Feasibility of Old Bark and Wood Waste Recycling
Abstract
1. Introduction
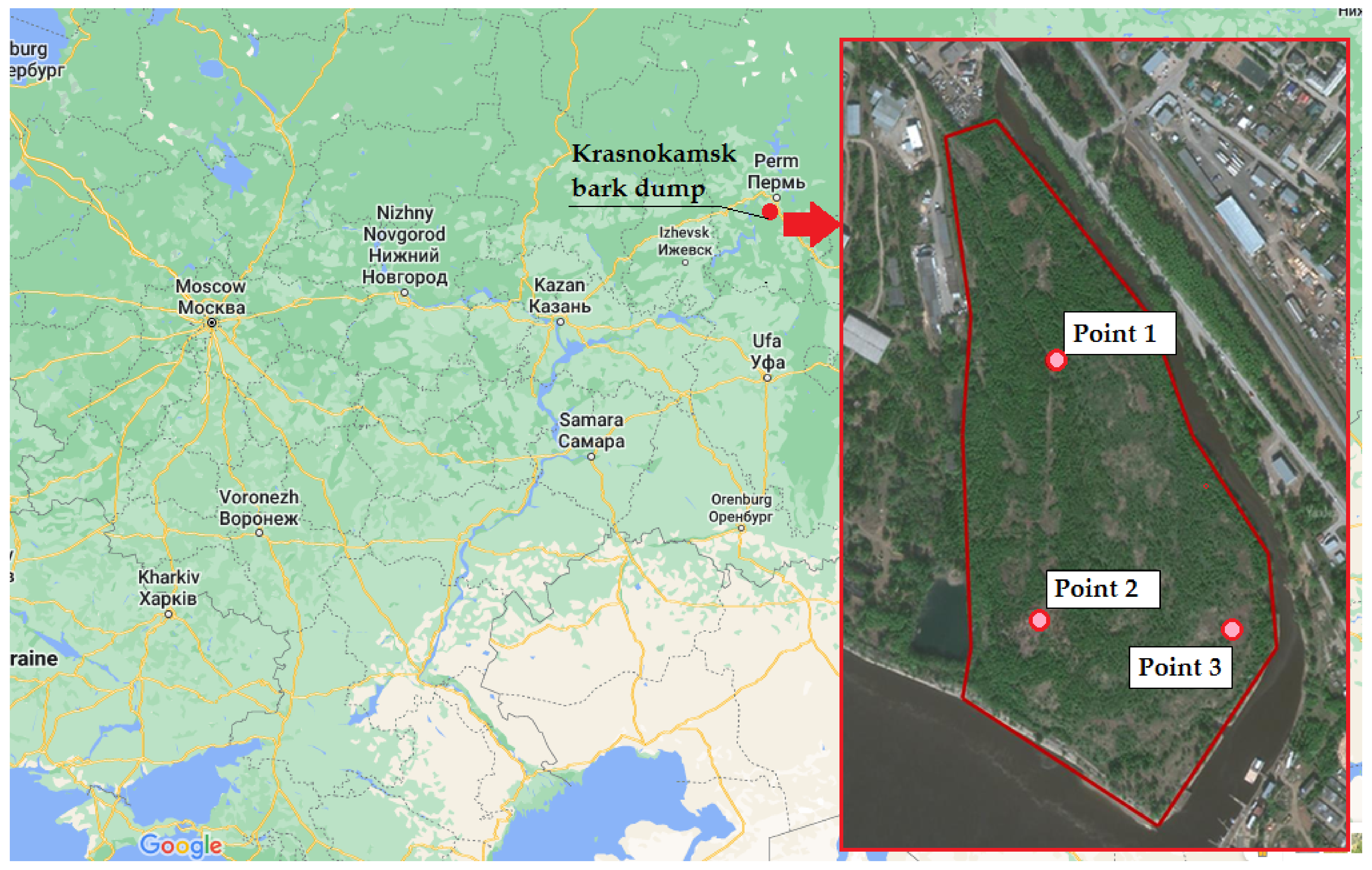
2. Materials and Methods
3. Results and Discussion
3.1. Evaluation of Agricultural Potential of BWW
3.2. Evaluation of BWW Thermal Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babich, O.O.; Kulikova, Y.V.; Sukhikh, S.A.; Kalashnikova, O.B.; Dolganyuk, V.F. Review of research in the field of development of technologies for direct production of liquid fuel from biomass. Herit. Sci. 2021, 80, 41–47. (In Russian) [Google Scholar] [CrossRef]
- Maksimov, A.Y.; Maksimova, Y.G.; Shilova, A.V.; Kolesova, O.V.; Simonetti, J. Study of the properties and microbiological composition of bark and wood waste from the Krasnokamsk bark dump. Bull. PNRPU. Chem. Eng. Biotechnol. 2018, 4, 98–112. (In Russian) [Google Scholar] [CrossRef]
- Giner-Santonja, G.; Suhr, M.; Klein, G.; Kourti, I.; Gonzalo, M.R.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper and Board; Publications Office of the European Union: Luxembourg, 2015; pp. 185–504. [Google Scholar] [CrossRef]
- Ministry of Natural Resources of Russia. On the State and Protection of the Environment of the Russian Federation in 2020. State-Donation Report; Ministry of Natural Resources of Russia: Moscow, Russia, 2021; pp. 245–318. (In Russian) [Google Scholar]
- BP Statistical Review of World Energy. 2021. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 10 May 2022).
