The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms
Abstract
:1. Introduction
2. Results
2.1. Contents of O2−, H2O2 Content, MDA, and EL Value
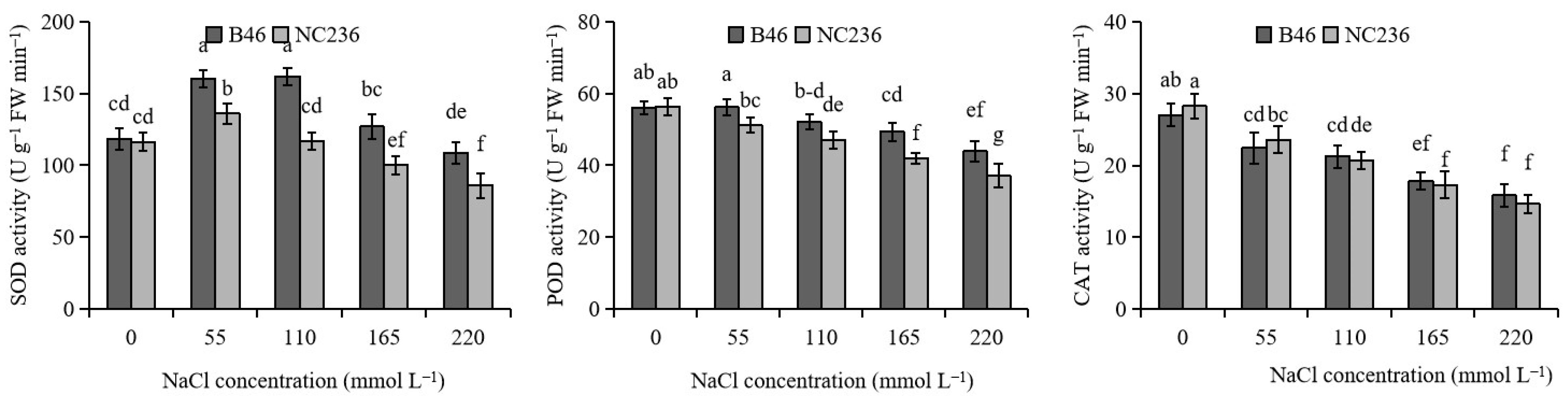
2.2. Activities of SOD, POD, and CAT
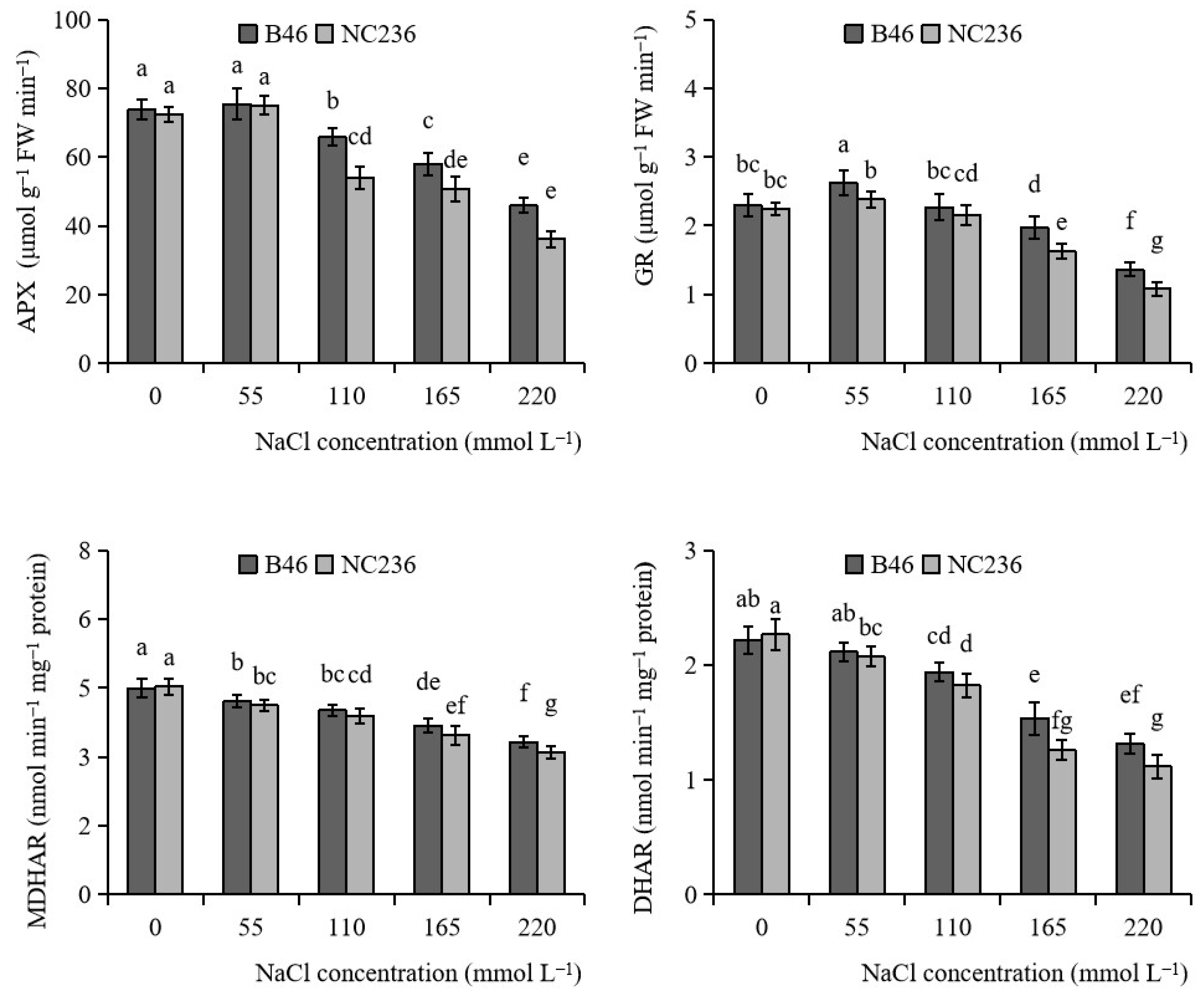
2.3. Enzyme Activities of AsA-GSH Cycle
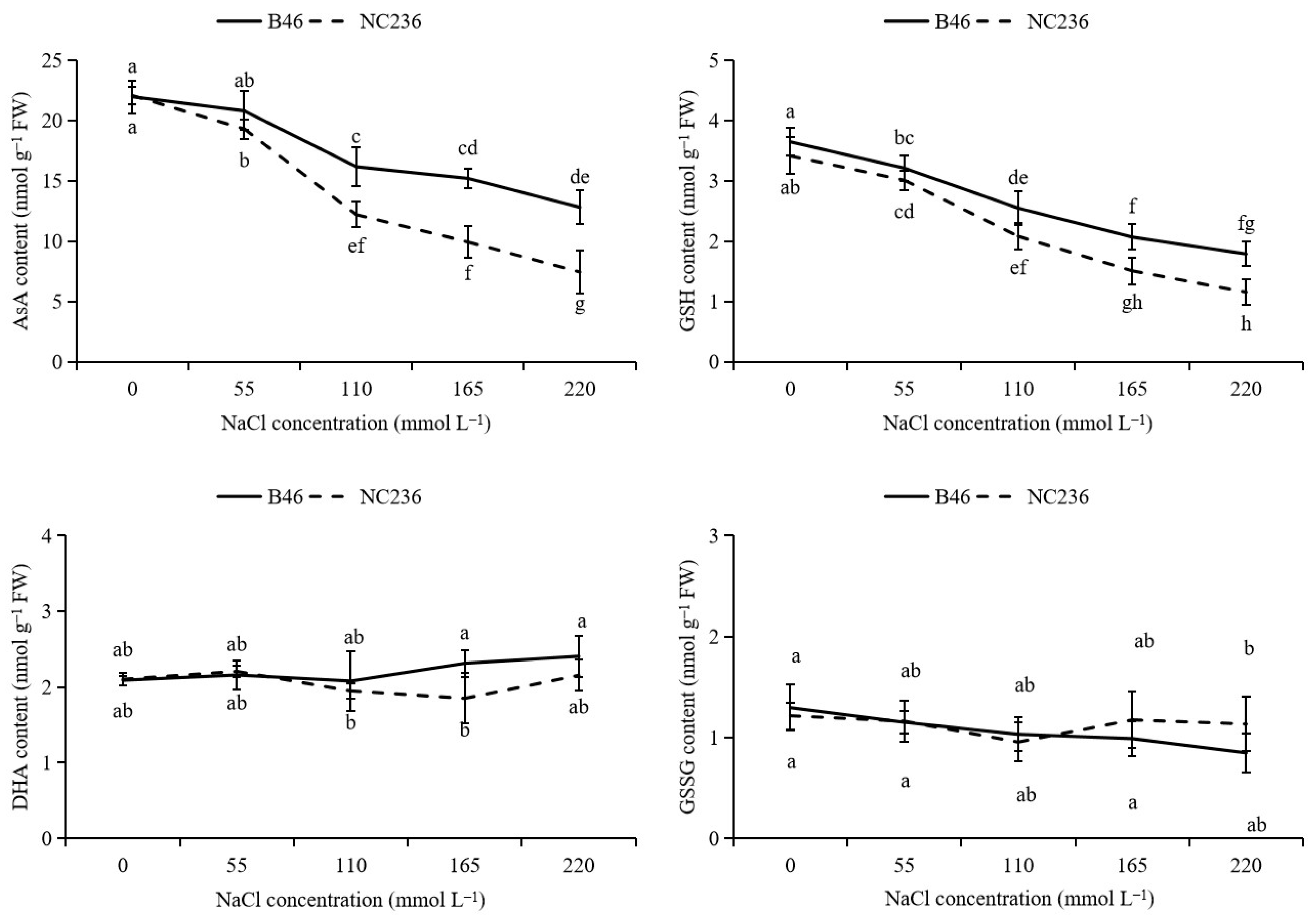
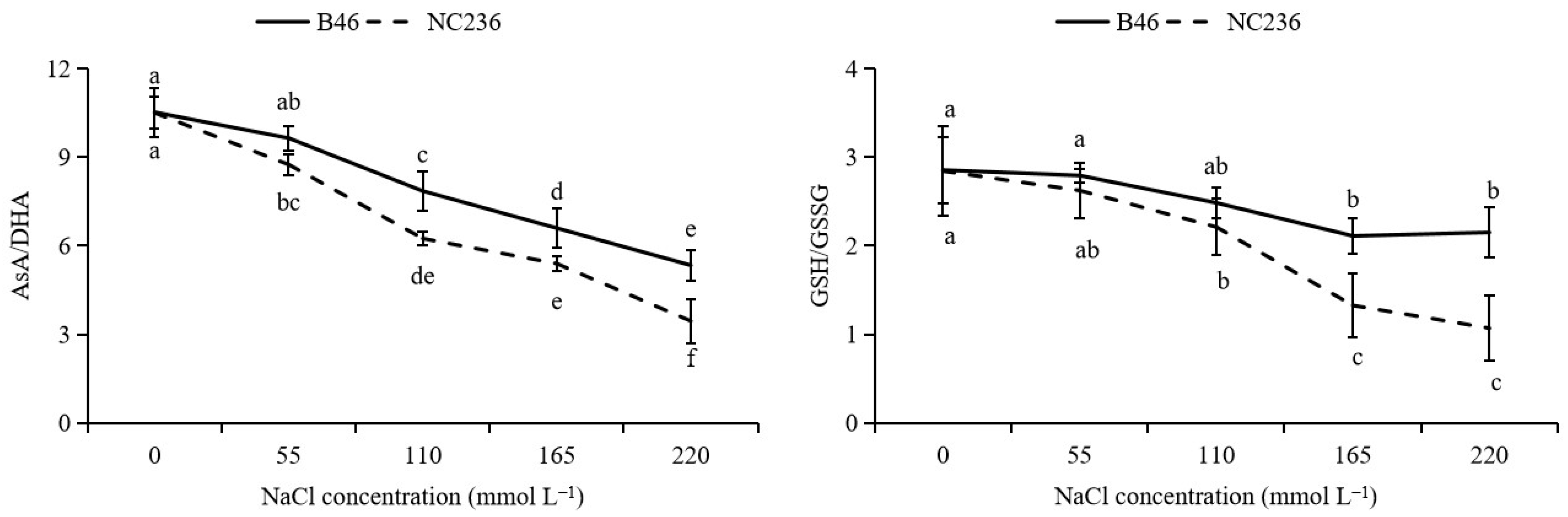
2.4. Contents of AsA, DHA, GSH, GSSG, Ratio of AsA/DHA and GSH/GSSG
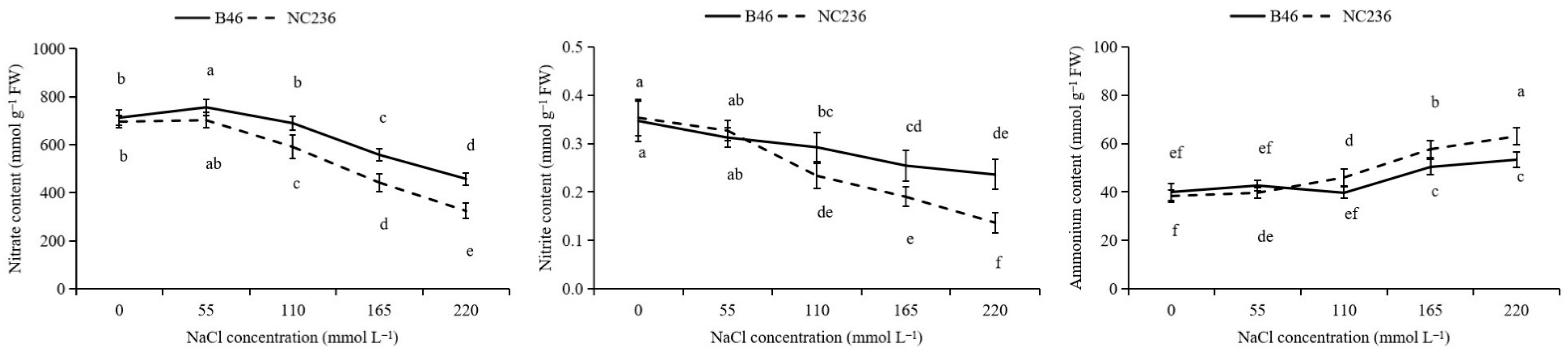
2.5. Contents of NO3−, NO2−, and NH4+
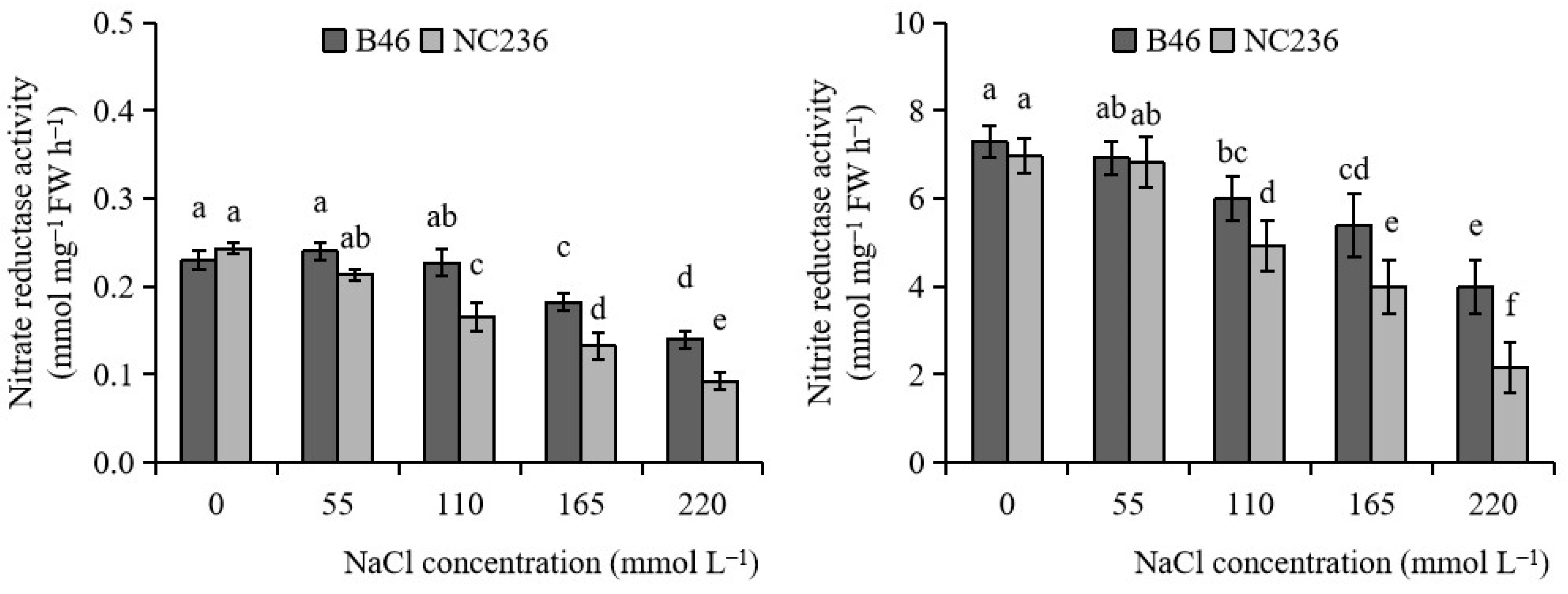
2.6. Activities of NR and NiR
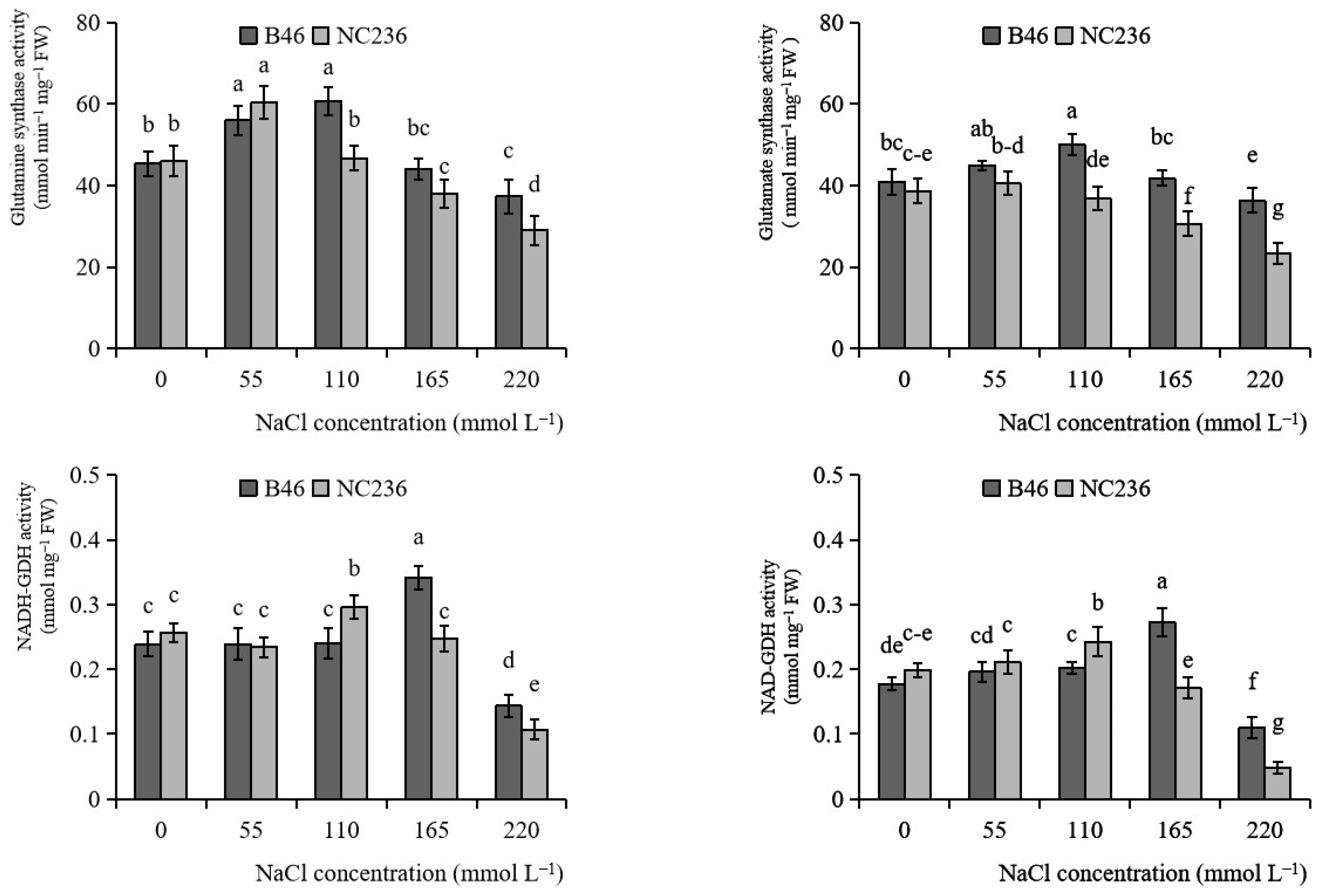
2.7. Activities of GS, GOGAT, NADH-GDH, and NAD-GDH
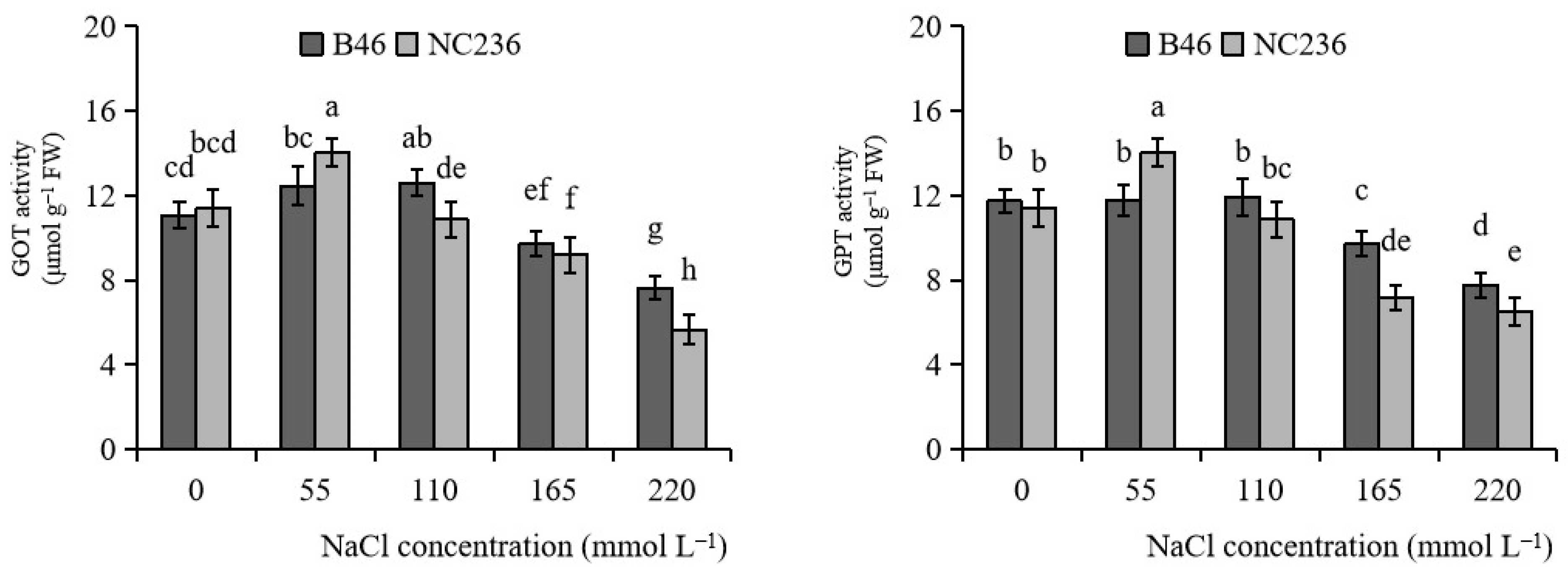
2.8. Activities of GOT and GPT
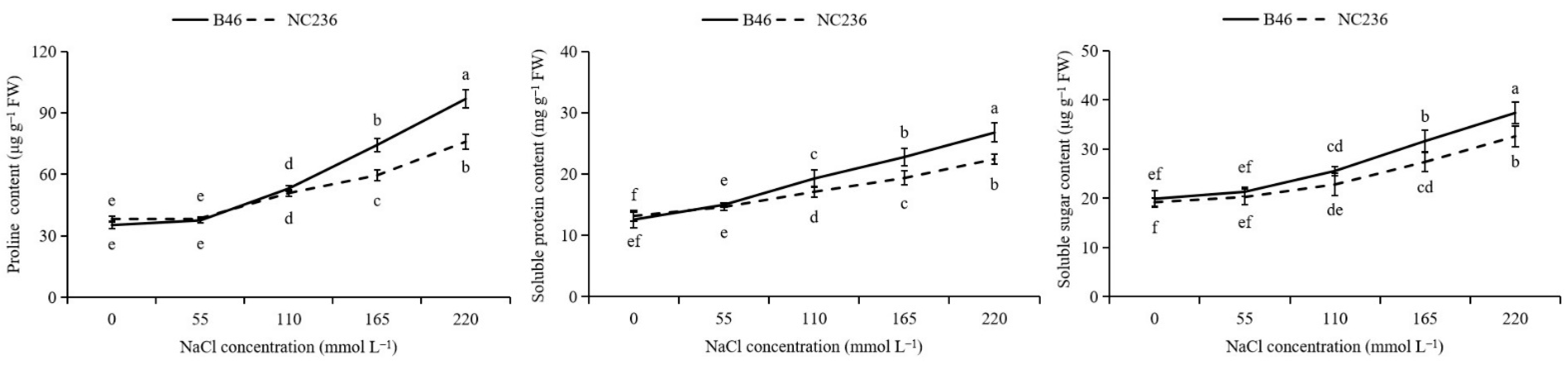
2.9. Contents of Proline, Soluble Protein, and Soluble Sugar
2.10. Contents of Na+, K+, and K+/Na+ Ratio
2.11. Contents of IAA, Z + ZR, and ABA
2.12. Ratio of IAA/(Z + ZR) and IAA/ABA
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Condition
4.2. Determination of O2− Content and H2O2 Content
4.3. Determination of MDA Content and Relative Conductivity (EL)
4.4. Determination of SOD, POD, CAT, APX, GR, MDHAR and DHAR Activities
4.5. Determination of AsA, DHA, GSSG, GSH, NO3−, NO2− and NH4+ Contents
4.6. Determination of NR, NiR, GS, GOGAT, GDH, GOT and GPT Activities
4.7. Determination of Osmoregulation Substance Contents
4.8. Determination of Na+ and K+ Contents
4.9. Determination of Hormone Contents
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Irfan, M.; Ahmad, A.; Hayat, S. Causes of salinity and plant manifestations to salt stress: A review. J. Enviro. Biol. 2011, 32, 667–685. [Google Scholar] [CrossRef] [Green Version]
