Myrtle-Functionalized Nanofibers Modulate Vaginal Cell Population Behavior While Counteracting Microbial Proliferation
Abstract
:1. Introduction
2. Results
2.1. Identification and Quantification of Phenolic Compounds
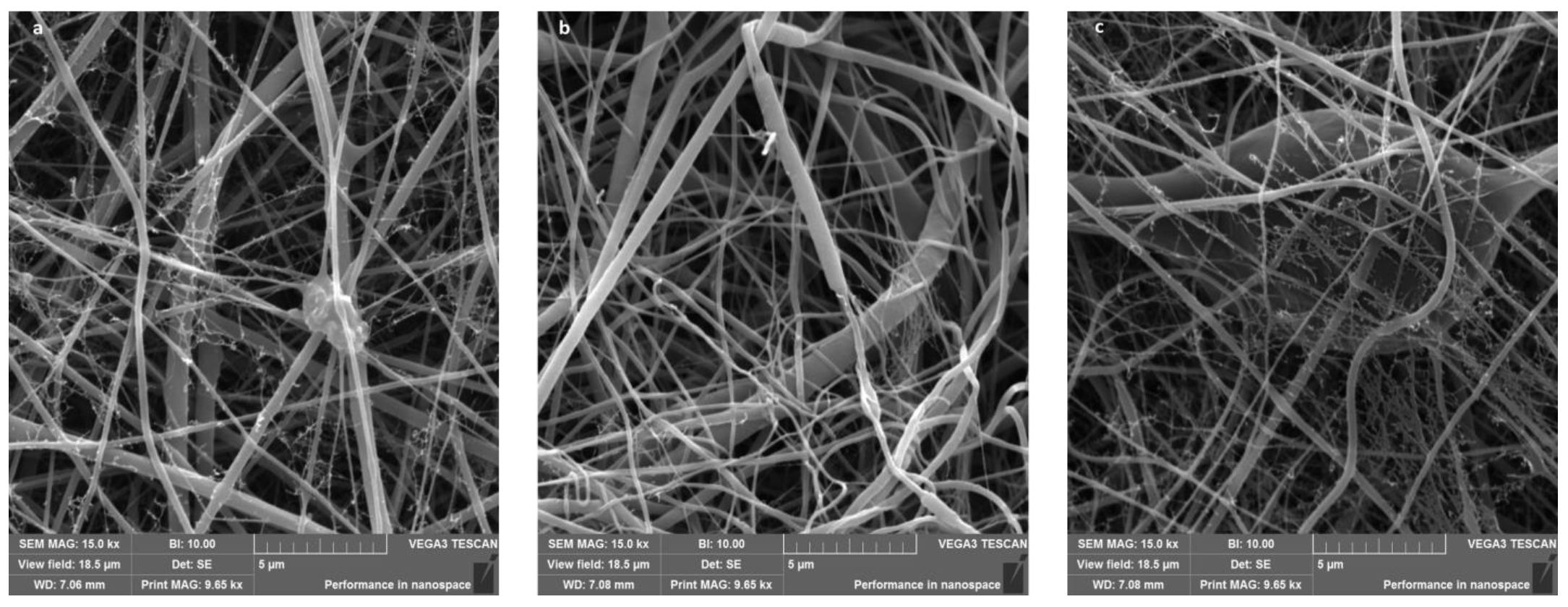
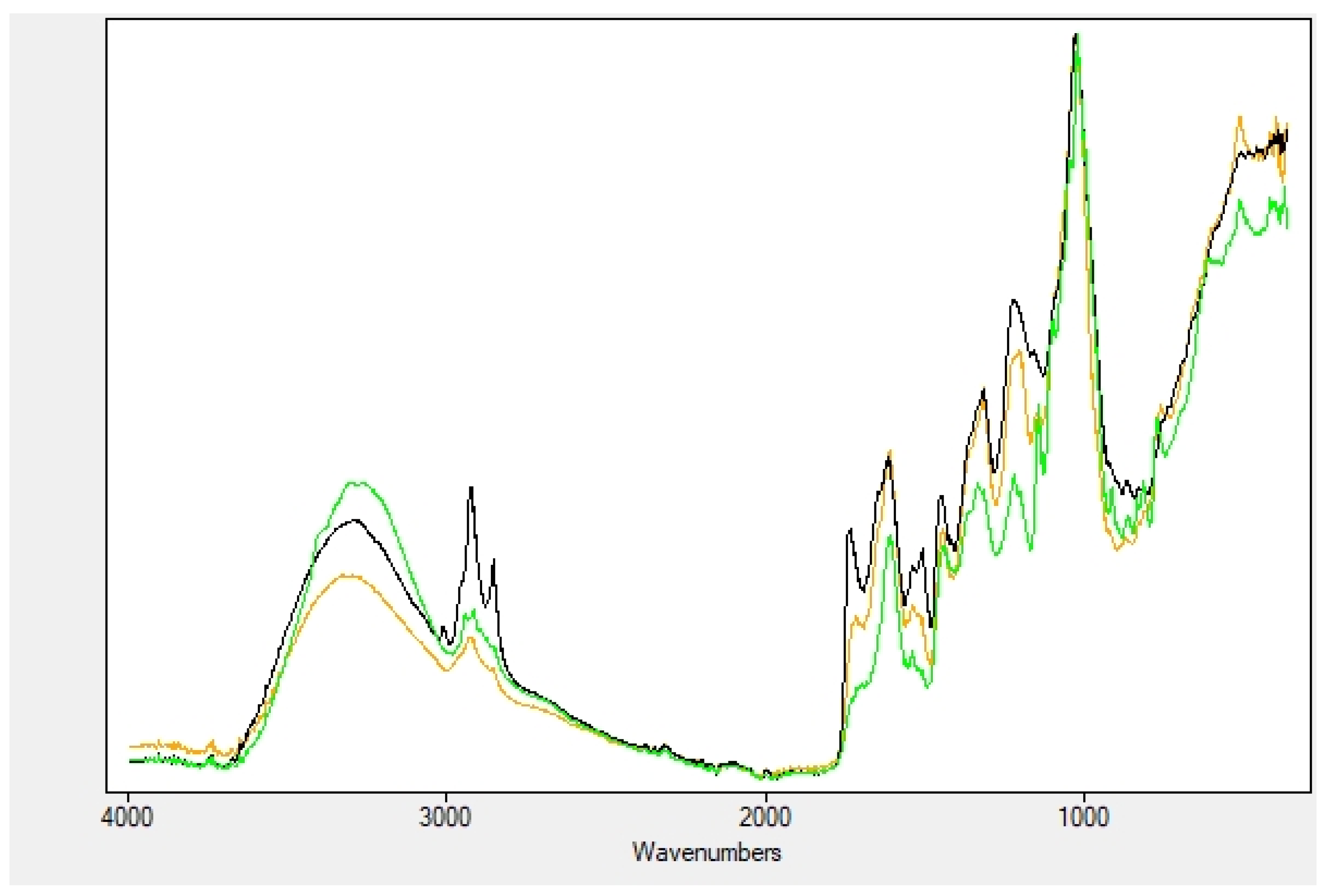
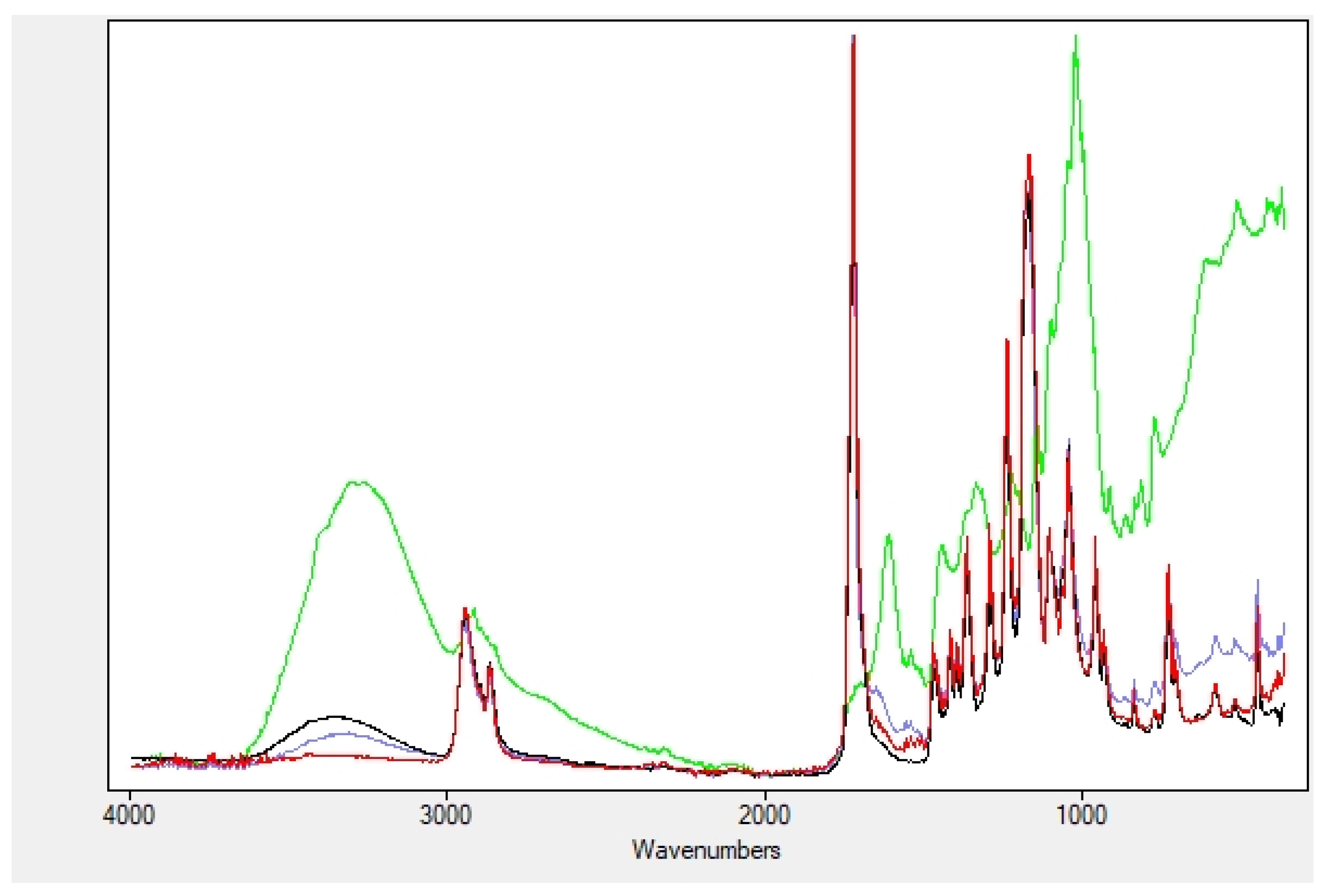
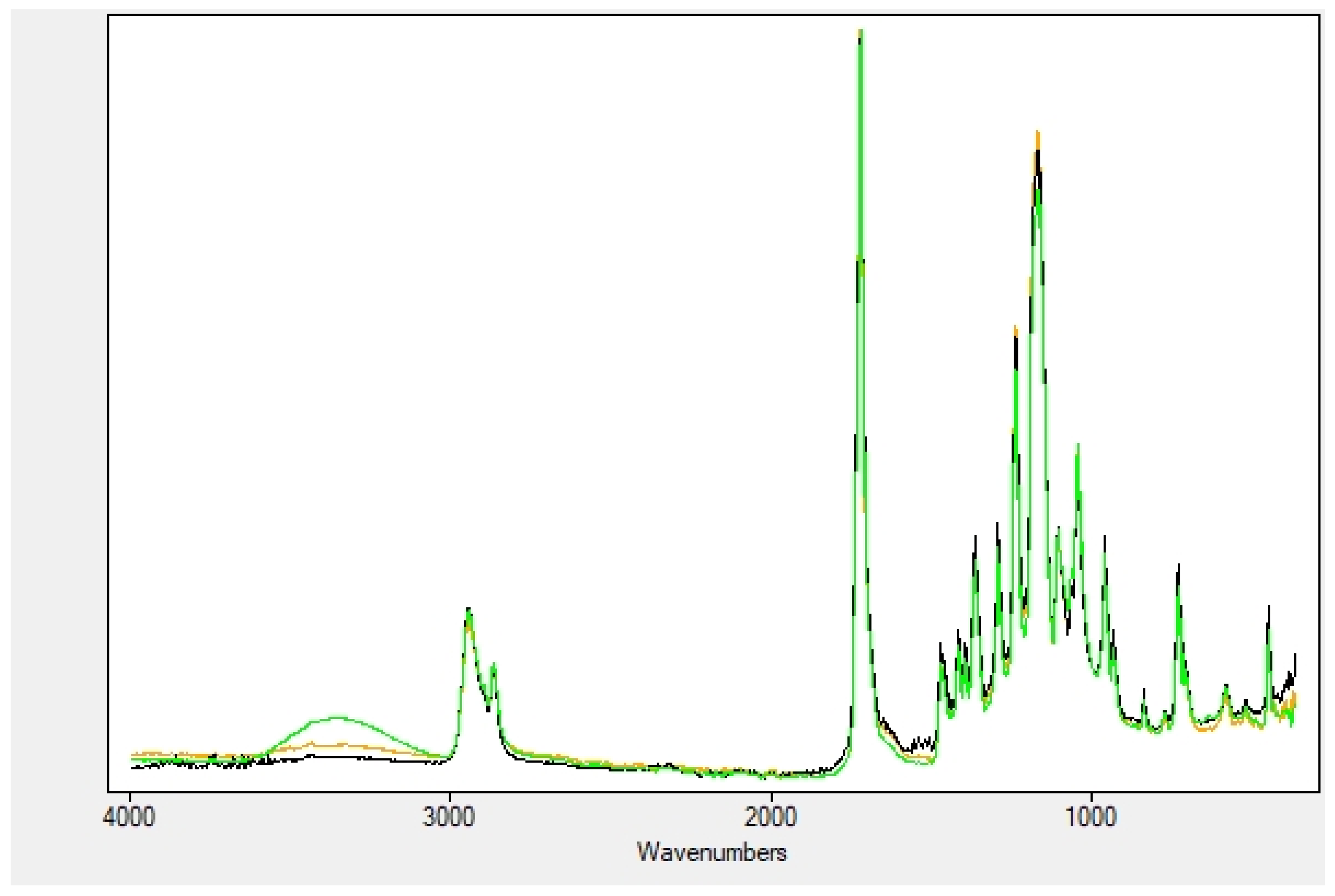
2.2. Nanofibers Characterization
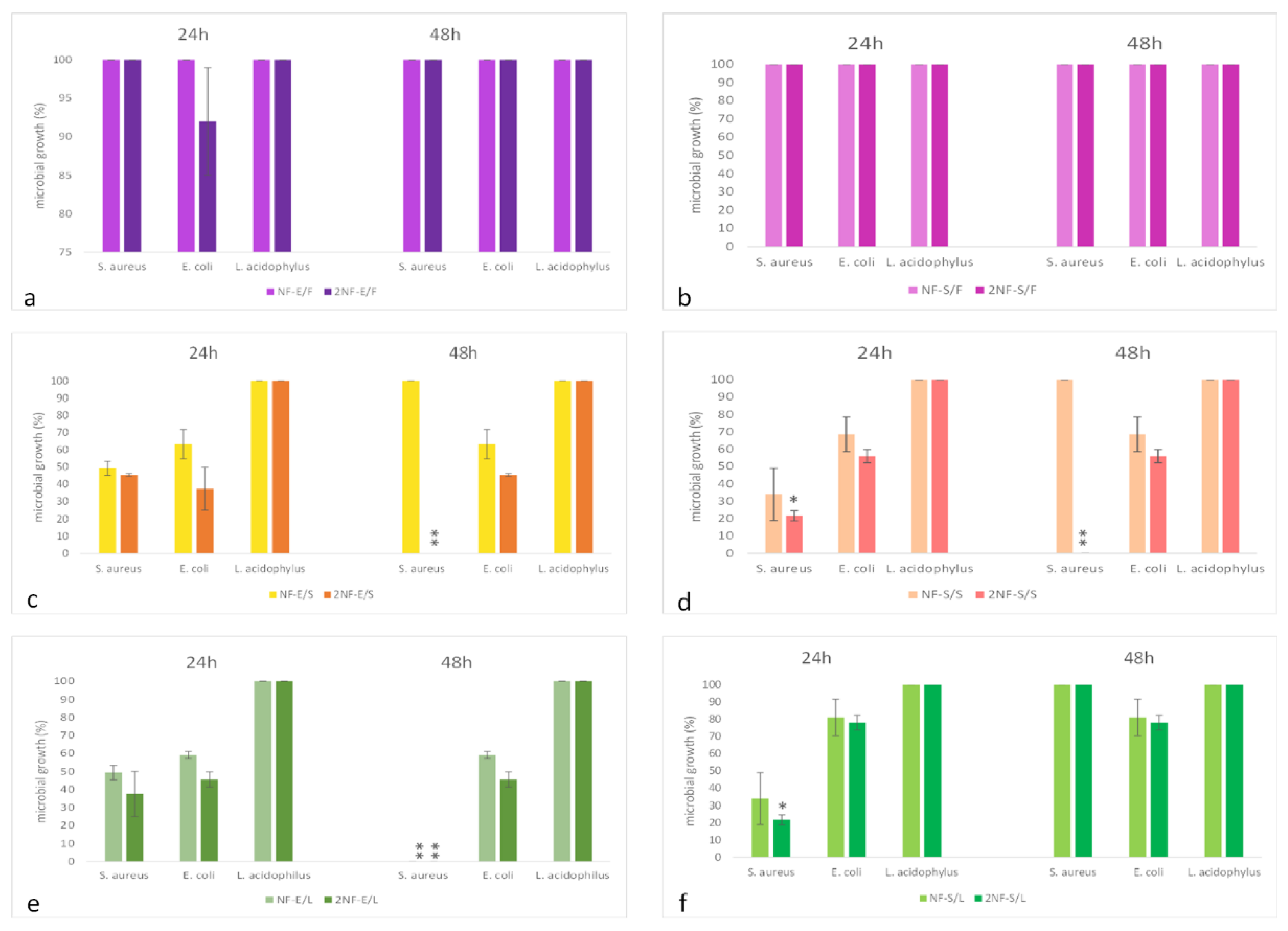
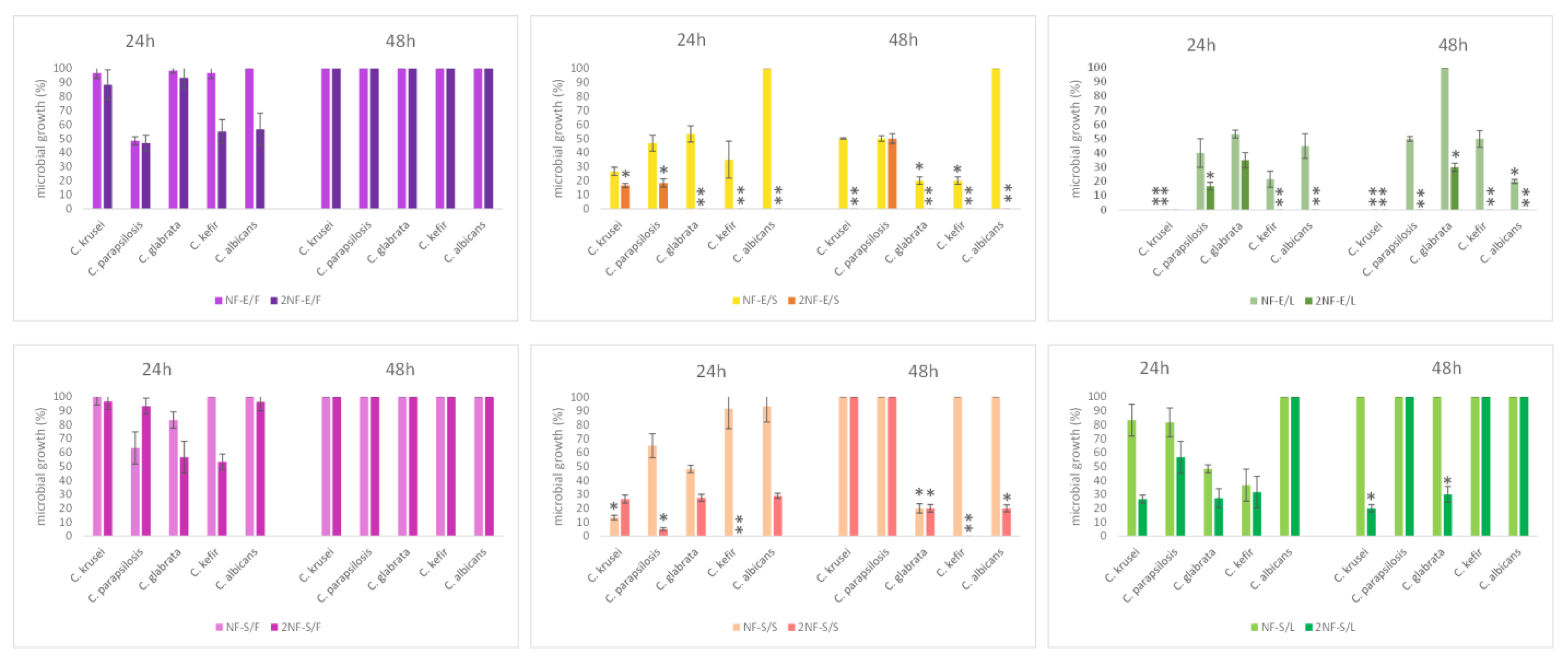
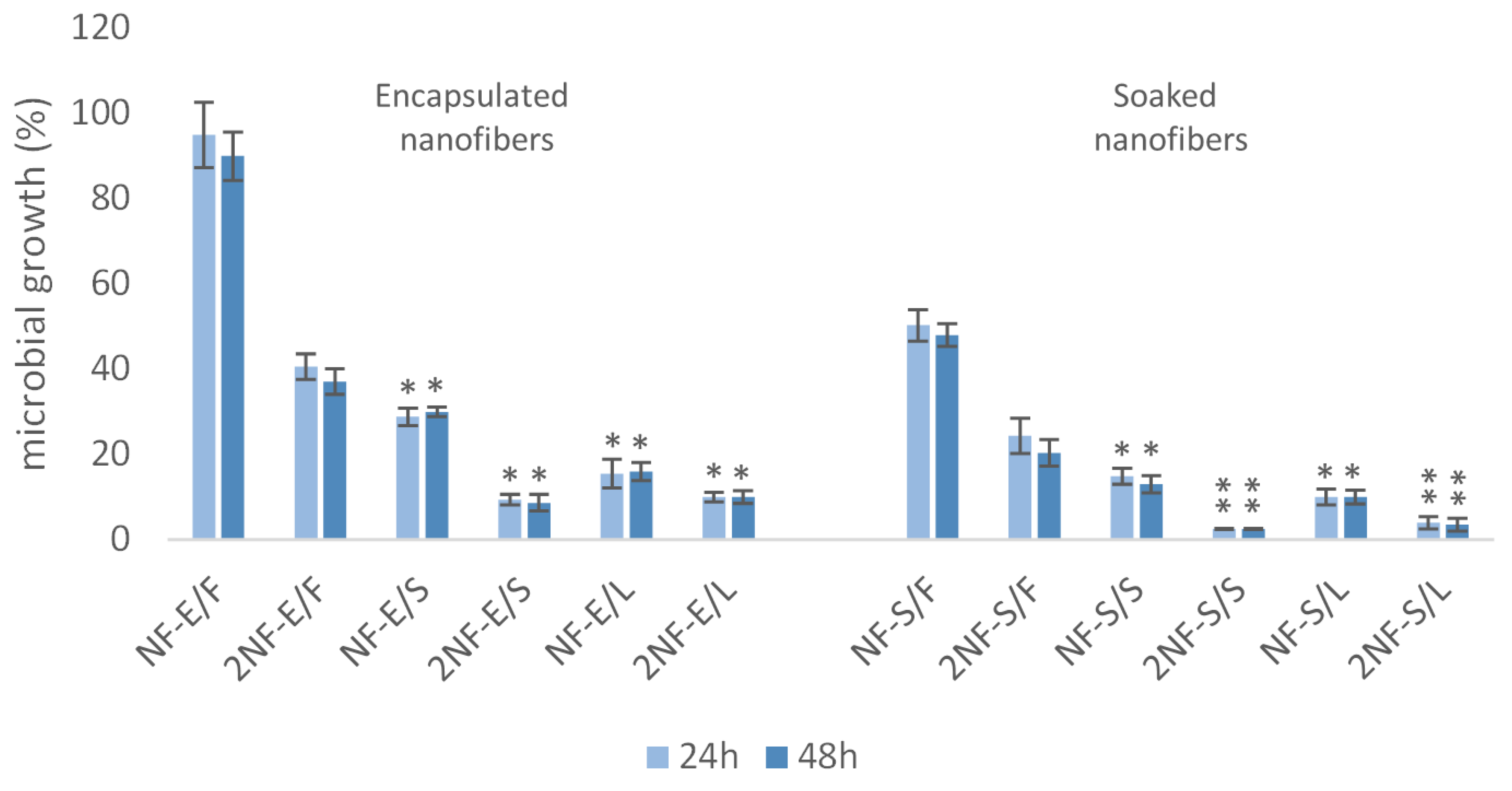
2.3. Nanofibers with Myrtle Extracts Exert Selective Antibacterial and Antifungal Action
2.4. Nanofibers with Myrtle Extracts Counteract Trichomonas vaginalis Strain Viability
2.5. Nanofibers with Myrtle Extracts Does Not Affect Eukaryotic Cell Viability
3. Discussion
4. Materials and Methods
4.1. Plant Material Preparation and Characterization
4.1.1. Chemicals
4.1.2. Plant Material
4.1.3. Preparation of Extracts
4.1.4. HPLC-DAD Analysis of Phenolic Compounds
4.2. Nanofibers Fabrication and Functionalization
4.2.1. Electrospinning of Nanofibers
4.2.2. Nanofibers Characterization
4.2.3. Preparation of Nanofibers Samples
4.3. Microorganisms Selected and Culturing Conditions
4.4. Human Cell Populations Choice and Culturing Conditions
4.5. In Vitro Antibacterial and Anticandidal Activity of Nanofibers with Myrtle Extracts
4.6. In Vitro Anti Trichomonas vaginalis Activity of Nanofibers with Myrtle Extracts
4.7. In Vitro Evaluation of Nanofibers Cytotoxicity on Human Cells HeLa, HFF-1 and SSCs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pendergrass, P.B.; Belovicz, M.W.; Reeves, C.A. Surface area of the human vagina as measured from vinyl polysiloxane casts. Gynecol. Obstet. Investig. 2003, 55, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Egedal, J.H.; Xie, G.; Packard, T.A.; Laustsen, A.; Neidleman, J.; Georgiou, K.; Pillai, S.K.; Greene, W.C.; Jakobsen, M.R.; Roan, N.R. Hyaluronic acid is a negative regulator of mucosal fibroblast-mediated enhancement of HIV infection. Mucosal Immunol. 