- Clemencon, R. The Two Sides of the Paris Climate Agreement: Dismal Failure or Historic Breakthrough? J. Env. Dev. 2016, 25, 3–24. [Google Scholar] [CrossRef]
- Liu, R.; Tian, W.; Kong, S.; Meng, Y.; Wang, H.; Zhang, J. Effects of inorganic and organic acid pretreatments on the hydrothermal liquefaction of municipal secondary sludge. Energ. Convers. Manag. 2018, 174, 661–667. [Google Scholar] [CrossRef]
- Mishra, A.; Ghosh, S. Bioethanol production from various lignocellulosic feedstocks by a novel «fractional hydrolysis» technique with different inorganic acids and coculture fermentation. Fuel 2019, 236, 544–553. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Dalmas, C.J.; Vandenberghe, L.P.S.; Carvalho, D.P.; Sydney, A.C.N.; Letti, L.A.J.; Karp, S.G.; Torres, L.A.Z.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Yu, Z.; Du, Y.; Shang, X.; Zheng, Y.; Zhou, J. Enhancing fermentable sugar yield from cassava residue using a two-step dilute ultra-low acid pretreatment process. Ind. Crops. Prod. 2018, 124, 555–562. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Chen, Z.; Jacoby, W.A.; Wan, C. Ternary deep eutectic solvents for effective biomass deconstruction at high solids and low enzyme loadings. Bioresour. Technol. 2019, 279, 126. [Google Scholar] [CrossRef]
- Kolesnikova, A.V. Analysis of the formation and use of wood waste at the enterprises of the Russian timber industry. Top. Issues Econ. Sci. 2013, 33, 116–120. (In Russian) [Google Scholar]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin-carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Dibyajyoti, H.M.; Kumar, P. Lignocellulosic conversion into value-added product. Process. Biochem. 2019, 89, 120–150. [Google Scholar] [CrossRef]
- Gu, B.J.; Dhumal, G.S.; Wolcott, M.P.; Ganjyal, G.M. Disruption of lignocellulosic biomass along the length of the screws with different screw elements in a twinscrew extruder. Bioresour. Technol. 2019, 275, 266–271. [Google Scholar] [CrossRef]
- Kamali, M.; Garmeio, T.; Costa, M.E.; Capela, I. Anaerobic digestion of pulp and paper mill wastes—An overview of the developments and improvement opportunities. Chem. Eng. J. 2016, 298, 162–182. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, S.; Li, J.; Wang, Q.; He, Z.; Feng, Y. Co-pyrolysis and co- hydrothermal liquefaction of seaweeds and rice husk: Comparative study towards enhanced biofuel production. J. Anal. Appl. Pyrol. 2018, 129, 162–170. [Google Scholar] [CrossRef]
- Huang, S.; Liu, T.; Peng, B.; Geng, A. Enhanced ethanol production from industrial lignocellulose hydrolysates by a hydrolysate-cofermenting Saccharomyces cerevisiae strain. Bioproc. Biosyst. Eng. 2019, 42, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Rynk, R.; Schwarz, M.; Richard, T.; Cotton, M.; Halbach, T.; Siebert, S. Compost feedstocks. Compost. Handb. 2022, 85, 103–157. [Google Scholar] [CrossRef]
- Kulikowska, D.; Sindrewicz, S. Effect of barley straw and coniferous bark on humification process during sewage sludge composting. Waste Manag. 2018, 79, 207–213. [Google Scholar] [CrossRef]
- Bohacz, J. Composts and Water Extracts of Lignocellulosic Composts in the Aspect of Fertilization, Humus-Forming, Sanitary, Phytosanitary and Phytotoxicity Value Assessment. Waste Biomass Valoris. 2019, 10, 334. [Google Scholar] [CrossRef]
- Pengqi, L.; Yufei, X.; Jian, L.; Xinjing, Q.; Shengyu, D.; Jun, Y. Effect of Different Microbial Inoculants and Particle Size on Compost of Acacia mangium. Chin. J. Trop. Crops 2019, 40, 39–44. [Google Scholar]
- Sang, S.; Zhuang, X.; Chen, H.; Qin, Y.; Cao, J.; Fan, F.; Lan, T. Effect of supramolecular structural changes during the crystalline transformation of cellulose on its enzymatic hydrolysis. Ind. Crops Prod. 2022, 180, 114687. [Google Scholar] [CrossRef]
- Novozhilov, E.V.; Sinelnikov, I.G.; Aksenov, A.S.; Chukhchin, D.G.; Tyshkunova, I.V.; Rozhkova, A.M.; Osipov, D.O.; Zorov, I.N.; Sinitsyn, A.P. Biocatalytic conversion of sulfate cellulose using complex biocatalysts based on recombinant Penicillium verruculosum enzyme preparations. Catal. Ind. 2015, 15, 78–83. [Google Scholar]
- Houfani, A.A.; Andersb, N.; Spiessb, A.C.; Baldrianc, P.; Benallaouaa, S. Insights from enzymatic degradation of cellulose and hemi cellulose to fermentable sugars—A review. Biomass Bioenerg. 2020, 134, 105481. [Google Scholar] [CrossRef]
- Kaltschmitt, M. Energy from Organic Materials (Biomass). In Encyclopedia of Sustainability Science and Technology, 2nd ed.; Springer: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Cedeno, R.F.; Belon de Siqueira, B.; Chavez, G.E.; Ulises, M.I.; Moreira, R.L.; Galán, J.; Masarin, F. Recovery of cellulose and lignin from Eucalyptus by-product and assessment of cellulose enzymatic hydrolysis. Renew. Energ. 2022, 193, 807–820. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Lazaridis, P.A.; MachAigner, A.; Matis, K.A.; Trianta, K.S. Enhancing lignocellulosic biomass hydrolysis by hydrothermal pretreatment, extraction of surface lignin, wet milling and production of cellulolytic enzymes. Chem. Sus. Chem. 2019, 12, 1179–1195. [Google Scholar] [CrossRef]
- Donev, E.; Gandla, M.L.; Jonsson, L.J.; Mellerowicz, E.J. Engineering non-cellulosic polysaccharides of wood for the biorefinery. Front. Plant. Sci. 2018, 9, 1537. [Google Scholar] [CrossRef]
- The Fifth Assessment Report (AR5) of the United Nations Intergovernmental Panel on Climate Change (IPCC). 2014. Available online: https://www.ipcc.ch/assessment-report/ar5/ (accessed on 10 May 2022).