- Jan, A.; Osman, M.B.; Amanullah. Response of Chickpea to Nitrogen Sources under Salinity Stress. J. Plant Nutr. 2013, 36, 1373–1382. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eprintsev, A.T.; Fedorin, D.N.; Cherkasskikh, M.V.; Igamberdiev, A.U. Effect of Salt Stress on the Expression and Promoter Methylation of the Genes Encoding the Mitochondrial and Cytosolic Forms of Aconitase and Fumarase in Maize. Int. J. Mol. Sci. 2021, 22, 6012. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zeng, Z.; Bai, X.; Qu, J.; Wang, Z. Root colonization and rhizospheric community structure of Arbuscular Mycorrhizal Fungi in BADH transgenic maize BZ-136 and its recipient under salt stress and neutral soil. Environ. Sci. Pollut. Res. 2021, 28, 66409–66419. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W. Studied on Secondary Salinization of the Greenhouse Soil and the Effects on Pepper Seedling under Salt Stress. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2010. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439. [Google Scholar] [CrossRef]
- Wei, G.; Zhu, Z.; Fang, X.; Li, J.; Cheng, J. The effects of NaCl stress on plant growth, chlorophyll fluorescence characteristics and active oxygen metabolism in seedings of two cucumber cultivars. Sci. Agric. Sin. 2004, 37, 1754–1759. [Google Scholar] [CrossRef]
- Han, B.; Sun, J.; Guo, S.; Jin, C. Effects of calcium on antioxidant enzymes activities of cucumber seedlings under salt stress. Acta Hortic. Sin. 2010, 37, 1937–1943. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 2007, 26, 2027–2038. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2010, 133, 481–489. [Google Scholar] [CrossRef]
- Daniel-Vedele, F.; Krapp, A.; Kaiser, W.M. Cellular Biology of Nitrogen Metabolism and Signaling. In Plant Cell Monographs; Springer: Berlin/Heidelberg, Germany, 2010; pp. 145–172. [Google Scholar]
- Yuan, Y.; Ou, J.; Wang, Z.; Zhang, C.; Zhou, Z.; Lin, Q. Regulation of carbon and nitrogen metabolisms in rice roots by 2-oxoglutarate at the level of hexokinase. Physiol. Plantarum. 2010, 129, 296–306. [Google Scholar] [CrossRef]