2021, 14, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Syed, S.; Jamaluddin, M.F.; Colino-Sanguino, Y.; Gallego-Ortega, D.; Tanwar, P.S. Cell Lineage Tracing Identifies Hormone-Regulated and Wnt-Responsive Vaginal Epithelial Stem Cells. Cell Rep. 2020, 30, 1463–1477.e7. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Lee, H.-S.; Kim, M.E.; Lee, J.S.; Park, K. Identification and localization of epithelial progenitor cells in the vagina. Int. J. Impot. Res. 2018, 31, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Bellu, E.; Cruciani, S.; Garroni, G.; Balzano, F.; Satta, R.; Montesu, M.; Fadda, A.; Mulas, M.; Sarais, G.; Bandiera, P.; et al. Natural Compounds and PCL Nanofibers: A Novel Tool to Counteract Stem Cell Senescence. Cells 2021, 10, 1415. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Gajer, P.; Brotman, R.M.; Ravel, J. Asymptomatic Bacterial Vaginosis Is Associated with Depletion of Mature Superficial Cells Shed from the Vaginal Epithelium. Front. Cell. Infect. Microbiol. 2020, 10, 106. [Google Scholar] [CrossRef]
- Mårdh, P.-A.; Rodrigues, A.G.; Genç, M.; Novikova, N.; de Oliveira, J.M.; Guaschino, S. Facts and myths on recurrent vulvovaginal candidosis—a review on epidemiology, clinical manifestations, diagnosis, pathogenesis and therapy. Int. J. STD AIDS 2002, 13, 522–539. [Google Scholar] [CrossRef]
- Palmeira-De-Oliveira, R.; Duarte, P.; Palmeira-De-Oliveira, A.; Das Neves, J.; Amaral, M.H.; Breitenfeld, L.; Martinez-De-Oliveira, J. Women’s experiences, preferences and perceptions regarding vaginal products: Results from a cross-sectional web-based survey in Portugal. Eur. J. Contracept. Reprod. Health Care 2015, 20, 259–271. [Google Scholar] [CrossRef]
- Hashemi, H.; Varshosaz, J.; Fazeli, H.; Sharafi, S.M.; Mirhendi, H.; Chadeganipour, M.; Yousefi, H.; Manoochehri, K.; Chermahini, Z.A.; Jafarzadeh, L.; et al. Rapid differential diagnosis of vaginal infections using gold nanoparticles coated with specific antibodies. Med. Microbiol. Immunol. 2019, 208, 773–780. [Google Scholar] [CrossRef]
- Paladine, H.L.; Desai, U.A. Vaginitis: Diagnosis and treatment. Am. Fam. Physician 2018, 97, 321–329. [Google Scholar]