- Aquila, A.; Twardowski, T.; Wohlgemuth, R. Bioeconomy for sustainable development. Biotechnol. J. 2019, 14, 1800638. [Google Scholar] [CrossRef]
- Yucai, H.; Cui-Luan, M.; Bin, Y. Pretreatment Process and Its Synergistic Effects on Enzymatic Digestion of Lignocellulosic. In Fungal Cellulolytic Enzymes; Springer: Berlin, Germany, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Zavyalov, A.V.; Rykov, S.V.; Lunina, N.A.; Sushkova, V.I.; Yarotsky, S.V.; Berezina, O.V. Plant polysaccharide xyloglucan and enzymes that hydrolyze it (review). Russ. J. Bioorg. Chem. 2019, 45, 845–859. [Google Scholar] [CrossRef]
- Castello, D.; Haider, M.; Rosendahl, L. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energ. 2019, 141, 420–430. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels: Their characteristics and analysis. Biomass. In Biopolymer-Based Materials, and Bioenergy; Elsevier: Berlin, Germany, 2019; pp. 277–325. [Google Scholar]
- Danquah, J.; Roberts, C.; Appiah, M. Elephant Grass (Pennisetum purpureum): A Potential Source of Biomass for Power Generation in Ghana. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- De Laporte, A.V.; Ripplinger, D.G. The effects of site selection, opportunity costs and transportation costs on bioethanol production. Renew. Energ. 2019, 131, 73–82. [Google Scholar] [CrossRef]
- Jellali, S.; El-Bassi, L.; Charabi, Y.; Usman, M.; Khiari, B.; Al-Wardy, M.; Jeguirim, M. Recent advancements on biochars enrichment with ammonium and nitrates from wastewaters: A critical review on benefits for environment and agriculture. J. Environ. Manag. 2022, 305, 114368. [Google Scholar] [CrossRef] [PubMed]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: London, UK, 2018; pp. 49–87. [Google Scholar]
- Venderbosch, R.H. Fast pyrolysis. Thermochemical processing of biomass: Conversion into fuels, chemicals and power. In Thermochemical Processing of Biomass; Brown, R., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 175–206. [Google Scholar]
- Matayeva, A.; Basile, F.; Cavani, F.; Bianchi, D.; Chiaberge, S. Development of upgraded bio-oil via liquefaction and pyrolysis. In Horizons in Sustainable Industrial Chemistry and Catalysis; Elsevier: London, UK, 2019; pp. 231–256. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Ding, H.; Zhu, X. Two-step pyrolysis of corncob for value-added chemicals and high-quality bio-oil: Effects of alkali and alkaline earth metals. Waste Manag. 2019, 87, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, Y.; Sukhikh, S.; Ivanova, S.; Babich, O.; Sliusar, N. Review of Studies on Joint Recovery of Macroalgae and Marine Debris by Hydrothermal Liquefaction. Appl. Sci. 2022, 12, 569. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Jiao, K.; Ning, X.; Zhang, J.; Guo, X.; Li, J.; Wang, C. Conversion mechanism and gasification kinetics of biomass char during hydrothermal carbonization. Renew. Energ. 2021, 173, 318–328. [Google Scholar] [CrossRef]
- Azzaz, A.; Khiari, B.; Jellali, S.; Ghimbeu, C.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sust. Energ. Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Marulanda, V.A.; Gutierrez, C.D.B.; Alzate, C.A.C. Thermochemical, Biological, Biochemical and Hybrid Conversion Methods of bio-derived molecules into renewable fuels. In Advanced Bioprocessing for Alternative Fuels; Elsevier: London, UK, 2019; pp. 59–81. [Google Scholar] [CrossRef]
- Moriarty, P.; Honnery, D. Global renewable energy resources and use in 2050. In Managing Global Warming; Elsevier: London, UK, 2019; pp. 221–235. [Google Scholar] [CrossRef]
- Kargbo, H.; Harris, J.S.; Phan, N.A. Dropin-fuel production from biomass Critical review on technoeconomic feasibility and sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Yang, J.; He, Q.S.; Corscadden, K.; Niu, H.; Lin, J.; Astatkie, T. Advanced models for the prediction of product yield in hydrothermal liquefaction via a mixture design of biomass model components coupled with process variables. Appl. Energy 2019, 233–234, 906–915. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404. [Google Scholar] [CrossRef]
- Tai, L.; Caprariis, B.; Scarsella, M.; de Filippis, P.; Marra, F. Improved Quality Bio-Crude from Hydrothermal Liquefaction of Oak Wood Assisted by Zero-Valent Metals. Energy Fuels 2021, 35, 10023–10034. [Google Scholar] [CrossRef]
- Xu, Y.H.; Li, M.F. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef] [PubMed]
- Santosa, D.M.; Wendt, L.M.; Wahlen, B.D.; Schmidt, A.J.; Billing, J.; Kutnyakov, I.V.; Hallen, R.T.; Thorson, M.R.; Oxford, T.L.; Anderson, D.B. Impact of storage and blending of algae and forest product residue on fuel blendstock production. Algal Res. 2022, 62, 102622. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Jia, S.; Mi, S.; Tong, Z.; Li, Z.; Li, M.; Zhang, Y.; Hu, Y.; Huang, Z. Effect of ethanol on Mulberry bark hydrothermal liquefaction and bio-oil chemical compositions. Energy 2018, 162, 460–475. [Google Scholar] [CrossRef]
- Stigsson, C.; Furusjö, E.; Börjesson, P. A model of an integrated hydrothermal liquefaction, gasification and Fischer-Tropsch synthesis process for converting lignocellulosic forest residues into hydrocarbons. Biores. Technol. 2022, 353, 126070. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Conradie, A.; Lester, E. Review of supercritical water gasification with lignocellulosic real biomass as the feedstocks: Process parameters, biomass composition, catalyst development, reactor design and its challenges. J. Chem. Eng. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Nunes, L. Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Res. Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Hossain, M. Promotional effects of Ce on Ni Ce/γAl2O3 for enhancement of H2 in hydrothermal gasification of biomass. Int. J. Hydrogen Energy 2018, 43, 6088–6095. [Google Scholar] [CrossRef]
- Tan, E.C. Sustainable Process Design for Biofuel Production via Syngas Conversion Pathway; No. NREL/PR-5100–74250; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2019. [Google Scholar]
- Water Quality–Determination of The Chemical Oxygen Demand. ISO 6060:1989. Available online: https://www.iso.org/standard/12260.html (accessed on 10 May 2022).