- Du, J.; Shu, S.; An, Y.; Zhou, H.; Guo, S.; Sun, J. Influence of exogenous spermidine on carbon–nitrogen metabolism under Ca(NO3)2 stress in cucumber root. Plant Growth Regul. 2017, 81, 103–115. [Google Scholar] [CrossRef]
- Lam, H.M.; Coschigano, K.T.; Oliveira, I.C.; Melo-Oliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P. Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: Implication of oxidative stress. Sci. Hortic.-Amst. 2011, 129, 321–328. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Gray, W.M. Hormonal Regulation of Plant Growth and Development. PLoS Biol. 2004, 2, e311. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Chen, Y.; Wu, C. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of Salinity on Endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed enhancement with cytokinins: Changes in growth and grain yield in salt stressed wheat plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Hadiarto, T.; Tran, L.P. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011, 30, 297–310. [Google Scholar] [CrossRef]
- Durgaprasad, K.M.R.; Muthukumarasamy, M.; Panneerselvam, R. Changes in protein metabolism induced by NaCl salinity in soybean seedlings. Indian J. Plant Physiol. 1996, 1, 98–101. [Google Scholar] [CrossRef]
- El-Samad, H.M.A.; Shaddad, M.A.K. Salt tolerance of soybean cultivars. Biol. Plant. 1997, 39, 263–269. [Google Scholar] [CrossRef]
- Xu, C.; Ling, F.; Xu, K.; Wu, Z.; Liu, X.; An, J.; Zhao, L. Effect of salt stress on photosynthetic characteristics and physiological and biochemical traits of different rice varieties. Chin. J. Rice Sci. 2013, 27, 280–286. [Google Scholar] [CrossRef]