- Ma, X.; Wu, M.; Wang, C.; Li, H.; Fan, A.; Wang, Y.; Han, C.; Xue, F. The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: A narrative review. Reprod. Health 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G. Definition and classification of abnormal vaginal flora. Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 355–373. [Google Scholar] [CrossRef] [PubMed]
- Tansarli, G.S.; Kostaras, E.K.; Athanasiou, S.; Falagas, M.E. Prevalence and treatment of aerobic vaginitis among non-pregnant women: Evaluation of the evidence for an underestimated clinical entity. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 34–43. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Bukar, M.; Mohammed, Y.; Mohammed, B.; Yahaya, M.; Audu, B.M.; Ibrahim, H.M. Prevalence of vaginal candidiasis among pregnant women with abnormal vaginal discharge in Maiduguri. Niger. J. Med. 2013, 22, 138–142. [Google Scholar]
- Diaz, N.; Lico, C.; Capodicasa, C.; Baschieri, S.; Dessì, D.; Benvenuto, E.; Fiori, P.L.; Rappelli, P. Production and Functional Characterization of a Recombinant Predicted Pore-Forming Protein (TVSAPLIP12) of Trichomonas vaginalis in Nicotiana benthamiana Plants. Front. Cell. Infect. Microbiol. 2020, 10, 581066. [Google Scholar] [CrossRef]
- Kissinger, P. Trichomonas vaginalis: A review of epidemiologic, clinical and treatment issues. BMC Infect. Dis. 2015, 15, 307. [Google Scholar] [CrossRef] [Green Version]
- Diaz, N.; Dessi, D.; Dessole, S.; Fiori, P.L.; Rappelli, P. Rapid detection of coinfections by Trichomonas vaginalis, Mycoplasma hominis, and Ureaplasma urealyticum by a new multiplex polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 2010, 67, 30–36. [Google Scholar] [CrossRef]
- Smith, J.D.; Garber, G.E. Trichomonas vaginalis Infection Induces Vaginal CD4+T-Cell Infiltration in a Mouse Model: A Vaccine Strategy to Reduce Vaginal Infection and HIV Transmission. J. Infect. Dis. 2015, 212, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Palmeira-De-Oliveira, R.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef]