- ASTM-D7348; Standard Test Methods for Loss on Ignition (LOI) of Solid Combustion Residues. ASTM International: West Conshohocken, PA, USA, 2021; pp. 1–7. [CrossRef]
- Binner, E.; Bohm, K.; Lechner, P. Large scale study on measurement of respiration activity (AT4) by Sapromat and OxiTop. Waste Manag. 2012, 32, 1752–1759. [Google Scholar] [CrossRef]
- NRCS-USDA. National Soil Survey Handbook. Available online: https://www.nrcs.usda.gov/wps/PA_NRCSConsumption/download?cid=stelprdb1270585&ext=pdf (accessed on 1 February 2022).
- Kondratyeva, M.A.; Bazukova, N.V. Mapping of soils since its inception to our days (on the example of the Perm region). Russ. J. Appl. Ecol. 2019, 3, 28–34. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106, Rome. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 10 May 2022).
- Romashkin, I.; Shorohova, E.; Kapitsa, E.; Galibina, N.; Nikerova, K. Carbon and nitrogen dynamics along the log bark decomposition continuum in a mesic old-growth boreal forest. Eur. J. Res. 2018, 137, 643–657. [Google Scholar] [CrossRef]
- Pognani, M.; Barrena, R.; Font, X.; Adani, F.; Scaglia, B.; Sánchez, A. Evolution of organic matter in a full–scale composting plant for the treatment of sewage sludge and biowaste by respiration techniques and pyrolysis–GC/MS. Bioresour. Technol. 2011, 102, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, C.; Wang, X.; Hai, L. Increased abundance of nitrogen transforming bacteria by higher C/N ratio reduces the total losses of N and C in chicken manure and corn stover mix composting. Bioresour. Technol. 2020, 297, 122410. [Google Scholar] [CrossRef] [PubMed]
- Akratos, C.; Tekerlekopoulou, A.; Vasiliadou, I.; Vayenas, D. Cocomposting of olive mill waste for the production of soil amendments. Olive Mill Waste 2017, 11, 161–182. [Google Scholar] [CrossRef]
- Dickson, N.; Richard, T.; Kozlowski, R. Composting to Reduce the Waste Stream: A Guide to Small Scale Food and Yard Waste Composting; NEARS: Ithaca, NY, USA, 1991; p. 46. [Google Scholar]
- Zhao, D.; Kane, M.; Teskey, R.; Markewitz, D.; Greene, D.; Borders, B. Impact of management on nutrients, carbon, and energy in aboveground biomass components of mid-rotation loblolly pine (Pinus taeda L.) plantations. Annu. For. Sci. 2014, 71, 843–851. [Google Scholar] [CrossRef][Green Version]
- Barta-Rajnai, E.; Wang, L.; Sebestyén, Z.; Barta, Z.; Khalil, R.; Skreiberg, Ø.; Grønli, M.; Jakab, E.; Czégény, Z. Comparative study on the thermal behavior of untreated and various torrefied bark, stem wood, and stump of Norway spruce. Appl. Energy 2017, 204, 1043–1054. [Google Scholar] [CrossRef]
