Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity
Abstract
:1. Introduction
2. Results
2.1. Morpho-Agronomic Variables
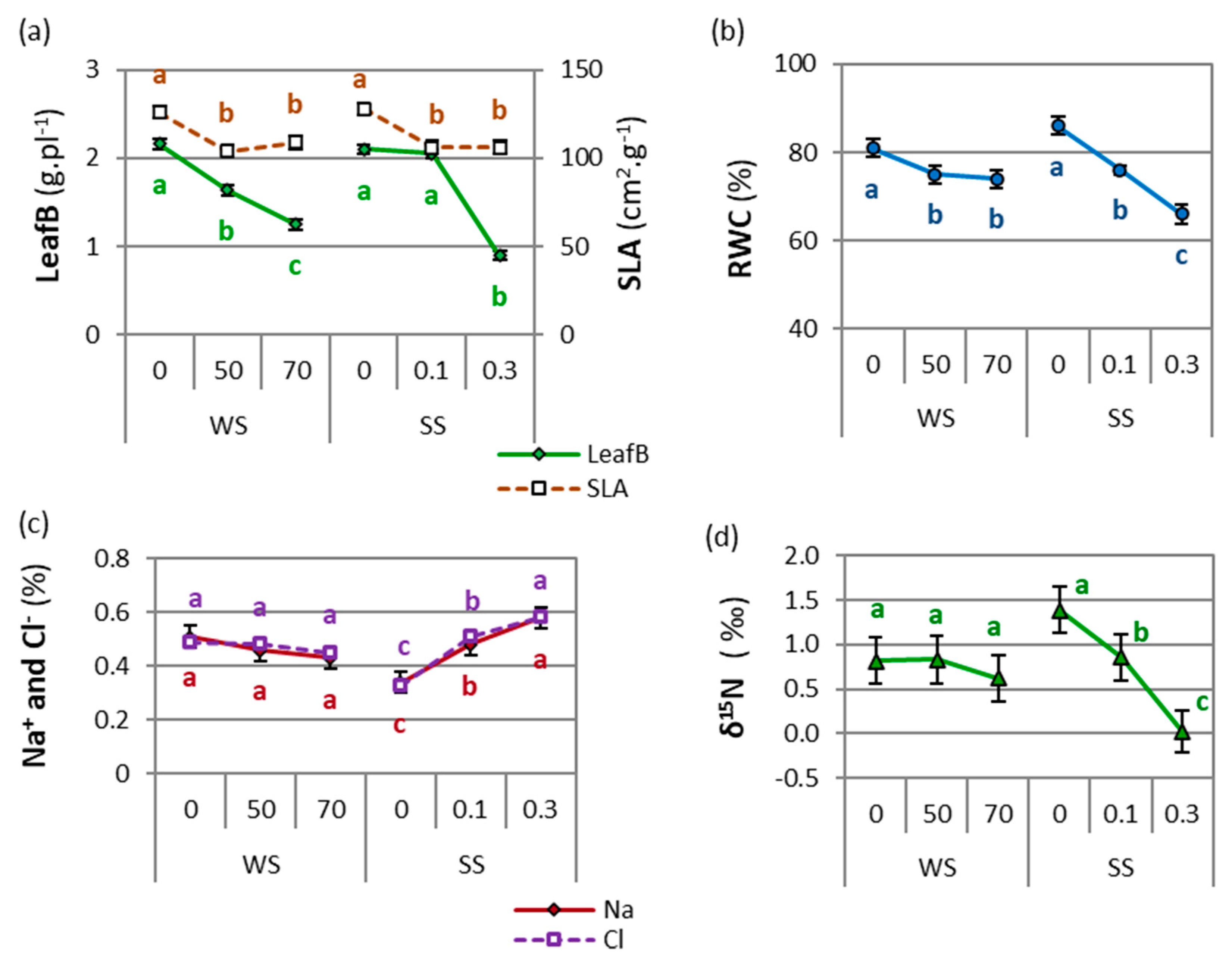
2.1.1. Drought and Salinity Effects
2.1.2. Accession Effects
2.2. Physiological and Isotopic Variables
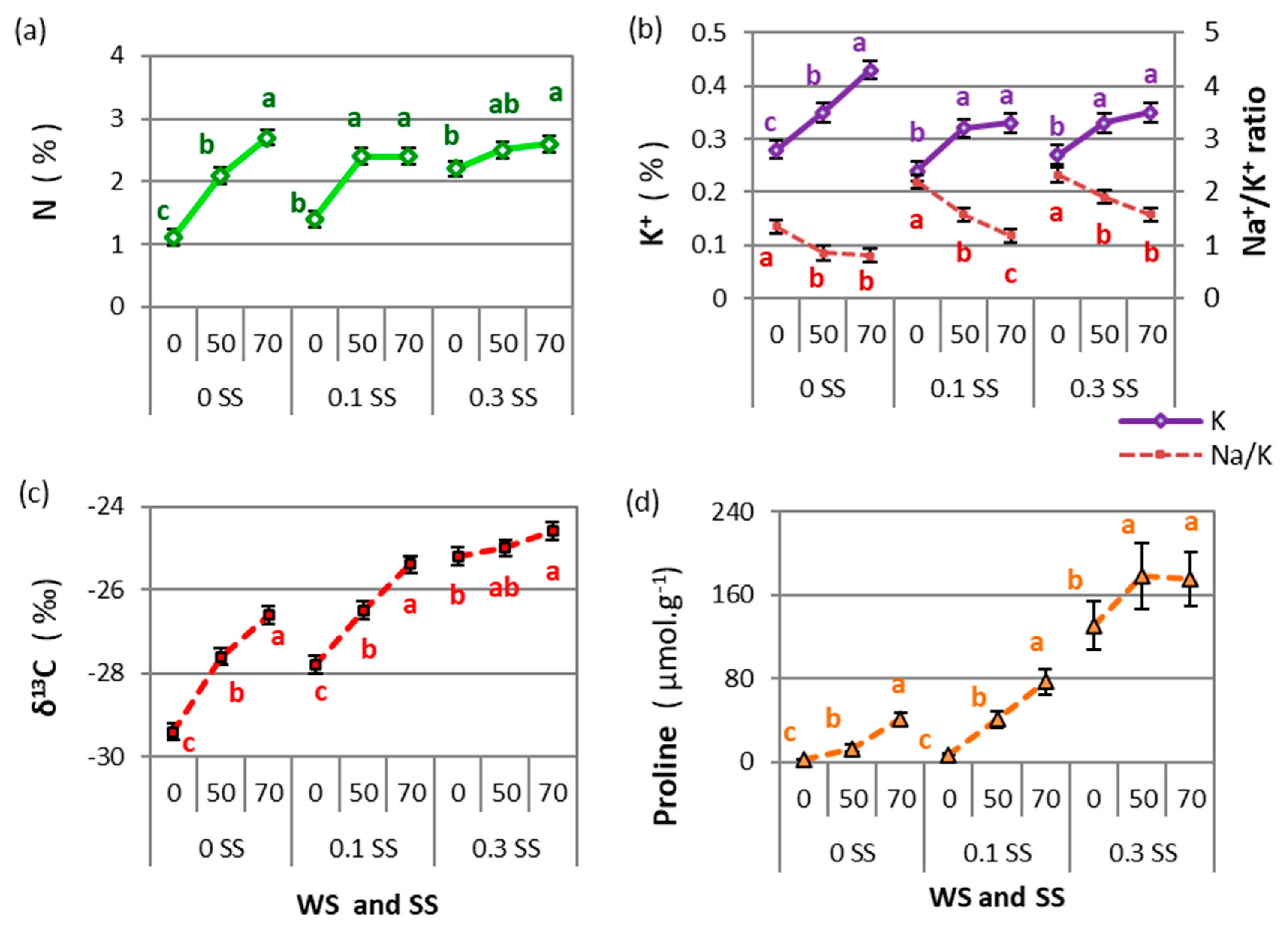
2.2.1. Drought and Salinity Effects
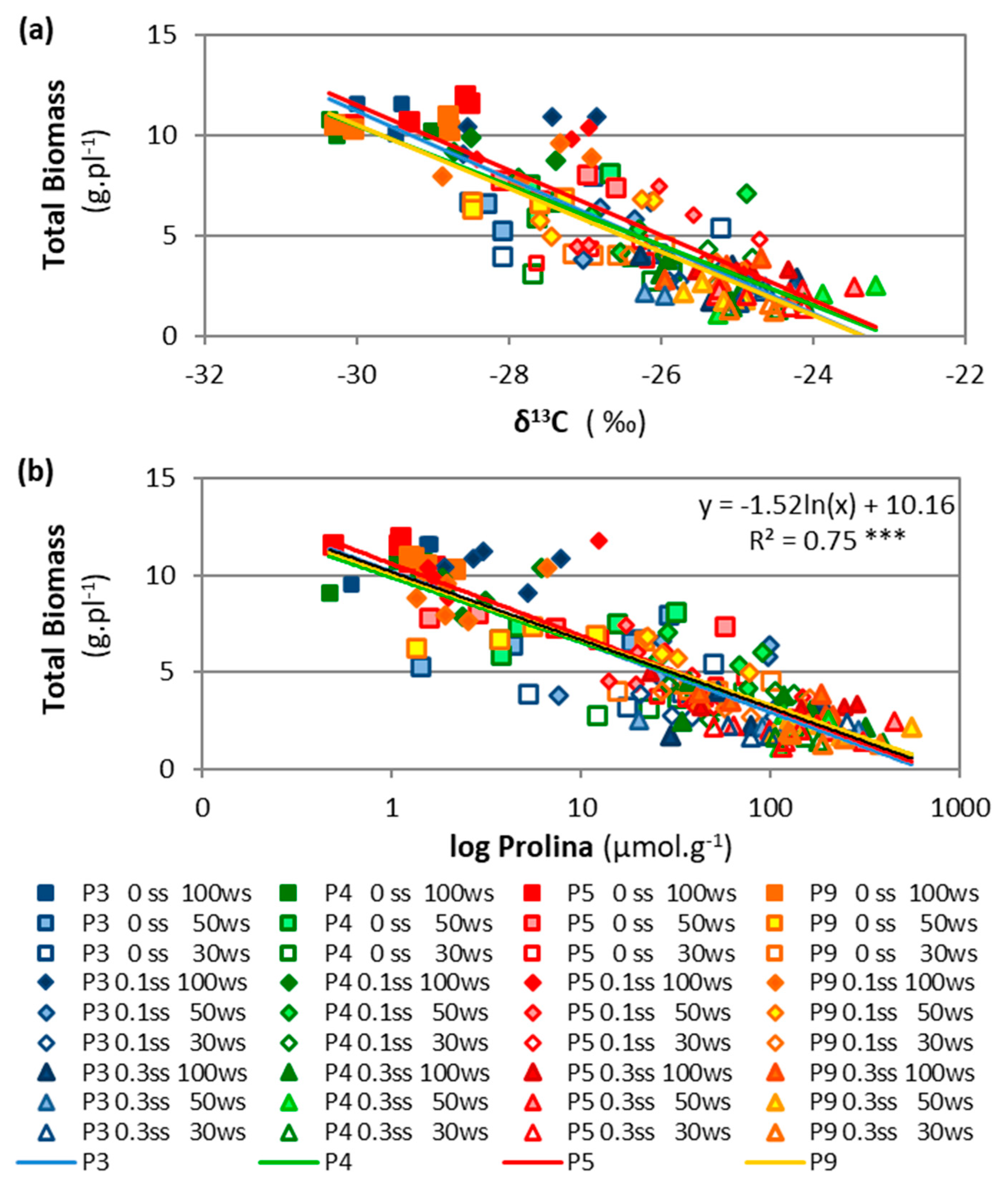
2.2.2. Accession Effects
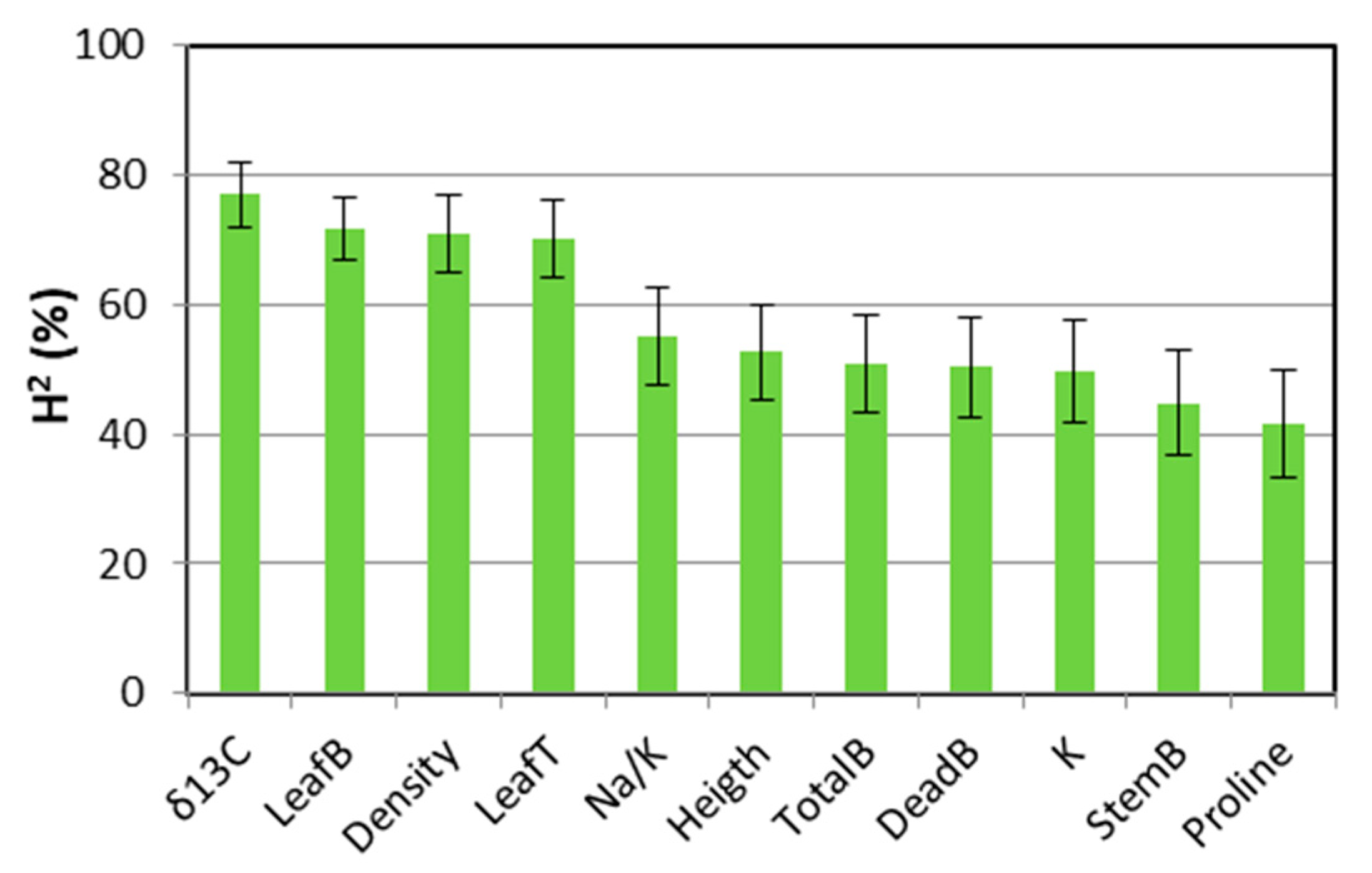
2.3. Broad-Sense Heritability
3. Discussion
3.1. Drought and Salinity in Tall Wheatgrass
3.2. Accession Variability and Heritability
4. Materials and Methods
4.1. Origin of the Germplasm and Experimental Conditions
4.2. Morpho-Agronomic Variables
4.3. Physiological and Isotopic Variables
4.4. Soil Electrical Conductivity
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Billah, M.; Aktar, S.; Brestic, M.; Zivcak, M.; Khaldun, A.B.M.; Uddin, M.S.; Bagum, S.A.; Yang, X.; Skalicky, M.; Mehari, T.G.; et al. Progressive genomic approaches to explore drought- and salt-induced oxidative stress responses in plants under changing climate. Plants 2021, 10, 1910. [Google Scholar] [CrossRef] [PubMed]
- Redden, R. New Approaches for Crop Genetic Adaptation to the Abiotic Stresses Predicted with Climate Change. Agronomy 2013, 3, 419–432. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Ruggeri, R.; Rossini, F.; Forte, P.; Cuccurullo, A.; Bitti, A. Harnessing genetic diversity of wild gene pools to enhance wheat crop production and sustainability: Challenges and opportunities. Diversity 2017, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Peng, Z.; Wang, M.; Li, F.; Lv, H.; Li, C.; Xia, G. A Proteomic Study of the Response to Salinity and Drought Stress in an Introgression Strain of Bread Wheat. Mol. Cell. Proteom. 2009, 8, 2676–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef] [Green Version]
- McGuire, P.E.; Dvoák, J. High salt-tolerance potential in wheatgrass. Crop Sci. 1981, 21, 702–705. [Google Scholar] [CrossRef]
- Mass, E.V. Salt tolerance of plants. Appl. Agric. Res. 1986, 1, 12–26. [Google Scholar]
- Borrajo, C.I.; Sanchez-Moreiras, A.M.; Reigosa, M.J. Morpho-physiological, biochemical and isotopic response of tall wheatgrass populations to salt stress. J. Agron. Crop Sci. 2021, 207, 236–248. [Google Scholar] [CrossRef]
- Falasca, S.L.; Miranda, C.; Alvarez, S.P. Agro-ecological zoning for tall wheatgrass (Thinopyrum ponticum) as a potential energy and forage crop in salt-affected and dry lands of Argentina. Arch. Crop Sci. 2017, 1, 10–19. [Google Scholar] [CrossRef]
- Ciria, C.S.; Barro, R.; Sanz, M.; Ciria, P. Long-term yield and quality performance of perennial energy grasses (Agropyron spp.) on marginal land. Agronomy 2020, 10, 1051. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.I.; Raman, A.; Hodgkins, D.S.; Mitchell, D.; Nicol, H.I. Physiological response and ion accumulation in two grasses one legume, andone saltbush under soil water and salinity stress. Ecohydrology 2015, 8, 1547–1559. [Google Scholar] [CrossRef]