- Brocklehurst, P.; Gordon, A.; Heatley, E.; Milan, S.J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst. Rev. 2013, 1, CD000262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogavac, M.; Karaman, M.; Janjušević, L.; Sudji, J.; Radovanović, B.; Novaković, Z.; Simeunović, J.; Bozin, B. Alternative treatment of vaginal infections—In vitro antimicrobial and toxic effects of Coriandrum sativum L. and Thymus vulgaris L. essential oils. J. Appl. Microbiol. 2015, 119, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bachmann, L.H.; Chan, P.A.; Johnston, C.M.; Muzny, C.A.; Park, I.; Reno, H.; Zenilman, J.M.; Bolan, G.A. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR. Recomm. Rep. 2021, 70, 1–187. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Bogavac, M.; Radovanović, B.; Sudji, J.; Tešanović, K.; Janjušević, L. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. Appl. Microbiol. 2017, 122, 1177–1185. [Google Scholar] [CrossRef]
- Martínez-Tomé, M.; Jiménez, A.M.; Ruggieri, S.; Frega, N.; Strabbioli, R.; Murcia, M.A. Antioxidant Properties of Mediterranean Spices Compared with Common Food Additives. J. Food Prot. 2001, 64, 1412–1419. [Google Scholar] [CrossRef]
- Bellu, E.; Garroni, G.; Cruciani, S.; Balzano, F.; Serra, D.; Satta, R.; Montesu, M.A.; Fadda, A.; Mulas, M.; Sarais, G.; et al. Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells 2020, 9, 2530. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M. Antimicrobial Polymer-Based Hydrogels for the Intravaginal Therapies—Engineering Considerations. Pharmaceutics 2021, 13, 1393. [Google Scholar] [CrossRef]
- das Neves, J.; Nunes, R.; Machado, A.; Sarmento, B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliv. Rev. 2014, 92, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Franco, A.M.; Tocci, N.; Guella, G.; Dell’Agli, M.; Sangiovanni, E.; Perenzoni, D.; Vrhovsek, U.; Mattivi, F.; Manca, G. Myrtle Seeds (Myrtus communis L.) as a Rich Source of the Bioactive Ellagitannins Oenothein B and Eugeniflorin D2. ACS Omega 2019, 4, 15966–15974. [Google Scholar] [CrossRef] [Green Version]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical Composition of Myrtle (Myrtus communis L.) Berries Essential Oils as Observed in a Collection of Genotypes. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef] [Green Version]