- Eyidogan, F.; Oz, M.T.; Yücel, M.; Oktem, H. Signal Transduction of Phytohormones Under Abiotic Stresses. In Phytohormones and Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–48. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.; Khan, M.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and Improving Salt Tolerance in Plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol Environ Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Plant height and evolutionary games. Trends Ecol. Evol. 2003, 18, 337–343. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, R. Oxidative DNA damage and telomere shortening. Prog. Biochem. Biophys. 2000, 27, 351–353. [Google Scholar] [CrossRef]
- Jia, X.; Deng, Y.; Sun, X.; Liang, L. Impacts of salt stress on the growth and physiological characteristics of Paspalum vaginatum. Acta Prataculturae Sin. 2015, 24, 204–212. [Google Scholar] [CrossRef]
- Han, Z.; Guo, S.; Jiao, Y.; Fan, H.; Li, J. Effect of NaCl Stress on growth and photosynthetic gas exchange of watermelon seedlings. Acta Bot. Boreal.-Occident. Sin. 2008, 28, 745–751. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, K.; Zhao, C.; Li, Z.; Wang, Y.; Fu, J.; Guo, L.; Li, W. Alleviation of the adverse effects of salt stress by regulating photosynthetic system and active oxygen metabolism in maize seedlings. Chin. Agric. Sci. 2014, 47, 3962–3972. [Google Scholar] [CrossRef]
- Rajesh, A.; Arumugam, R.; Venkatesalu, V. Growth and Photosynthetic Characteristics of Ceriops Roxburghiana under NaCl Stress. Photosynthetica 1998, 35, 285–287. [Google Scholar] [CrossRef]
- Li, R. Studies on the Mechanism of Salt Resistance of Two Halophytes and Amelioration of Saline Soil by Salt-Resistance Plants. Ph.D. Thesis, Nankai University, Tianjin, China, 2010. [Google Scholar]
- Wang, Y.; Sun, G.; Wang, J.; Cao, W.; Liang, J.; Yu, Z. Relationships among MDA content, plasma membrane permeability and the chlorophyll fluorescence parameters of Puccinellia tenuiflora seedlings under NaCl stress. Acta Ecol. Sin. 2006, 26, 122–129. [Google Scholar] [CrossRef]
