting bryophyte regeneration in peatlands.
4. Materials and Methods
4.1. Study Species, Spore Capsule Collection and Spore Suspension Preparation
Two hummock species, Sphagnum fuscum (Schimp.) Klinggr. and S. magellanicum Brid., and two hollow species, S. angustifolium C. Jensen and S. squarrosum Crome. were selected for the study. At the end of July 2020, mature spore capsules of Sphagnum angustifolium, S. magellanicum, S. squarrosum and S. fuscum were collected from Tangbei, Hani and Dongfanghong peatlands, Northeastern China, respectively.
During collection, more than 20 shoots belonging to different populations in each peatland were chosen. Spore capsules were put in sealed PVC bottles in the field and later stored in a refrigerator at 4 °C in darkness before onset of the experiment.
Spore capsules of each species were surface sterilized with 75% ethanol for 2 min and were then crushed in a beaker with 6 mL distilled water to make a spore suspension. We put 2 mL of spore suspension into small bags made of polyamide filter fabrics with 7 µm mesh size.
4.2. Experimental Design
The whole experiment included two factors, heat and heat + smoke. There were three replicates and each with more than 300 spores for each species. In total, 96 spore bags (4 species × 8 treatments × 3 replicates) were prepared.
4.2.1. Heat Treatments
In addition to the control treatment (20 °C: average daily temperature for the growing season in the Changbai Mountains), spore bags of each species were exposed to the following temperatures for 10 min: 40 °C, 60 °C and 100 °C [32]. The temperatures of 40 °C and 60 °C and exposure time of 10 min were chosen according to the soil temperature characteristics at different depths under the simulated burning conditions [32,59]. We chose 100 °C to represent a case of an extremely high temperature. The temperature treatments were performed in a pre-heated drying oven with stable heat settings and each replicate underwent the dry heat shock separately to guarantee its independence [38].
4.2.2. Heat + Smoke Treatments
In this study, we performed an experiment combining heat followed by the exposure to smoke. In addition to the control, spore bags (each bag contains at least 300 spores) were exposed to 40 °C, 60 °C and 100 °C for 10 min.
Sphagnum fuscum shoots were collected to prepare a smoke-water solution by igniting its dry material (390 g) in a 3 L kettle [37]. The smoke was pumped into a PET bottle containing 300 mL of distilled water through a tube for two hours, and the prepared solution (pH 3.68 ± 0.01, n = 4) was considered as the 100% smoke–water (SW) stock solution [17,37]. In this experiment, we used the concentrations of smoke–water:distilled water (SW:DW, v:v) = 1:1 that stimulated best spore germination of peatland bryophytes [37]. Distilled water acted as the control. In addition to the control (imbibed in distilled water), heated spores were soaked in the smoke–water solution for 24 h and then put to germinate.
4.3. Germination Experiment
An improved Rudolph nutrient solution [30,60] was used as the culture solution. The culture solution and the agar powder were mixed and sterilized at a ratio of 50:1 to prepare the medium in Petri dishes. Spore suspension was poured evenly onto the medium for cultivation in a growth chamber (PRX-450C, Ningbo Saifu Experimental Instrument Co. Ltd., Ningbo, China). The cultivation conditions were 27 °C:22 °C under a 16 h:8 h, light:dark photoperiod, with light 60:0 μmol m−2 s−1, and a relative air humidity constant at 60% [31]. We observed spore germination twice, on days 11 and 21 of cultivation. The number of germinated (spores with filamentous protonema) and ungerminated spores were counted to produce a germination proportion.
4.4. Spore Viability Test
Viability of the spores was estimated by conducting methylene blue dyeing with three replicates of about 300 spores for each species [37,61]. Methylene blue is an alkaline dye which combines with nucleic acid to make the nucleus of viable spores blue [37]. Spores dyed blue were scored as alive while spores remaining undyed or only lightly dyed were scored as dead.