- Safaeijavan, R.; Soleimani, M.; Divsalar, A.; Eidi, A.; Ardeshirylajimi, A. Biological behavior study of gelatin coated PCL nanofiberous electrospun scaffolds using fibroblasts. Arch. Adv. Biosci. 2013, 5. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Choudhury, H.; Abdul-Aziz, A.; Bhattamisra, S.K.; Gorain, B.; Carine, T.; Wee Toong, T.; Yi, N.J.; Win Yi, L. Promising Drug Delivery Approaches to Treat Microbial Infections in the Vagina: A Recent Update. Polymers 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Takada, K.; Komine-Aizawa, S.; Kuramochi, T.; Ito, S.; Trinh, Q.D.; Pham, N.T.K.; Sasano, M.; Hayakawa, S. Lactobacillus crispatusaccelerates re-epithelialization in vaginal epithelial cell line MS74. Am. J. Reprod. Immunol. 2018, 80, e13027. [Google Scholar] [CrossRef] [PubMed]
- Berendika, M.; Drozdek, S.D.; Odeh, D.; Oršolić, N.; Dragičević, P.; Sokolović, M.; Garofulić, I.E.; Đikić, D.; Jurčević, I.L. Beneficial Effects of Laurel (Laurus nobilis L.) and Myrtle (Myrtus communis L.) Extract on Rat Health. Molecules 2022, 27, 581. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.; Seyfan, A.; Jalalinezhad, S.; Nasery, F. Antibacterial effect of myrtus communis hydro-alcoholic extract on pathogenic bacteria. Zahedan J. Res. Med. Sci. 2013, 15, 19–24. [Google Scholar]
- Koohsari, H.; Ghaemi, E.; Sheshpoli, M.S.; Jahedi, M.; Zahiri, M. The investigation of antibacterial activity of selected native plants from North of Iran. J. Med. Life 2015, 8, 38–42. [Google Scholar]
- Le, N.T.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, D.; Sanna, G.; Carta, A.; Rappelli, P.; Diaz, N.; et al. Biological Activities of Essential Oils from Leaves of Paramignya trimera (Oliv.) Guillaum and Limnocitrus littoralis (Miq.) Swingle. Antibiotics 2020, 9, 207. [Google Scholar] [CrossRef]