- Vane, C.H.; Drage, T.C.; Snape, C.E. Bark decay by the white-rot fungus Lentinula edodes: Polysaccharide loss, lignin resistance and the unmasking of suberin. Int. Biodeterior. Biodegrad. 2006, 57, 14–23. [Google Scholar] [CrossRef]
- Vane, C.H.; Martin, S.C.; Abbott, G.D. Degradation of Lignin in Wheat Straw during Growth of the Oyster Mushroom (Pleurotus ostreatus) Using Off-line Thermochemolysis with Tetramethylammonium Hydroxide and Solid-State 13C NMR. J. Agric. Food Chem. 2001, 49, 2709–2716. [Google Scholar] [CrossRef]
- Kulikova, Y.; Sukhikh, S.; Kalashnikova, O.; Chupakhin, E.; Ivanova, S.; Chubarenko, B.; Gorbunova, J.; Babich, O. Assessment of the Resource Potential of Baltic Sea Macroalgae. Appl. Sci. 2022, 12, 3599. [Google Scholar] [CrossRef]
- González, J.F.; Cuello, T.B.; Calderón, A.J.; Calderón, M.; González, J.; Carmona, D. Cultivation of Autochthonous Microalgae for Biomass Feedstock: Growth Curves and Biomass Characterization for Their Use in Biorefinery Products. Energies 2021, 14, 4567. [Google Scholar] [CrossRef]
- Goldšteins, L.; Dzenis, M.G.; Valdmanis, R.; Zaķe, M.; Arshanitsa, A. Thermo-Chemical Conversion of Microwave Activated Biomass Mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 355, 12018. [Google Scholar] [CrossRef]
- Portnov, D.; Subbotin, D.; Kazakov, A.; Zavorin, A. The Peat and Wood Gasification at Different Conditions of the Pyrolysis Process. MATEC Web Conf. 2015, 37, 1043. [Google Scholar] [CrossRef]
- Therasme, O.; Eisenbies, M.; Volk, T. Overhead Protection Increases Fuel Quality and Natural Drying of Leaf-On Woody Biomass Storage Piles. Forests 2019, 10, 390. [Google Scholar] [CrossRef]
- Cai, H.; Yang, K.; Zhang, Q.; Zhao, K.; Gu, S. Pyrolysis Characteristics of Typical Biomass Thermoplastic Composites. Res. Phys. 2017, 7, 3230–3235. [Google Scholar] [CrossRef]
- Hendriyana, M. Effect of Equivalence Ratio on the Rice Husk Gasification Performance Using Updraft Gasifier with Air Suction Mode. J. Bahan Alam Terbarukan 2020, 9, 30–35. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Yadav, S.; Chandra, R. Detection and assessment of the phytotoxicity of residual organic pollutants in sediment contaminated with pulp and paper mill effluent. Environ. Monit. Assess. 2018, 190, 581. [Google Scholar] [CrossRef]




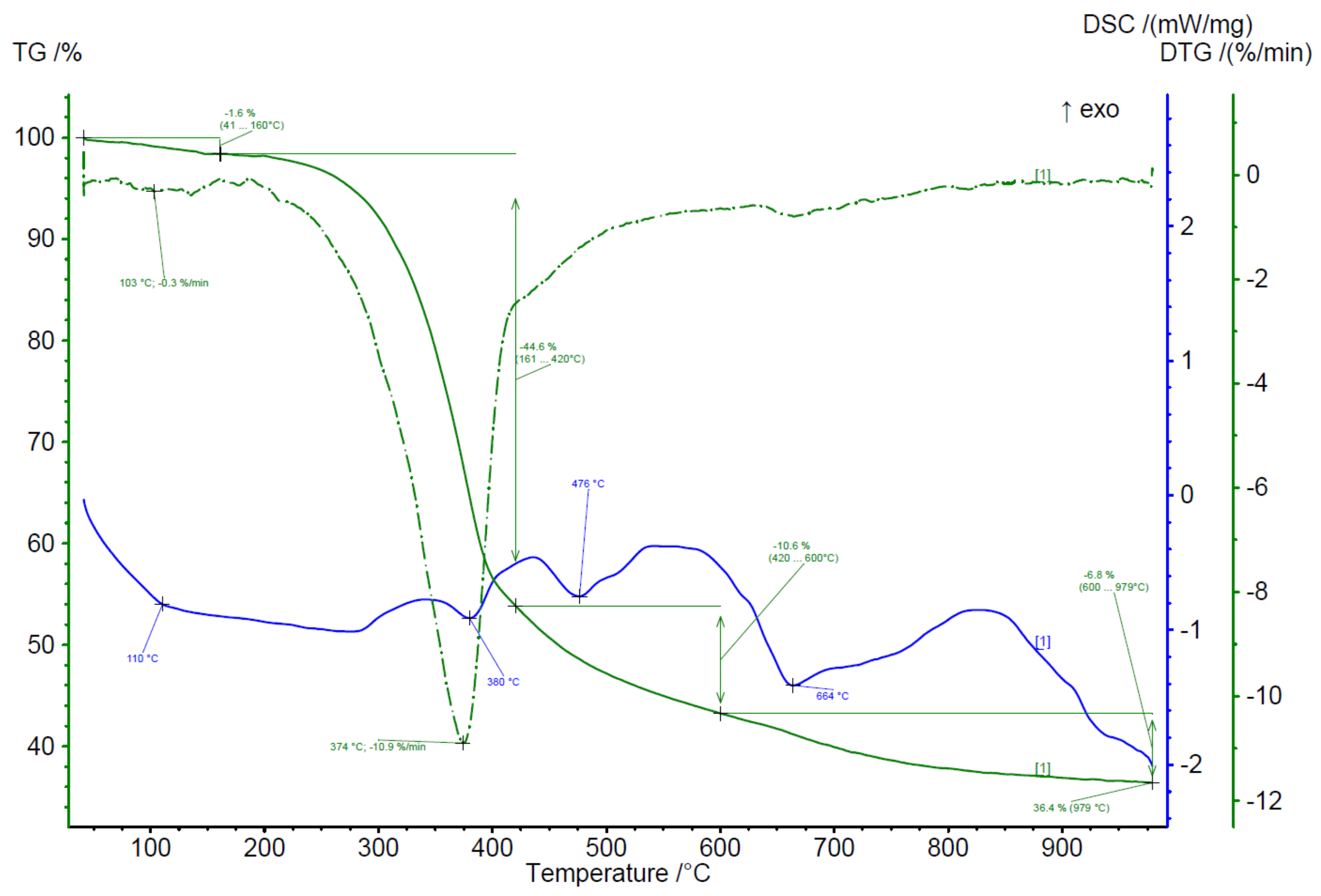
| Parameter | Value |
|---|---|
| Initial temperature: | 30/40 °C |
| Dynamic segment: | 1000 °C |
| Heating rate | 20 degrees/min |