4.5. Data Processing and Statistical Analysis
4.5.1. Data Processing
The spore germination percentage (GP), germination speed (GS), viability (V, dyeing percentage), and heat and heat + smoke treatment effect index (TEI) are calculated as follows:
where n is the number of germinated spores within a cultivation period; N is the total number of observed spores; ns is the number of spores stained blue; GP11d and GP21d are GP at the 11th and 21st d, respectively.
where C is the control group value and T is the treatment group value. When TEI > 0, the effect is promotive, and when TEI < 0, it is inhibitive. The absolute value of TEI represents the magnitude of the effect.
GP (%) = (n/N) × 100%;
GS = GP11d/GP21d;
V (%) = (ns/N) × 100%.
GS = GP11d/GP21d;
V (%) = (ns/N) × 100%.
When T ≥ C, TEI = 1 − C/T;
When T < C, TEI = T/C − 1;
When T < C, TEI = T/C − 1;
4.5.2. Statistical Analysis
All statistical analyses were performed with SPSS for windows 26.0 (SPSS Inc., New York, NY, USA). We applied a two-way ANOVA to examine the main and interactive effects of species and heat on spore germination (GP) and spore viability (V). One-way ANOVAs were used to test the effect of heat singly and in combination with smoke on spore germination (GP), germination speed (GS) and treatment effect index (TEI) of each species. Tukey’s tests were performed to identify the difference in spore germination, viability, and treatment effect index among different species and treatments. The significance level was set to α = 0.05. Since the germination of all species under the treatment of 100 °C high temperature was zero, we did not use the data from this treatment when analyzing the effect of heat on spore germination.
Author Contributions
S.Y.: Conceptualization, data curation, formal analysis, investigation, methodology, software, writing—original draft. S.S.: Investigation, writing—review and editing. B.F.: Data curation and investigation. M.S.: Investigation, writing—review and editing. Z.-J.B.: Conceptualization, methodology, funding acquisition, project administration, supervision, visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Nature Science Foundation of China (No. 41871046, 41471043 and 32060050), and the Jilin Provincial Science and Technology Development Project (20210402032GH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the article. Additional data are available on request from the corresponding author.
Acknowledgments
We thank the anonymous reviewers and the editor for their comments and suggestions that helped improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keeley, J.E.; Fotheringham, C.J.; Baer-Keeley, M. Determinants of postfire recovery and succession in Mediterranean climate shrublands of California. Ecol. Appl. 2005, 15, 1515–1534. [Google Scholar] [CrossRef]
- Bowman, D.M.; Balch, J.K.; Artaxo, P.; Bond, W.J.; Carlson, J.M.; Cochrane, M.A.; D’Antonio, C.M.; DeFries, R.S.; Doyle, J.C.; Harrison, S.P.; et al. Fire in the Earth system. Science 2009, 324, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Ribeiro, E. Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 2017, 26, 889–897. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef]
- Koch, J.M.; Bell, D.T. Leaf scorch in Xanthorrhoea gracilis as an index of fire intensity. Aust. For. Res. 1980, 10, 113–119. [Google Scholar]
- Lamont, B.B.; He, T. Fire-proneness as a prerequisite for the evolution of fire-adapted traits. Trends Plant Sci. 2017, 22, 278–288. [Google Scholar] [CrossRef]