- Donadu, M.G.; Le, N.T.; Ho, D.V.; Doan, T.Q.; Le, A.T.; Raal, A.; Usai, M.; Marchetti, M.; Sanna, G.; Madeddu, S.; et al. Phytochemical Compositions and Biological Activities of Essential Oils from the Leaves, Rhizomes and Whole Plant of Hornstedtia bella Škorničk. Antibiotics 2020, 9, 334. [Google Scholar] [CrossRef]
- Dessì, D.; Rappelli, P.; Diaz, N.; Cappuccinelli, P.; Fiori, P.L. Mycoplasma hominis and Trichomonas vaginalis: A unique case of symbiotic relationship between two obligate human parasites. Front. Biosci. 2006, 11, 2028–2034. [Google Scholar] [CrossRef] [Green Version]
- Margarita, V.; Fiori, P.L.; Rappelli, P. Impact of Symbiosis Between Trichomonas vaginalis and Mycoplasma hominis on Vaginal Dysbiosis: A Mini Review. Front. Cell. Infect. Microbiol. 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, V.; Knezevic, P. Antimicrobial and antioxidative activity of extracts and essential oils of Myrtus communis L. Microbiol. Res. 2014, 169, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian cell behavior on hydrophobic substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Fadda, A.; Sarais, G.; Lai, C.; Sale, L.; Mulas, M. Control of postharvest diseases caused by Penicillium spp. with myrtle leaf phenolic extracts: In vitro and in vivo study on mandarin fruit during storage. J. Sci. Food Agric. 2021, 101, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Sarais, G.; D’Urso, G.; Lai, C.; Pirisi, F.M.; Pizza, C.; Montoro, P. Targeted and untargeted mass spectrometric approaches in discrimination betweenMyrtus communiscultivars from Sardinia region. J. Mass Spectrom. 2016, 51, 704–715. [Google Scholar] [CrossRef]
- Bellu, E.; Garroni, G.; Balzano, F.; Satta, R.; Montesu, M.; Kralovic, M.; Fedacko, J.; Cruciani, S.; Maioli, M. Isolating stem cells from skin: Designing a novel highly efficient non-enzymatic approach. Physiol. Res. 2019, 68, S385–S388. [Google Scholar] [CrossRef]
- Reker, D.; Blum, S.M.; Steiger, C.; Anger, K.E.; Sommer, J.M.; Fanikos, J.; Traverso, G. “Inactive” ingredients in oral medications. Sci. Transl. Med. 2019, 11, eaau6753. [Google Scholar] [CrossRef]