| Furnace gas flow rate | 40 mL/min air/argon |
| Pan | PtRh20 85 µL, with lead |
| Depth, m | Age, Years | Humidity,% | AT4, mgO2/kg | pH | LOI,% | C,% | H,% | N,% | S,% | O,% | C:N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Well 1 | |||||||||||
| 1 | 10 | 62.02 | 7.89 | 5.44 | 97.91 | 49.96 | 6.669 | 0 | 0.024 | 41.26 | - |
| 3 | 15 | 66.26 | 6.6 | 6.82 | 98.71 | 49.54 | 6.562 | 0 | 0 | 42.61 | - |
| 5 | 17 | 62.99 | 4.3 | 6.89 | 97.25 | 49.21 | 6.565 | 0 | 0 | 41.48 | - |
| Well 2 | |||||||||||
| 1 | 9 | 60.79 | 3.59 | 6.12 | 69.23 | 46.15 | 5.858 | 0 | 0.211 | 38.78 | - |
| 3 | 10 | 65.2 | 4.1 | 6.85 | 98.9 | 49.67 | 6.342 | 0.23 | 0.885 | 41.77 | 216 |
| 5 | 15 | 67.76 | 7.83 | 7.02 | 93.05 | 48.97 | 6.349 | 0.16 | 0.929 | 36.64 | 306 |
| 7 | 17 | 69.6 | 6.95 | 91.87 | 49.22 | 6.345 | 0.23 | 1.228 | 34.85 | 214 | |
| 9 | 29 | 71.25 | 4.8 | 6.61 | 93.49 | 48.48 | 5.922 | 0.65 | 0.885 | 37.55 | 75 |
| 11 | 40 | 69.85 | 1.34 | 4.70 | 84.29 | 44.14 | 5.143 | 1.3 | 0.3 | 33.41 | 34 |
| 13 | 50 | 74.95 | 4.17 | 6.58 | 91.32 | 49.57 | 6.183 | 0.55 | 0.472 | 34.55 | 90 |
| 15 | 60 | 74.77 | 3.88 | 6.18 | 92.32 | 49.13 | 6.154 | 0.59 | 0.523 | 35.92 | 83 |
| 17 | 67 | 68.03 | 7.13 | 6.44 | 93.07 | 49.26 | 6.02 | 0.66 | 0.231 | 36.90 | 75 |
| 19 | 73 | 71.5 | 1.85 | 7.04 | 96.54 | 49.59 | 6.089 | 0.61 | 0.126 | 40.13 | 81 |
| 21 | 78 | 73 | - | 7.78 | 94.55 | 48.91 | 6.151 | 0.56 | 0.141 | 38.79 | 87 |
| Well 3 | |||||||||||
| 1 | 17 | 62.72 | 1.28 | 7.55 | 67.33 | 43.91 | 2.947 | 0.42 | 0.588 | 22.41 | 105 |
| 3 | 29 | 70.91 | 1.61 | 7.76 | 90.44 | 46.89 | 5.318 | 0.58 | 0.068 | 37.58 | 81 |
| 5 | 40 | 69.49 | 2.73 | 7.59 | 82.87 | 47.76 | 5.305 | 0.53 | 0.053 | 29.22 | 90 |
| 7 | 50 | 66.08 | 1.6 | 6.9 | 66.93 | 44.73 | 4.955 | 0.49 | 0.481 | 17.74 | 91 |
| 9 | 60 | 69.68 | 4.89 | 7.3 | 95.07 | 48.28 | 5.525 | 0.56 | 0.088 | 40.62 | 86 |
| 11 | 67 | 70.38 | 2.29 | 7.51 | 85.47 | 40.48 | 4.592 | 0.52 | 0.236 | 80.12 | 78 |
| 13 | 73 | 70.15 | 1.72 | 7.57 | 90.59 | 47.11 | 5.066 | 0.24 | 0.024 | 38.15 | 196 |
| 15 | 78 | 66.53 | 1.2 | 7.2 | 87.65 | 47.2 | 5.437 | 0.37 | 0.058 | 34.59 | 128 |
| 17 | 82 | 70.58 | 1.17 | 7.05 | 87.74 | 48.4 | 5.287 | 0.35 | 0.23 | 33.47 | 138 |
| 19 | 85 | 73.07 | - | 7.59 | 86.47 | 47.86 | 5.435 | 0.38 | 0.26 | 32.54 | 126 |
| Podzolic soils 1 [64,65,66] | 5.7 | 16.0 | 12.1 | n/d | 1.37 | n/d | n/d | 11 | |||
| Dark humus soils 2 [64,65,66] | 7.3 | 49.2 | 50.8 | 1.27 | n/d | n/d | 23 | ||||
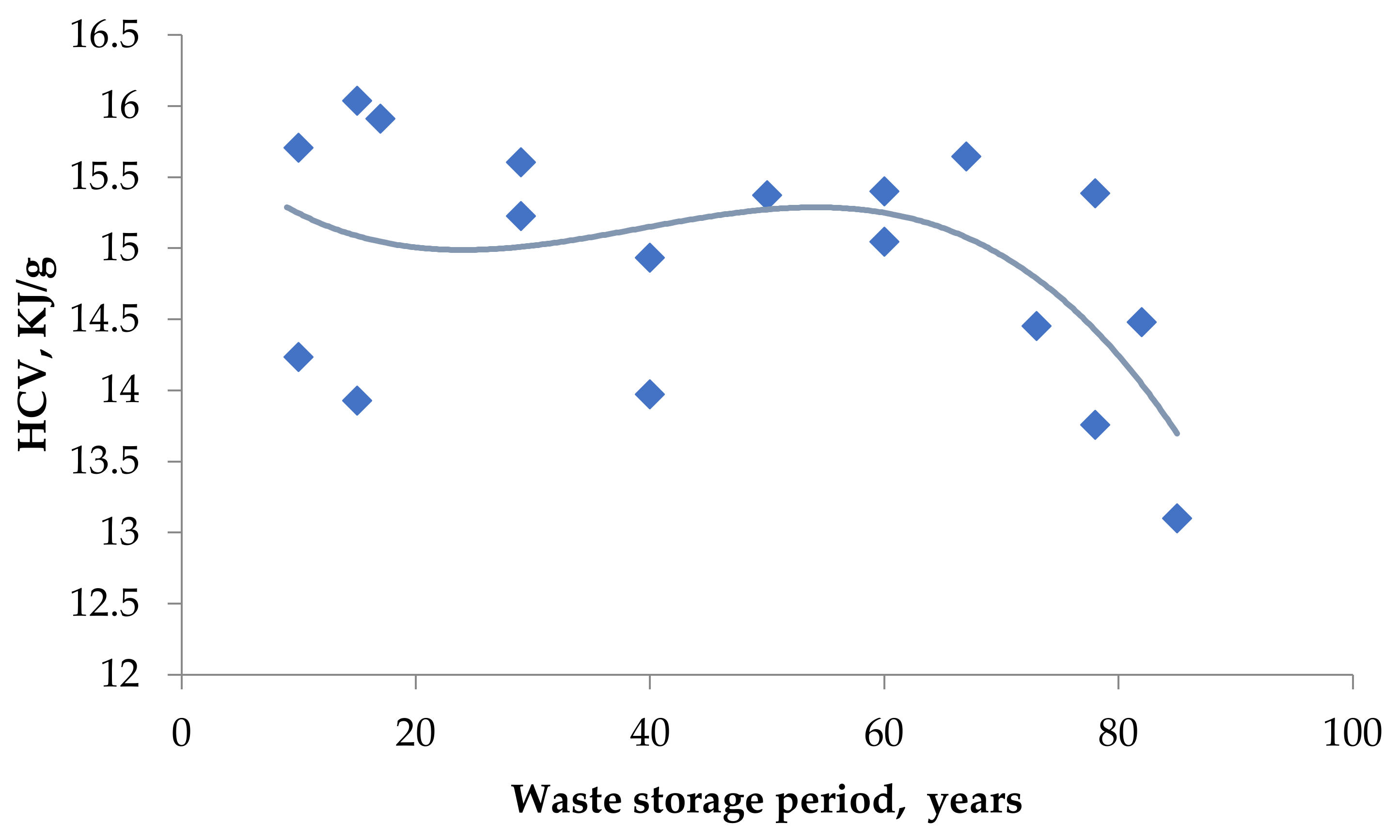