- Zacharias, P.J.K. The effect of fire on germination in five common veld grasses. J. Grassl. Soc. S. Afr. 1988, 5, 229–230. [Google Scholar] [CrossRef]
- Reyes, O.; Trabaud, L. Germination behavior of 14 Mediterranean species in relation to fire factors: Smoke and heat. Plant Ecol. 2008, 202, 113–121. [Google Scholar] [CrossRef]
- Nelson, D.C.; Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Regulation of seed germination and seedling growth by chemical signals from burning vegetation. Annu. Rev. Plant Biol. 2012, 63, 107–130. [Google Scholar] [CrossRef]
- Van Staden, J.; Brown, N.A.; Jäger, A.K.; Johnson, T.A. Smoke as a germination cue. Plant Species Biol. 2000, 15, 167–178. [Google Scholar] [CrossRef]
- Herranz, J.M.; Ferrandis, P.; Martínez Sánchez, J.J. Influence of heat on seed germination of nine woody Cistaceae species. Int. J. Wildland Fire 1998, 9, 173–182. [Google Scholar] [CrossRef]
- Moreira, B.; Tormo, J.; Estrelles, E.; Pausas, J.G. Disentangling the role of heat and smoke as germination cues in Mediterranean Basin flora. Ann. Bot. 2010, 105, 627–635. [Google Scholar] [CrossRef]
- De Lange, J.H.; Boucher, C. Autecological studies on Audouinia capitata (Bruniaceae). I. Plant-derived smoke as a seed germination cue. S. Afr. J. Bot. 1990, 56, 700–703. [Google Scholar] [CrossRef]
- Dixon, K.W.; Roche, S.; Pate, J.S. The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 1995, 101, 185–192. [Google Scholar] [CrossRef]
- Brown, N.A.C.; Van Staden, J.; Daws, M.I.; Johnson, T. Patterns in the seed germination response to smoke in plants from the Cape Floristic Region, South Africa. S. Afr. J. Bot. 2003, 69, 514–525. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Van Staden, J.; Jager, A.K.; Light, M.E.; Burger, B.V. Isolation of the major germination cue from plant-derived smoke. S. Afr. J. Bot. 2004, 70, 654–659. [Google Scholar] [CrossRef]
- Flematti, G.R.; Merritt, D.J.; Piggott, M.J.; Trengove, R.D.; Smith, S.M.; Dixon, K.W.; Ghisalberti, E.L. Burning vegetation produces cyanohydrins that liberate cyanide and stimulate seed germination. Nat. Commun. 2011, 2, 1–6. [Google Scholar] [CrossRef]
- Auld, T.D.; Denham, A.J. How much seed remains in the soil after a fire? Plant Ecol. 2006, 187, 15–24. [Google Scholar] [CrossRef]
- Hu, F.; Tang, X.R.; Yang, J.; Chen, Y.F.; Kong, C.H. Ecophysiological effects of plant-derived smoke and heat on plants. Acta Ecol. Sin. 2006, 26, 594–600. [Google Scholar]
- Jefferson, L.; Pennacchio, M.; Havens-Young, K. Ecology of Plant-Derived Smoke: Its Use in Seed Germination; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Tieu, A.; Dixon, K.W.; Meney, K.A.; Sivasithamparam, K. The interaction of heat and smoke in the release of seed dormancy in seven species from southwestern Western Australia. Ann. Bot. 2001, 88, 259–265. [Google Scholar] [CrossRef]
- Jeffery, D.J.; Holmes, P.M.; Rebelo, A.G. Effects of dry heat on seed germination in selected indigenous and alien Legume species in South Africa. S. Afr. J. Bot. 1988, 54, 28–34. [Google Scholar] [CrossRef]
- Williams, P.R.; Congdon, R.A.; Grice, A.C.; Clarke, P.J. Fire-related cues break seed dormancy of legumes in tropical eucalypt savannas of north-eastern Australia. Austral Ecol. 2003, 28, 507–514. [Google Scholar] [CrossRef]