| Compound | λmax Uv-Vis (nm) | Concentration (mg/kgDW ± SD; n = 3) | ||
|---|---|---|---|---|
| Peel + Pulp | Seeds | Leaves | ||
| Phenolic acids Gallic acid Ellagic acid Hydrolysable tannins ($) Flavonols myricetin-3-O-galactoside myricetin-3-O-rhamnoside quercetin-3-O-glucoside quercetin-3-O-rhamnoside quercetin 3-O-galactoside vitexin Anthocyanins cyanidin 3-O-glucoside petunidin 3-O-glucoside peonidin 3-O-glucoside malvidin 3-O-glucoside | 280 360 280+360 360 360 360 360 360 360 520 520 520 520 | 353.4 ± 22.2 450.3 ± 20.5 8612.0 ± 416.6 - - - - - - 2.8 ± 0.2 5.6 ± 0.6 2.5 ± 0.3 13.1 ± 0.4 | 797.0 ± 60.5 1050.3 ± 112.8 25,016.2 ± 359.8 - 49.7 ± 1.4 - 160.6 ± 14.5 31.0 ± 2.2 45.3 ± 3.1 - - - - | 1199.3 ± 70.4 - 21,858.3 ± 1099.3 1926.4 ± 6.2 3902.9 ± 117.7 104.1 ± 4.6 192.0 ± 7.2 85.9 ± 3.0 280.0 ± 13.5 - - - - |
| Nanofibers | Average Diameter [nm] | Contact Angle |
|---|---|---|
| PCL | 320 ± 190 | 129.2 ± 2.8° |
| PCL and Gelatin | 129 ± 197 | 33.2 ± 3.5° |
| PCL and Gelatin encapsulated with leaves extract | 104 ± 88 | 109.9 ± 6° |
| PCL and Gelatin encapsulated with seeds extract | 147 ± 156 | 122.8 ± 5.7° |
| PCL and Gelatin encapsulated with fruit extract | 166 ± 177 | 117.3 ± 5.8° |
| OH Stretching | Amide I | Amide II | |
|---|---|---|---|
| Extracts | 3300 | ||
| NF | 1648 | 1537 | |
| Soaked NF | 3360 | 1644 | 1530 |
| Encapsulated NF | 3290 | 1657 | 1545 |
| Name of the Treatment | Treatment |
|---|---|
| NF-E/F | One 6 mm disc of nanofibers made of PCL with Gelatin encapsulated with myrtle fruits extract |
| 2NF-E/F | Two 6 mm discs of nanofibers made of PCL with Gelatin encapsulated with myrtle fruits extract |
| NF-E/S | One 6 mm disc of nanofibers made of PCL with Gelatin encapsulated with myrtle seeds extract |
| 2NF-E/S | Two 6 mm discs of nanofibers made of PCL with Gelatin encapsulated with myrtle seeds extract |
| NF-E/L | One 6 mm disc of nanofibers made of PCL with Gelatin encapsulated with myrtle leaves extract |
| 2NF-E/L | Two 6 mm discs of nanofibers made of PCL with Gelatin encapsulated with myrtle leaves extract |