- Vogel, K.P.; Moore, J. Forage yield and quality of tall wheatgrass accessions in the USDA germplasm collection. Crop Sci. 1998, 38, 509–512. [Google Scholar] [CrossRef]
- Borrajo, C.I.; Sanchez-Moreiras, A.M.; Reigosa, M.J. Morpho-physiological responses of tall wheatgrass populations to different levels of water stress. PLoS ONE 2018, 13, e0209281. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.C. Testing salt tolerance variability among tall wheatgrass lines. Agron. J. 1978, 70, 719–722. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, L.; Mao, P.C.; Tian, X.X. Salt tolerance in two tall wheatgrass species is associated with selective capacity for K+ over Na+. Acta Physiol. Plant. 2015, 37, 1708. [Google Scholar] [CrossRef]
- Otondo, J.; Borrajo, C.I.; Bandera, R. Forrajeras templadas y subtropicales en suelos afectados por sales de regiones húmedas y subhúmedas de la Argentina. In Suelos Afectados Por Sales Con Énfasis En La Argentina; Bandera, R., Ed.; Orientación Gráfica: Buenos Aires, Argentina, 2019; pp. 139–167. [Google Scholar]
- Bennett, S.J.; Barrett-Lennard, E.G.; Colmer, T.D. Salinity and waterlogging as constraints to saltland pasture production: A review. Agric. Ecosyst. Environ. 2009, 129, 349–360. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Flexas, J.; Niinemets, U.; Gallé, A.; Barbour, M.M.; Centritto, M.; Diaz-Espejo, A.; Douthe, C.; Galmés, J.; Ribas-Carbo, M.; Rodriguez, P.L.; et al. Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth. Res. 2013, 117, 45–59. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Sade, N.; Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Taleisnik, E.; Rodríguez, A.A.; Bustos, D.; Erdei, L.; Ortega, L.; Senn, M.E. Leaf expansion in grasses under salt stress. J. Plant Physiol. 2009, 166, 1123–1140. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A Vital Regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Gazanchian, A.; Hajheidari, M.; Sima, N.K.; Salekdeh, G.H. Proteome response of Elymus elongatum to severe water stress and recovery. J. Exp. Bot. 2007, 58, 291–300. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Giannakoula, A.; Dordas, C.; Skiada, V.; Papadopoulou, K. Carbon assimilation, isotope discrimination, proline and lipid peroxidation contribution to barley (Hordeum vulgare) salinity tolerance. Plants 2021, 10, 299. [Google Scholar] [CrossRef]
- Yousfi, S.; Serret, M.D.; Márquez, A.J.; Voltas, J.; Araus, J.L. Combined use of δ13C, δ18O and δ15N tracks nitrogen metabolism and genotypic adaptation of durum wheat to salinity and water deficit. New Phytol. 2012, 194, 230–244. [Google Scholar] [CrossRef]
- Sadaqat Shah, S.; Li, Z.; Yan, H.; Shi, L.; Zhou, B. Comparative study of the effects of salinity on growth, gas exchange, n accumulation and stable isotope signatures of forage oat (Avena sativa L.) genotypes. Plants 2020, 9, 1025. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Condon, A.G.; Richards, R.A.; Farquhar, G.D. Selection for reduced carbon-isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 2002, 42, 739–745. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Cabrera-Bosquet, L.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T. Comparative performance of δ13C, δ18O and δ15N for phenotyping durum wheat adaptation to a dryland environment. Funct. Plant Biol. 2013, 40, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liang, Z.S.; Xu, X.; Li, S.H.; Jing, J.H.; Monneveux, P. Monneveux E Relationships between carbon isotope discrimination and leaf morphophysiological traits in spring-planted spring wheat under drought and salinity stress in Northern China. Aust. J. Agric. Res. 2008, 59, 941–949. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A. Broad sense heritability and genotype x environment interaction for carbon isotope discrimination in field-grown wheat. Aust. J. Agric. Res. 1992, 43, 921–934. [Google Scholar] [CrossRef]
- Alonso, S.I.; Borrajo, C.I. Colecta y evaluación inicial de poblaciones de Agropiro alargado naturalizadas en la región pampeana Argentina. In Avances De Investigaciones En Recursos Genéticos En El Cono Sur; Clausen, A., Condón, F., Berretta, A., Eds.; Procisur: Montevideo, Uruguay, 2007; Volume 2, pp. 9–22. [Google Scholar]
- Borrajo, C.I. Generación Y Expansión De Los Órganos Foliares De Agropiro Alargado En Función Del Material Genético Y La Disponibilidad De Nitrógeno. Master’s Thesis, Universidad Nacional de Mar del Plata, Balcarce, Argentina, 1998; p. 75. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer Science+Business Media LCC: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Tambussi, E.; Bort, J.; Araus, J. Water use efficiency in C3 cereals under Mediterranean conditions: A review of physiological aspects. Ann. Appl. Biol. 2007, 150, 307–321. [Google Scholar] [CrossRef]
- Johnson, R.C.; Bassett, L.M. Carbon isotope discrimination and water use efficiency in four cool-season grasses. Crop Sci. 1991, 31, 157–162. [Google Scholar] [CrossRef]
- Blum, A. Towards a conceptual ABA ideotype in plant breeding for water limited environments. Funct. Plant Biol. 2015, 42, 502–513. [Google Scholar] [CrossRef]
- Ortega, L.; Taleisnik, E. Elongation growth in leaf blades of Chloris gayana under saline conditions. J. Plant Physiol. 2003, 167, 517–522. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Durand, J.L.; Gastal, F. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 2010, 30, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M. Nitrogen containing compounds and adaptation of plants to salinity stress. Biol. Plant. 2003, 43, 491–500. [Google Scholar] [CrossRef]
- Reigosa, M.J. Handbook of Plant Ecophysiology Techniques; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; Available online: https://link.springer.com/content/pdf/10.1007/0-306-48057-3.pdf (accessed on 20 January 2020).