| Waste Storage Period, Years | Atm. | Number of Main Stages | t1 | t2 | tmax | ∆m, % | Ash, % | HHV, KJ/g | |
|---|---|---|---|---|---|---|---|---|---|
| Well 1 | 10 | O2 | 1 | 148 | 372 | 341 | 52.6 | 4.8 | 15.71 |
| 2 | 371 | 600 | 433 | 35.5 | |||||
| Ar | 1 | 176 | 409 | 374 | 50.5 | 29.7 | |||
| 15 | O2 | 1 | 162 | 374 | 342 | 53.5 | 4.5 | 16.04 | |
| 2 | 375 | 600 | 429 | 35 | |||||
| Ar | 1 | 176 | 417 | 380 | 51.7 | 29.9 | |||
| 17 | O2 | 1 | 154 | 376 | 349 | 56.7 | 4.1 | 15.92 | |
| 2 | 376 | 516 | 402 | 33.7 | |||||
| Ar | 1 | 174 | 415 | 379 | 52.4 | 26.9 | |||
| Well 2 | 9 | O2 | 1 | 157 | 376 | 332 | 49.6 | 9 | 13.21 |
| 2 | 376 | 600 | 438 | 34.8 | |||||
| Ar | 1 | 166 | 412 | 364 | 50.9 | 31.4 | |||
| 10 | O2 | 1 | 156 | 376 | 330 | 56.8 | 7.7 | 14.24 | |
| 2 | 377 | 600 | 478 | 34.5 | |||||
| Ar | 1 | 171 | 415 | 378 | 58.8 | 24.8 | |||
| 15 | O2 | 1 | 152 | 364 | 323 | 53 | 9.2 | 13.93 | |
| 2 | 364 | 600 | 489 | 36.8 | |||||
| Ar | 1 | 150 | 413 | 357 | 54.4 | 26.1 | |||
| 17 | O2 | 1 | 146 | 380 | 295 | 53.4 | 7 | 13.84 | |
| 2 | 380 | 600 | 450 | 36.6 | |||||
| Ar | 1 | 133 | 392 | 309 | 50.1 | 27.1 | |||
| 29 | O2 | 1 | 150 | 371 | 335 | 49.1 | 9.8 | 15.23 | |
| 2 | 372 | 625 | 386 | 36 | |||||
| Ar | 1 | 170 | 416 | 375 | 46.4 | 34.2 | |||
| 40 | O2 | 1 | 172 | 364 | 329 | 37.3 | 17.9 | 13.97 | |
| 2 | 365 | 600 | 535 | 36.6 | |||||
| Ar | 1 | 178 | 438 | 369 | 35.2 | 45.7 | |||
| 50 | O2 | 1 | 160 | 378 | 338 | 55.4 | 5.7 | 15.38 | |
| 2 | 379 | 600 | 391 | 34.1 | |||||
| Ar | 1 | 166 | 417 | 375 | 50.8 | 31.3 | |||
| 60 | O2 | 1 | 160 | 376 | 336 | 53.3 | 8.5 | 15.05 | |
| 2 | 375 | 601 | 378 | 35.1 | |||||
| Ar | 1 | 169 | 411 | 380 | 50.9 | 30.9 | |||
| 67 | O2 | 1 | 164 | 382 | 339 | 53.5 | 7 | 15.65 | |
| 2 | 386 | 600 | 402 | 34.9 | |||||
| Ar | 1 | 172 | 416 | 377 | 48.8 | 32.1 | |||
| 73 | O2 | 1 | 151 | 381 | 340 | 67.4 | 15.2 | 18.86 | |
| 2 | 382 | 600 | 423 | 42 | |||||
| Ar | 1 | 174 | 418 | 378 | 52.6 | 29.7 | |||
| Well 2 | 78 | O2 | 1 | 151 | 376 | 337 | 55.2 | 5.6 | 15.39 |
| 2 | 376 | 600 | 390 | 32.7 | |||||
| Ar | 1 | 170 | 417 | 376 | 49.8 | 31.6 | |||
| Well 3 | 17 | O2 | 1 | 174 | 380 | 338 | 27.9 | 48.2 | 7.71 |
| 2 | 380 | 600 | 538 | 17.9 | |||||
| Ar | 1 | 177 | 419 | 377 | 22.2 | 62.2 | |||
| 29 | O2 | 1 | 149 | 378 | 337 | 55 | 9.2 | 15.61 | |
| 2 | 378 | 600 | 390 | 31.3 | |||||
| Ar | 1 | 168 | 421 | 379 | 51.1 | 32.6 | |||
| 40 | O2 | 1 | 161 | 382 | 339 | 54.3 | 9.7 | 14.94 | |
| 2 | 383 | 600 | 395 | 29.6 | |||||
| Ar | 1 | 161 | 421 | 374 | 47.1 | 33.9 | |||