| NF-S/F | One 6 mm disc of nanofibers made of PCL with Gelatin soaked with myrtle fruit extract |
| 2NF-S/F | Two 6 mm discs of nanofibers made of PCL with Gelatin soaked with myrtle fruit extract |
| NF-S/S | One 6 mm disc of nanofibers made of PCL with Gelatin soaked with myrtle seeds extract |
| 2NF-S/S | Two 6 mm discs of nanofibers made of PCL with Gelatin soaked with myrtle seeds extract |
| NF-S/L | One 6 mm disc of nanofibers made of PCL with Gelatin soaked with myrtle leaves extract |
| 2NF-S/L | Two 6 mm discs of nanofibers made of PCL with Gelatin soaked with myrtle leaves extract |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellu, E.; Diaz, N.; Kralovič, M.; Divin, R.; Sarais, G.; Fadda, A.; Satta, R.; Montesu, M.A.; Medici, S.; Brunetti, A.; et al. Myrtle-Functionalized Nanofibers Modulate Vaginal Cell Population Behavior While Counteracting Microbial Proliferation. Plants 2022, 11, 1577. https://doi.org/10.3390/plants11121577
Bellu E, Diaz N, Kralovič M, Divin R, Sarais G, Fadda A, Satta R, Montesu MA, Medici S, Brunetti A, et al. Myrtle-Functionalized Nanofibers Modulate Vaginal Cell Population Behavior While Counteracting Microbial Proliferation. Plants. 2022; 11(12):1577. https://doi.org/10.3390/plants11121577
Chicago/Turabian StyleBellu, Emanuela, Nicia Diaz, Martin Kralovič, Radek Divin, Giorgia Sarais, Angela Fadda, Rosanna Satta, Maria Antonia Montesu, Serenella Medici, Antonio Brunetti, and et al. 2022. "Myrtle-Functionalized Nanofibers Modulate Vaginal Cell Population Behavior While Counteracting Microbial Proliferation" Plants 11, no. 12: 1577. https://doi.org/10.3390/plants11121577
APA StyleBellu, E., Diaz, N., Kralovič, M., Divin, R., Sarais, G., Fadda, A., Satta, R., Montesu, M. A., Medici, S., Brunetti, A., Barcessat, A. R. P., Jarošíková, T., Rulc, J., Amler, E., Margarita, V., Rappelli, P., & Maioli, M. (2022). Myrtle-Functionalized Nanofibers Modulate Vaginal Cell Population Behavior While Counteracting Microbial Proliferation. Plants, 11(12), 1577. https://doi.org/10.3390/plants11121577