- Rasband, W.S. ImageJ 1.50i [Program File]; National Institutes of Health: Bethesda, MD, USA, 1997–2016. Available online: http://imagej.nih.gov/ij/ (accessed on 12 July 2020).
- SAS. The SAS System for Windows. Release 9.1; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Singh, B.D. Plant Breeding Principles and Methods; Kalyani Publishers: Ludhiana, India, 2001. [Google Scholar]

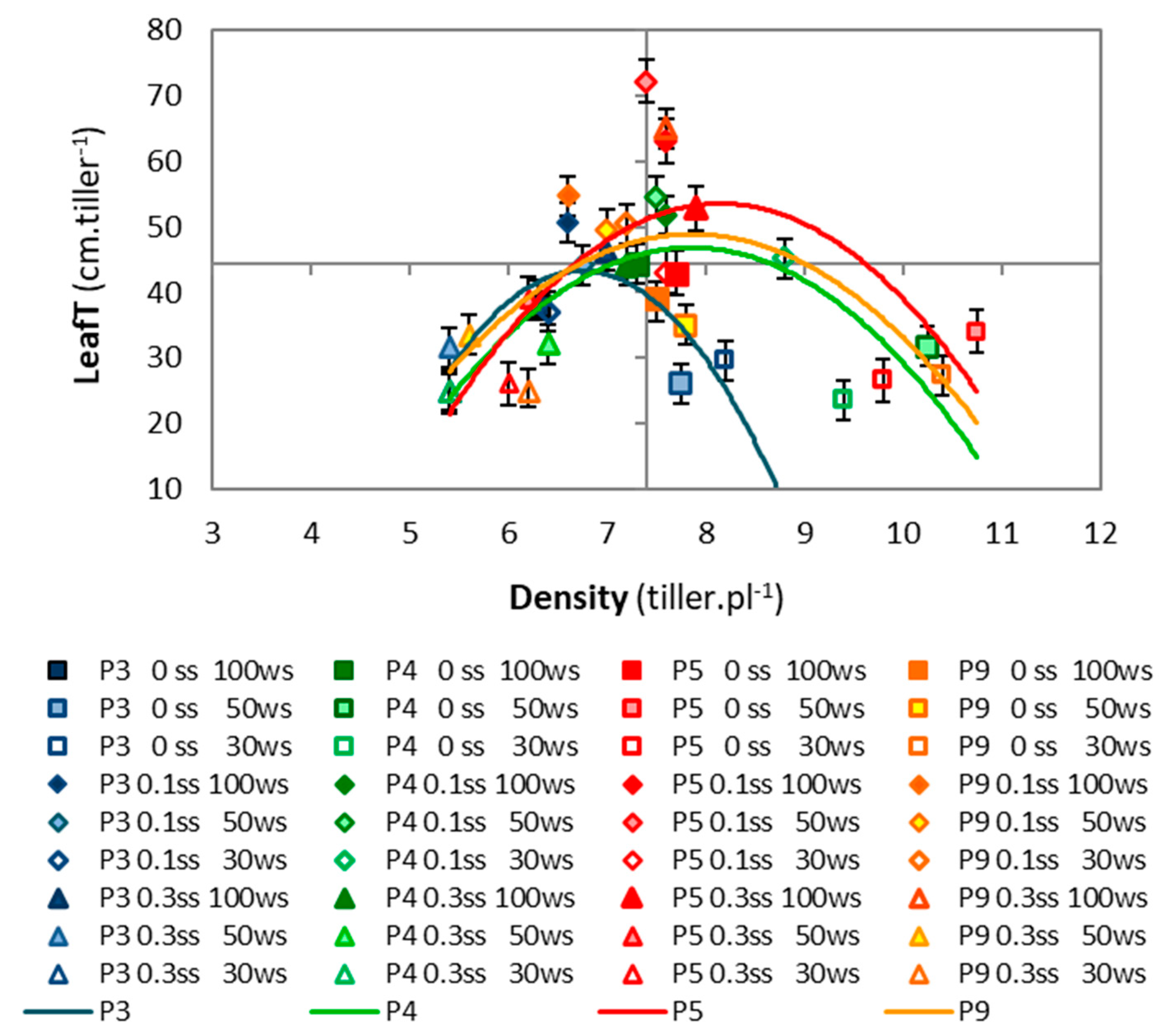


| Density | LeafT | LeafB | Dead% | StemB | TotalB | Height | ETA | WUE | Spike% | Na+/K+ | δ13C | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession | (tiller.pl−1) | (cm.tiller−1) | (g.pl−1) | (%) | (g.pl−1) | (g.pl−1) | (cm.pl−1) | (mLH2O pot−1) | mg.mL−1H2O | 0 SS | 0.1 SS | 0.3 SS | (‰) | |
| P3 | 6.8 b | 36.4 b | 1.57 b | 19.5 ab | 2.93 a | 5.27ab | 66.9 a | 1893 ab | 2.66 b | 53 a | 42 b | 8 a | 1.44 b | −26.55 b |
| P4 | 7.7 a | 39.2 ab | 1.64 b | 20.0 a | 2.61 b | 5.09 b | 63.2 ab | 1849 b | 2.63 b | 30 b | 46 b | 10 a | 1.70 a | −26.48 ab |
| P5 | 7.9 a | 44.1 a | 1.91 a | 18.7 ab | 2.73 ab | 5.53a | 61.8 b | 1861 b | 2.83 a | 40 b | 34 c | 8 a | 1.40 b | −26.21 a |
| P9 | 7.3 ab | 42.2 a | 1.62 b | 16.8 b | 2.91 a | 5.21b | 64.4 ab | 1904 a | 2.62 b | 40 b | 55 a | 9 a | 1.58 ab | −26.60 b |
| ee | 0.3 | 2.5 | 0.07 | 1.4 | 0.12 | 0.13 | 1.6 | 42 | 0.06 | 4.1 | 4.0 | 3.6 | 0.09 | 0.16 |
| Accession | P3 | P4 | P5 | P9 |
|---|---|---|---|---|
| Latitude, longitude | 38°30′ S, 58°45′ W | 39°24′ S, 65°36′ W | 39°24′ S, 65°36′ W | 38°44′ S, 62°33′ W |