- Lukenbach, M.C.; Devito, K.J.; Kettridge, N.; Petrone, R.M.; Waddington, J.M. Hydrogeological controls on post-fire moss recovery in peatlands. J. Hydrol. 2015, 530, 405–418. [Google Scholar] [CrossRef]
- Gabriel, M.; Martin, L.; Serge, P. Impact of fire on long-term vegetation dynamics of ombrotrophic peatlands in northwestern Québec, Canada. Quat. Res. 2012, 77, 110–121. [Google Scholar]
- Marcisz, K.; Galka, M.; Pietrala, P.; Miotk-Szpiganowicz, G.; Obremska, M.; Tobolski, K.; Lamentowicz, M. Fire activity and hydrological dynamics in the past 5700 years reconstructed from Sphagnum peatlands along the oceanic-continental climatic gradient in northern Poland. Quat. Sci. Rev. 2017, 177, 145–157. [Google Scholar] [CrossRef]
- Clymo, R.S.; Hayward, P.M. The ecology of Sphagnum. In Bryophyte Ecology; Springer: Dordrecht, The Netherlands, 1982; pp. 229–289. [Google Scholar]
- During, H.J. Diaspore banks. Bryologist 2001, 104, 92–97. [Google Scholar] [CrossRef]
- Sundberg, S.; Rydin, H. Habitat requirements for establishment of Sphagnum from spores. J. Ecol. 2002, 90, 268–278. [Google Scholar] [CrossRef]
- Bu, Z.J.; Sundberg, S.; Feng, L.; Li, H.K.; Zhao, H.Y.; Li, H.C. The Methuselah of plant diaspores: Sphagnum spores can survive in nature for centuries. New Phytol. 2017, 214, 1398–1402. [Google Scholar] [CrossRef]
- Guo, H.B.; Xu, X.Y.; Bu, Z.J.; Feng, L.; Lu, X.Y.; Wang, J.Y.; Chen, Y.D.; Yusup, S.; Lu, F. Effects of high temperature during burning on Sphagnum spore germinability: A simulated experimental study. Chin. J. Ecol. 2019, 38, 2369–2376. [Google Scholar]
- Johnson, P.N. Vegetation recovery after fire on a southern New Zealand peatland. N. Z. J. Bot. 2001, 39, 251–267. [Google Scholar] [CrossRef]
- Benscoter, B.W. Post-fire bryophyte establishment in a continental bog. J. Veg. Sci. 2006, 17, 647–652. [Google Scholar] [CrossRef]
- Clarke, P.J.; Keith, D.A.; Vincent, B.E.; Letten, A.D. Post-grazing and post-fire vegetation dynamics: Long-term changes in mountain bogs reveal community resilience. J. Veg. Sci. 2015, 26, 278–290. [Google Scholar] [CrossRef]
- Blier-Langdeau, A.; Guêné-Nanchen, M.; Hugron, S.; Rocherfort, L. The resistance and short-term resilience of a restored extracted peatland ecosystems post-fire: An opportunistic study after a wildfire. Restor. Ecol. 2021, e13545. [Google Scholar] [CrossRef]
- Yusup, S.; Sundberg, S.; Ooi, M.K.; Zhang, M.M.; Sun, Z.Q.; Rydin, H.; Wang, M.; Feng, L.; Chen, X.; Bu, Z.J. Smoke promotes germination of peatland bryophyte spores. Ecology, 2022; submitted. [Google Scholar]
- Zirondi, H.L.; Silveira, F.A.; Fidelis, A. Fire effects on seed germination: Heat shock and smoke on permeable vs impermeable seed coats. Flora 2019, 253, 98–106. [Google Scholar] [CrossRef]
- Bradstock, R.A.; Auld, T.D. Soil temperatures during experimental bushfires in relation to fire intensity: Consequences for Legume germination and fire management in south-eastern Australia. J. Appl. Ecol. 1995, 32, 76–84. [Google Scholar] [CrossRef]
- Zhang, G.F.; Hua, D.F.; Huang, B.Q.; Zhang, H.H.; Yu, T.; Meng, L.Y.; Su, W.H. Effects of high temperature treatment on seed germination of Dodonaea viscosa (L.) Jacq. Agric. Sci. Technol. 2017, 18, 82–86. [Google Scholar]
- Webster, R.E.; Waterworth, W.M.; Stuppy, W.; West, C.E.; Ennos, R.; Bray, C.M.; Pritchard, H.W. Biomechanical, biochemical, and morphological mechanisms of heat shock-mediated germination in Carica papaya seed. J. Exp. Bot. 2016, 67, 6373–6384. [Google Scholar] [CrossRef]