| 50 | O2 | 1 | 161 | 381 | 336 | 42 | 31.6 | 11.17 | |
| 2 | 380 | 600 | 381 | 21.7 | |||||
| Ar | 1 | 162 | 428 | 364 | 35.7 | 48.2 | |||
| 60 | O2 | 1 | 151 | 380 | 340 | 55.8 | 6.7 | 15.402 | |
| 2 | 380 | 600 | 395 | 31.3 | |||||
| Ar | 1 | 170 | 419 | 378 | 47.8 | 31.4 | |||
| 67 | O2 | 1 | 150 | 372 | 337 | 44.1 | 22.1 | 10.966 | |
| 2 | 372 | 600 | 381 | 25.7 | |||||
| Ar | 1 | 161 | 418 | 372 | 37.1 | 46 | |||
| 73 | O2 | 1 | 162 | 377 | 335 | 49.5 | 15.5 | 14.457 | |
| 2 | 377 | 600 | 413 | 30.9 | |||||
| Ar | 1 | 162 | 417 | 370 | 45.8 | 36.1 | |||
| 78 | O2 | 1 | 155 | 378 | 338 | 56.4 | 8.5 | 13.761 | |
| 2 | 379 | 600 | 387 | 29.7 | |||||
| Ar | 1 | 161 | 419 | 378 | 51.9 | 30.4 | |||
| 82 | O2 | 1 | 158 | 372 | 335 | 50.9 | 10.6 | 14.483 | |
| 2 | 372 | 600 | 407 | 34.4 | |||||
| Ar | 1 | 161 | 420 | 374 | 44.6 | 36.4 | |||
| 85 | O2 | 1 | 158 | 380 | 336 | 62.1 | 4.7 | 13.102 | |
| 2 | 380 | 600 | 437 | 29.1 | |||||
| Ar | 1 | 154 | 420 | 377 | 51.2 | 31.1 |
| Sample | Source | Content, % | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | HHV, MJ/kg | Ash, % | ||
| BWW | Own research | 46.2 | 4.99 | 0.44 | 0.21 | 48.1 | 14.85 | 11.7 |
| Furcellaria | [76] | 37.52 | 5.82 | 3.60 | 3.00 | 50.05 | 9.13 | 7.8 |
| Scenedesmus sp. | [77] | 46.3 | 6.81 | 3.28 | 0.28 | 21.5 | 7.0 | |
| Peat pellets | [78] | 58.83 | 5.12 | 1.11 | 36.93 | 21.24 | 3.02 | |
| Coniferous wood | [78] | 48.56 | 11.84 | 0.7 | 0.06 | 38.85 | 19.52 | 0.64 |
| Poplar | [79,80] | 51.60 | 6.00 | 0.60 | 0.02 | 41.70 | 18.3 | 3.77 |
| Rice husk | [81,82] | 49.40 | 6.20 | 0.30 | 0.40 | 43.70 | 15.72 | 3.98 |
| Wheat straw | [78] | 46.62 | 5.09 | 1.31 | 0.11 | 42.72 | 18.47 | 4.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, Y.; Sukhikh, S.; Babich, O.; Yuliya, M.; Krasnovskikh, M.; Noskova, S. Feasibility of Old Bark and Wood Waste Recycling. Plants 2022, 11, 1549. https://doi.org/10.3390/plants11121549
Kulikova Y, Sukhikh S, Babich O, Yuliya M, Krasnovskikh M, Noskova S. Feasibility of Old Bark and Wood Waste Recycling. Plants. 2022; 11(12):1549. https://doi.org/10.3390/plants11121549
Chicago/Turabian StyleKulikova, Yuliya, Stanislav Sukhikh, Olga Babich, Margina Yuliya, Marina Krasnovskikh, and Svetlana Noskova. 2022. "Feasibility of Old Bark and Wood Waste Recycling" Plants 11, no. 12: 1549. https://doi.org/10.3390/plants11121549
APA StyleKulikova, Y., Sukhikh, S., Babich, O., Yuliya, M., Krasnovskikh, M., & Noskova, S. (2022). Feasibility of Old Bark and Wood Waste Recycling. Plants, 11(12), 1549. https://doi.org/10.3390/plants11121549