| Nearest town, province | Necochea, Buenos Aires | Lamarque, Río Negro | Lamarque, Río Negro | Bahía Blanca, Buenos Aires |
| Köppen § | Cfb | BSk | BSk | Cfa |
| Climate | Temperate oceanic | Semiarid | Semiarid | Temperate transitional |
| Precipitation | 840 mm year−1 | 266 mm year−1 | 266 mm year−1 | 583 mm year−1 |
| Great Group Soil | Argiudolls. | Torrifluvents. | Torrifluvents. | Haplustolls. |
| Soil type, pH # | Non-saline/non-alkaline soil, pH ≅ 7.0 | Saline–alkaline soil, pH: 9.0 | Non-saline/non-alkaline soil, pH: 7.5 | Saline alkaline soil, pH: 9.5 |
| Environment | Roadside grassland | Grassland, with Distichlis spicata | Roadside grassland of irrigated fields | Natural grasslands, with Distichlis spicata |
| Collection BAL † | Nu + Alo 338 | CIB 118 | CIB 117 | CIB 114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrajo, C.I.; Sánchez-Moreiras, A.M.; Reigosa, M.J. Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity. Plants 2022, 11, 1548. https://doi.org/10.3390/plants11121548
Borrajo CI, Sánchez-Moreiras AM, Reigosa MJ. Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity. Plants. 2022; 11(12):1548. https://doi.org/10.3390/plants11121548
Chicago/Turabian StyleBorrajo, Celina I., Adela M. Sánchez-Moreiras, and Manuel J. Reigosa. 2022. "Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity" Plants 11, no. 12: 1548. https://doi.org/10.3390/plants11121548
APA StyleBorrajo, C. I., Sánchez-Moreiras, A. M., & Reigosa, M. J. (2022). Ecophysiological Responses of Tall Wheatgrass Germplasm to Drought and Salinity. Plants, 11(12), 1548. https://doi.org/10.3390/plants11121548