- Fu, Y.; Gao, S.; Yang, K.; Yin, K. Effects of salt stress on several physiological and biochemical indicators in seedling of salt-tolerant line and salt-sensitive line of maize (Zea mays L.). Plant Physiol. J. 2011, 47, 459–462. [Google Scholar] [CrossRef]
- Mishra, P.; Bhoomika, K.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Hossain, M.A.; Fujita, M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: Oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 2015, 252, 461–475. [Google Scholar] [CrossRef]
- Liu, H.; He, X.; Ma, Z.; Xu, W.; Liu, M.; Liu, H. Effects of exogenous GSH on the growth and AsA-GSH cycle in tamato seedlings under NaCl stress. J. Shihezi Univ. Nat. Sci. 2014, 32, 265–271. [Google Scholar] [CrossRef]
- Yang, H. Effects of osmotic stress and salt stress on nitrate reductase and nitrite reductase activities in Buckwheat. Crops 2013, 3, 53–55. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Y.; Zhang, X.; Ye, M.; Zhang, Q. Responses of growth and physiology metabolism of Ophiopogon Japonicus to NaCl stress. Acta Agrestia Sin. 2016, 24, 93–100. [Google Scholar] [CrossRef]
- Liu, W.; Pan, T.; Ke, Y. Effect of salt stress on nitrogen metabolism in the leaves of sweet potato. J. Fujian Agric. Univ. 1998, 27, 490–494. [Google Scholar]
- Sun, F.; Wang, D.; Chen, H.; Sun, X.; Zhao, J. Effects of NaCl stress on nitrogen metabolism in Chinese cabbage leaves. Jiangsu Agric. Sci. 2012, 40, 160–162. [Google Scholar] [CrossRef]
- Wei, C. Study on the Regulation Mechanism of Calcium on Potato under NaCl Stress. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Gong, M.; Zhao, F.; Wu, S.; Wang, L.; Liu, Y. Influences of NaCl stress on nitrate uptake and related enzyme activities of barley seedings. Plant Physlol. Commun. 1990, 6, 13–16. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of Proline and Glycinebetaine on Vicia Faba Responses to Salt Stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Tammam, A.A.E.; Alhamd, M.F.A.; Hemeda, M.M. Study of salt tolerance in wheat (Triticum aestium L.) cultivar Banysoif 1. Aust. J. Crop Sci. 2008, 1, 115–125. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Blackman, S.A.; Wettlaufer, S.H.; Obendorf, R.L.; Leopold, A.C. Maturation proteins and sugars in desiccation tolerance of developing soybean seeds. Plant Physiol. 1992, 100, 225. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Akhter, M.; Elsabagh, A.E.; Liu, L. Comparative studies on growth and physiological responses to saline and alkaline stresses of Foxtail millet (Setaria italica L.) and Proso millet (Panicum miliaceum L.). Aust. J. Crop Sci. 2011, 5, 1269–1277. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Y.; Zhong, Z.; Wu, X.; Zhang, M. Effects of salt stress on growth and accumulation of osmoregulatory substances in color leaf grass. Acta Pratacul. Sin. 2013, 22, 211–218. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Effects of silicon on enzyme activity and sodium, potassium and calcium concentration in barley under salt stress. Plant Soil 1999, 209, 217. [Google Scholar] [CrossRef]
- Matsushita, N.; Matoh, T. Characterization of Na+ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 2010, 83, 170–176. [Google Scholar] [CrossRef]
- Axelrod, J.; Reisine, T.D. Stress hormones: Their interaction and regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, K.; Li, X. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J. Plant Physiol. 2009, 166, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Kovács, E.; Sárvári, E.; Nyitrai, P.; Darók, J.; Keresztes, A. Structural-functional changes in detached cucumber leaves, and modelling these by hormone-treated leaf discs. Plant Biol. 2006, 9, 85–92. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Huang, J. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Wang, A.; Luo, G. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 1990, 84, 2895–2898. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The Presence of Glutathione and Glutathione Reductase in Chloroplasts: A Proposed Role in Ascorbic Acid Metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Reactive oxygen species, ascorbate-Glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 2013, 250, 585–600. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plant. 2010, 98, 685–692. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid1. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Barro, F.; Fontes, A.G.; Maldonado, J.M. Organic nitrogen content and nitrate and nitrite reductase activities in tritordeum and wheat grown under nitrate or ammonium. Plant Soil 1991, 135, 251–256. [Google Scholar] [CrossRef]
- Ida, S.; Morita, Y. Purification and general properties of spinach leaf nitrite reductase. Plant Cell Physiol. 1973, 14, 661–671. [Google Scholar] [CrossRef]
- O’Neal, D.; Joy, K.W. Glutamine synthetase of pea leaves. I. Purification, stabilization, and pH optima. Arch. Biochem. Biophys. 1973, 159, 113–122. [Google Scholar] [CrossRef]
- Groat, R.G.; Vance, C.P. Root Nodule Enzymes of Ammonia Assimilation in Alfalfa (Medicago sativa L.). Plant Physiol. 1981, 67, 1198–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, C.G.; Chen, L.P.; Wang, Y.; Liu, J.; Xu, G.L.; Li, T. High Temperature at Grain-filling Stage Affects Nitrogen Metabolism Enzyme Activities in Grains and Grain Nutritional Quality in Rice. Rice Sci. 2011, 18, 210–216. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Xiao, Q.; Zheng, H.; Chen, Y.; Huang, W.; Zhu, Z. Effects of salinity on the growth and proline, soluble sugar and protein contents of Spartina alterniflora. Chin. J. Ecol. 2005, 24, 373–376. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, S.; Chen, M.; Song, X.; Wang, B. Effect of sodium chloride stress on growth of sweet potato plantlets in vitro and ion content. J. Anhui Agric. Sci. 2008, 36, 15333–15335. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Zuo, S.; Meng, Y.; Li, C.; Li, W.; Zhang, Z.; Wei, W. Effects of thidiazuron and ethephon on the grain filling and dehydration characteristics of maize in Northeast China. Arch. Agron. Soil Sci. 2020, 4, 886–902. [Google Scholar] [CrossRef]

