Genome Size Variation in Dianthus sylvestris Wulfen sensu lato (Caryophyllaceae)
Abstract
:1. Introduction
2. Results
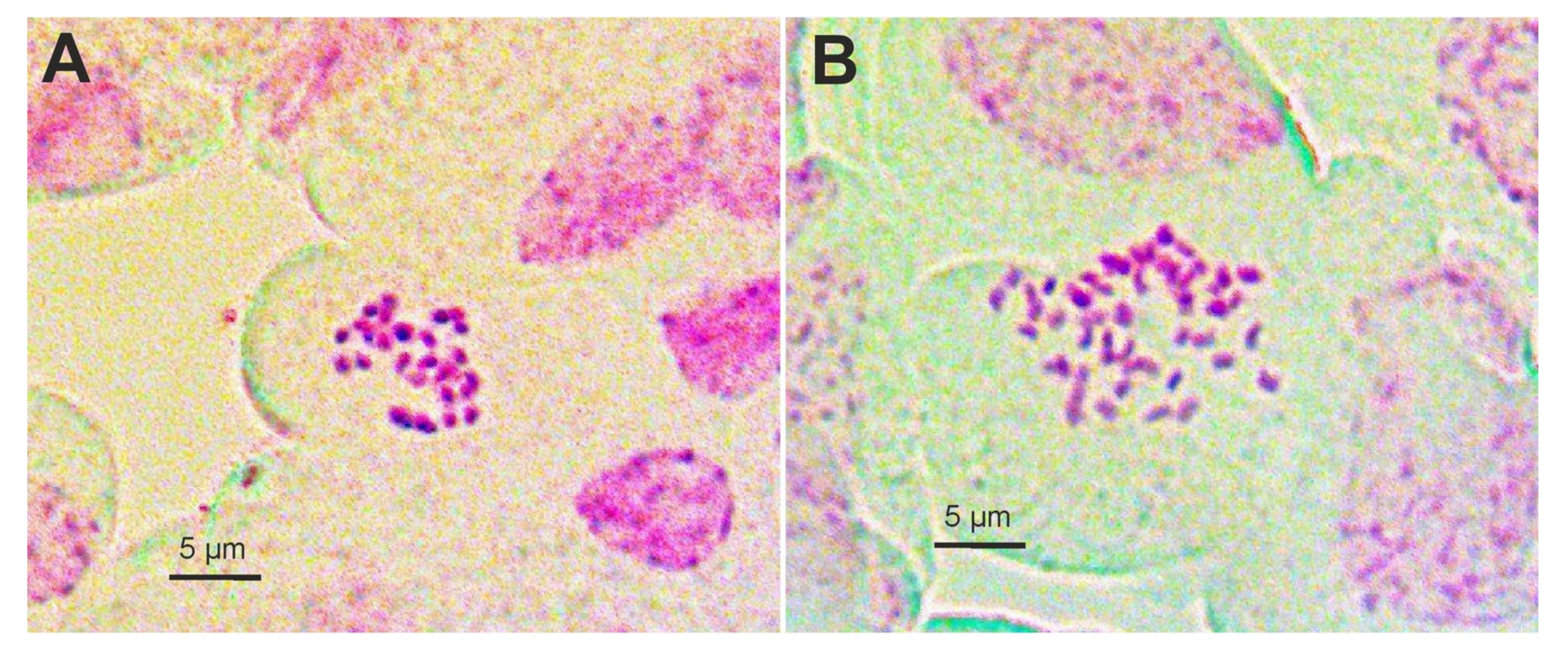
2.1. Chromosome Numbers
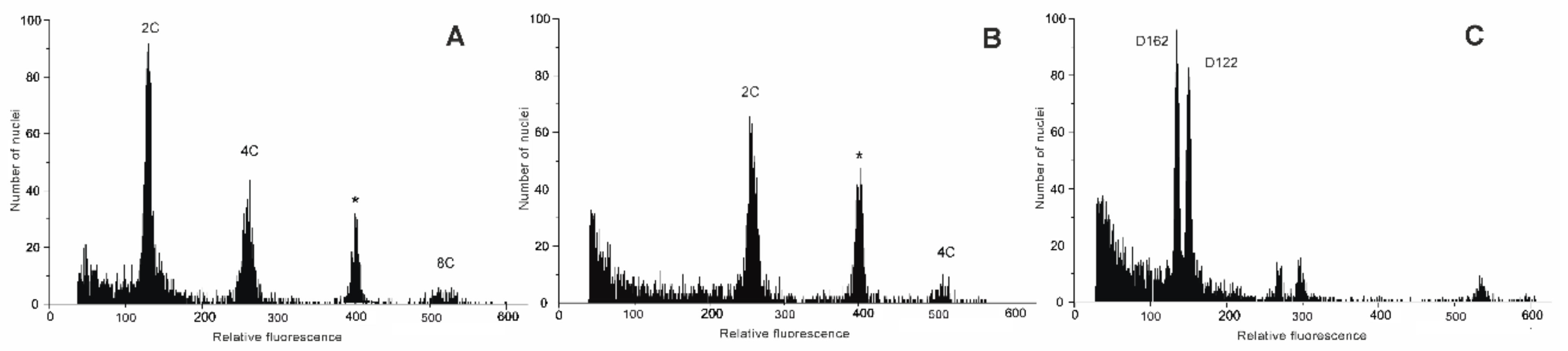
2.2. Relative Genome Size Estimation and DNA Ploidy Level
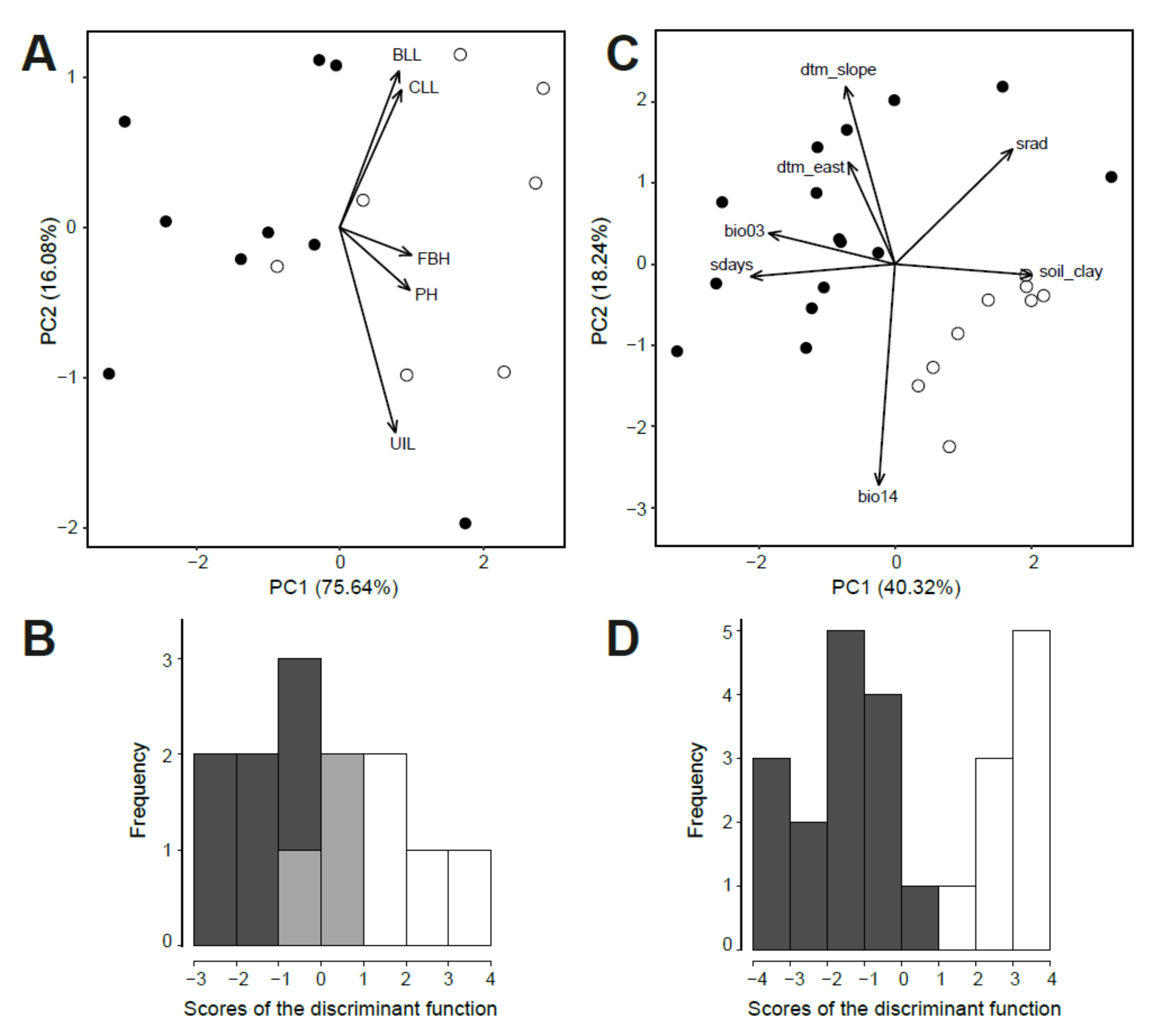
2.3. Morphological and Environmental Differences between Diploids and Tetraploids
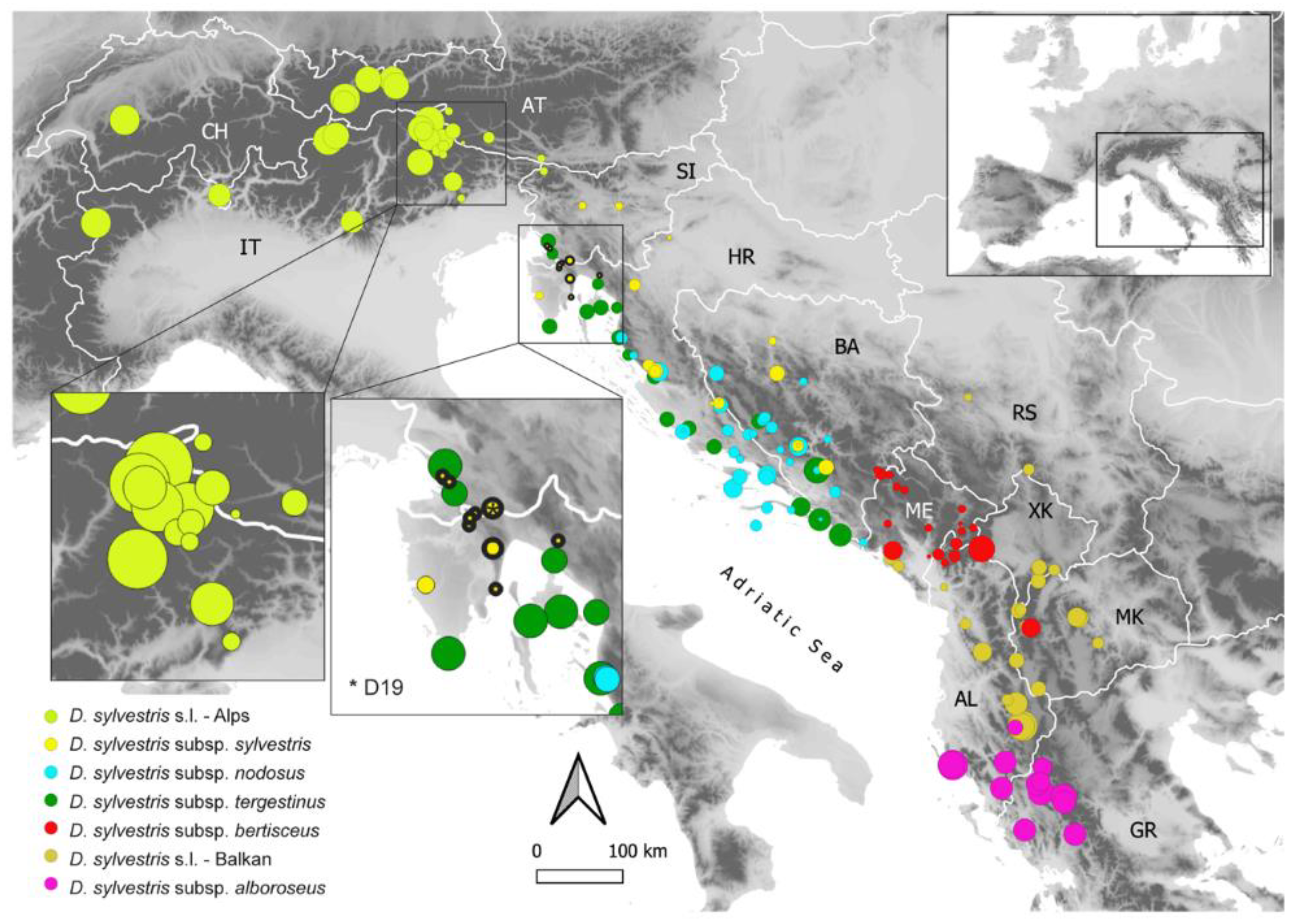
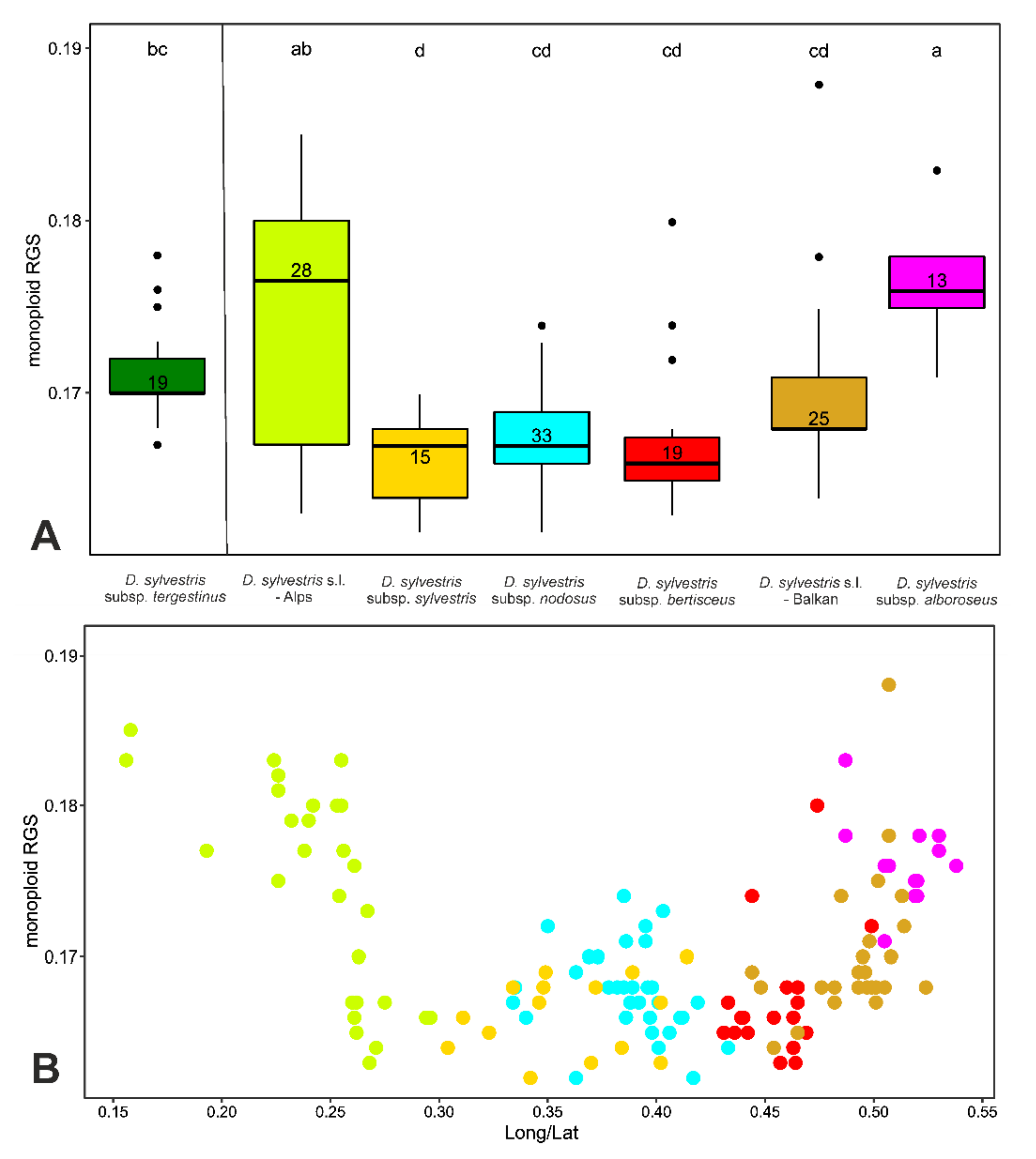
2.4. RGS Variation across Intraspecific Entities
3. Discussion
3.1. Tetraploidization within D. sylvestris Populations in the Northern Balkan Peninsula
3.2. Geographic and Intraspecific Variation of RGS within Diploid D. sylvestris s.l.
4. Materials and Methods
4.1. Plant Material
4.2. Chromosome Counts
4.3. Flow Cytometry