- Keeley, J.E.; Fotheringham, C.J. Trace gas emissions and smoke-induced seed germination. Science 1997, 276, 1248–1250. [Google Scholar] [CrossRef]
- Baldwin, I.T.; Staszak-Kozinski, L.; Davidson, R. Up in smoke I. Smoke-derived germination cues for post-fire annual, Nicotiana attenuata Toor ex Watson. J. Chem. Ecol. 1994, 20, 2345–2371. [Google Scholar] [CrossRef]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef]
- Preston, C.A.; Becker, R.; Baldwin, I.T. Is ‘NO’ news good news? Nitrogen oxides are not components of smoke that elicits germination in two smoke-stimulated species, Nicotiana attenuata and Emmenanthe penduliflora. Seed Sci. Res. 2004, 14, 73–79. [Google Scholar] [CrossRef]
- Light, M.E.; van Staden, J. The nitric oxide specific scavenger carboxy-PTIO does not inhibit smoke stimulated germination of Grand Rapids lettuce seeds. S. Afr. J. Bot. 2003, 69, 217–219. [Google Scholar] [CrossRef][Green Version]
- Footitt, S.; Cohn, M.A. Developmental arrest: From sea urchins to seeds. Seed Sci. Res. 2001, 11, 3–16. [Google Scholar] [CrossRef]
- Abella, S.; Springer, J.; Covington, W. Seed banks of an Arizona Pinus ponderosa landscape: Responses to environmental gradients and fire cues. Can. J. For. Res. 2007, 37, 552–567. [Google Scholar] [CrossRef]
- Figueroa, J.; Cavieres, L.; Gomez-Gonzalez, S.; Molina-Montenegro, M.; Jaksic, F. Do heat and smoke increase emergence of exotic and native plants in the matorral of central Chile? Acta Oecologica 2009, 35, 335–340. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Mojzes, A.; Csontos, P.; Kalapos, T. Is the positive response of seed germination to plant-derived smoke associated with plant traits? Acta Oecologica 2015, 65, 24–31. [Google Scholar] [CrossRef]
- Carthey, A.J.; Tims, A.; Geedicke, I.; Leishman, M.R. Broad-scale patterns in smoke-responsive germination from the south-eastern Australian flora. J. Veg. Sci. 2018, 29, 737–745. [Google Scholar] [CrossRef]
- Nungesser, M.K. Modelling microtopography in boreal peatlands: Hummocks and hollows. Ecol. Model. 2003, 165, 175–207. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Clymo, R.S.; Duckett, J.G. Regeneration of Sphagnum. New Phytol. 1986, 102, 589–614. [Google Scholar] [CrossRef]
- Sundberg, S.; Rydin, H. Experimental evidence for a persistent spore bank in Sphagnum. New Phytol. 2000, 148, 105–116. [Google Scholar] [CrossRef]
- Thompson, D.K.; James, M. Wildfire effects on vadose zone hydrology in forested boreal peatland microforms. J. Hydrol. 2013, 486, 48–56. [Google Scholar] [CrossRef]
- Smith, S.M.; Newman, S.; Garrett, P.B.; Leeds, J.A. Differential effects of surface and peat fire on soil constituents in a degraded wetland of the northern Florida Everglades. J. Environ. Qual. 2001, 30, 1998–2005. [Google Scholar] [CrossRef]
- Ramírez-Trejo, M.D.R.; Pérez-García, B.; Orozco-Segovia, A. Analysis of fern spore banks from the soil of three vegetation types in the central region of Mexico. Am. J. Bot. 2004, 91, 682–688. [Google Scholar] [CrossRef]
- Rudolph, H.; Kirchhoff, M.; Gliesmann, S. Sphagnum culture techniques. In Methods in Bryology, Proceedings of the Bryological Methods Workshop, Mainz, Germany, 1988; Hattori Botanical Laboratory: Nichinan, Japan, 1988; pp. 25–34. [Google Scholar]
- Bai, X.S.; Bu, Z.J.; Liu, W.J.; Shuayib, Y.; Xu, X.Y. A preliminary comparative study on rapid viability determination of peatland bryophyte spores. Guihaia 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).