| NaCl Concentration (mmol L−1) | B46 | NC236 | ||||
|---|---|---|---|---|---|---|
| Na+ Content (%) | K+ Content (%) | K+/Na+ Content (%) | Na+ Content (%) | K+ Content (%) | K+/Na+ Ratio (%) | |
| 0 | 0.74 ± 0.10 e | 5.48 ± 0.21 a | 7.44 ± 0.78 a | 0.78 ± 0.10 e | 5.49 ± 0.17 a | 7.08 ± 0.85 a |
| 55 | 1.53 ± 0.12 d | 5.28 ± 0.32 ab | 3.45 ± 0.07 b | 1.63 ± 0.05 d | 5.23 ± 0.11 b | 3.20 ± 0.16 b |
| 110 | 2.15 ± 0.26 c | 5.10 ± 0.05 bc | 2.38 ± 0.19 c | 2.35 ± 0.06 c | 5.06 ± 0.05 bc | 2.15 ± 0.07 c |
| 165 | 2.67 ± 0.15 b | 4.94 ± 0.09 cd | 1.86 ± 0.12 cd | 3.15 ± 0.08 b | 4.75 ± 0.11 d | 1.51 ± 0.02 d |
| 220 | 3.19 ± 0.16 a | 4.79 ± 0.13 d | 1.51 ± 0.10 d | 3.62 ± 0.11 a | 4.49 ± 0.13 e | 1.24 ± 0.01 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Gong, S.; Fu, L.; Hu, G.; Li, G.; Hu, S.; Yang, J. The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms. Plants 2022, 11, 1547. https://doi.org/10.3390/plants11121547
Wang M, Gong S, Fu L, Hu G, Li G, Hu S, Yang J. The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms. Plants. 2022; 11(12):1547. https://doi.org/10.3390/plants11121547
Chicago/Turabian StyleWang, Mingquan, Shichen Gong, Lixin Fu, Guanghui Hu, Guoliang Li, Shaoxin Hu, and Jianfei Yang. 2022. "The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms" Plants 11, no. 12: 1547. https://doi.org/10.3390/plants11121547
APA StyleWang, M., Gong, S., Fu, L., Hu, G., Li, G., Hu, S., & Yang, J. (2022). The Involvement of Antioxidant Enzyme System, Nitrogen Metabolism and Osmoregulatory Substances in Alleviating Salt Stress in Inbred Maize Lines and Hormone Regulation Mechanisms. Plants, 11(12), 1547. https://doi.org/10.3390/plants11121547