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greilhuber, J.; Doležel, J.; Lysák, M.A.; Bennett, M.D. The Origin, Evolution and Proposed Stabilization of the Terms ‘Genome Size’ and ‘C-Value’ to Describe Nuclear DNA Contents. Ann. Bot. 2005, 95, 255–260. [Google Scholar] [CrossRef]
- Doležel, J.; Bartoš, J. Plant DNA Flow Cytometry and Estimation of Nuclear Genome Size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suda, J.; Krahulcová, A.; Trávníček, P.; Rosenbaumová, R.; Peckert, T.; Krahulec, F. Genome Size Variation and Species Relationships in Hieracium Sub-Genus Pilosella (Asteraceae) as Inferred by Flow Cytometry. Ann. Bot. 2007, 100, 1323–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šmarda, P.; Bureš, P.; Horová, L.; Rotreklová, O. Intrapopulation Genome Size Dynamics in Festuca pallens. Ann. Bot. 2008, 102, 599–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slovák, M.; Vít, P.; Urfus, T.; Suda, J. Complex Pattern of Genome Size Variation in a Polymorphic Member of the Asteraceae. J. Biogeogr. 2009, 36, 372–384. [Google Scholar] [CrossRef]
- Ladner, J.; Mayfield, M.H.; Prather, L.A.; Ferguson, C.J. Polyploidy and Genome Size Variation in Phlox nana (Polemoniaceae) from the Pecos Plains of New Mexico and the Davis Mountains of West Texas, USA. J. Bot. Res. Inst. Tex. 2017, 11, 351–362. [Google Scholar] [CrossRef]
- Pellicer, J.; Hidalgo, O.; Dodsworth, S.; Leitch, I. Genome Size Diversity and Its Impact on the Evolution of Land Plants. Genes 2018, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Nunvářová Kabátová, K.; Kolář, F.; Jarolímová, V.; Krak, K.; Chrtek, J. Does Geography, Evolutionary History or Ecology Drive Ploidy and Genome Size Variation in the Minuartia verna Group (Caryophyllaceae) across Europe? Plant Syst. Evol. 2019, 305, 1019–1040. [Google Scholar] [CrossRef]
- Hodálová, I.; Mártonfiová, L.; Skokanová, K.; Majerová, M.; Somlyay, L.; Mereďa, P. The Utility of Genome Size in Plant Identification: A Case Study on Sesleria (Poaceae) from Croatia and Slovenia. Plant Syst. Evol. 2020, 306, 87. [Google Scholar] [CrossRef]
- Caković, D.; Cresti, L.; Stešević, D.; Schönswetter, P.; Frajman, B. High Genetic and Morphological Diversification of the Euphorbia verrucosa Alliance (Euphorbiaceae) in the Balkan and Iberian Peninsulas. Taxon 2021, 70, 286–307. [Google Scholar] [CrossRef]
- Weiss, H.; Dobeš, C.; Schneeweiss, G.M.; Greimler, J. Occurrence of Tetraploid and Hexaploid Cytotypes between and within Populations in Dianthus Sect. Plumaria (Caryophyllaceae). New Phytol. 2002, 156, 85–94. [Google Scholar] [CrossRef]
- Kolár, F.; Stech, M.; Trávnícek, P.; Rauchová, J.; Urfus, T.; Vít, P.; Kubesová, M.; Suda, J. Towards Resolving the Knautia arvensis Agg. (Dipsacaceae) Puzzle: Primary and Secondary Contact Zones and Ploidy Segregation at Landscape and Microgeographic Scales. Ann. Bot. 2009, 103, 963–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niketić, M.; Siljak-Yakovlev, S.; Frajman, B.; Lazarević, M.; Stevanović, B.; Tomović, G.; Stevanović, V. Towards Resolving the Systematics of Cerastium Subsection Cerastium (Caryophyllaceae): A Cytogenetic Approach. Bot. J. Linn. Soc. 2013, 172, 205–224. [Google Scholar] [CrossRef] [Green Version]
- Frajman, B.; Schönswetter, P.; Weiss-Schneeweiss, H.; Oxelman, B. Origin and Diversification of South American Polyploid Silene Sect. Physolychnis (Caryophyllaceae) in the Andes and Patagonia. Front. Genet. 2018, 9, 639. [Google Scholar] [CrossRef]
- Frajman, B.; Rešetnik, I.; Weiss-Schneeweiss, H.; Ehrendorfer, F.; Schönswetter, P. Cytotype Diversity and Genome Size Variation in Knautia (Caprifoliaceae, Dipsacoideae). BMC Evol. Biol. 2015, 15, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarević, M.; Kuzmanović, N.; Lakušić, D.; Alegro, A.; Schönswetter, P.; Frajman, B. Patterns of Cytotype Distribution and Genome Size Variation in the Genus Sesleria Scop. (Poaceae). Bot. J. Linn. Soc. 2015, 179, 126–143. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.; Costa, L.; Guignard, M.S.; Van-Lume, B.; Pellicer, J.; Gagnon, E.; Leitch, I.J.; Lewis, G.P. Do Tropical Plants Have Smaller Genomes? Correlation between Genome Size and Climatic Variables in the Caesalpinia Group (Caesalpinioideae, Leguminosae). Perspect. Plant Ecol. 2019, 38, 13–23. [Google Scholar] [CrossRef]
- Trávníček, P.; Čertner, M.; Ponert, J.; Chumová, Z.; Jersáková, J.; Suda, J. Diversity in Genome Size and GC Content Shows Adaptive Potential in Orchids and is Closely Linked to Partial Endoreplication, Plant Life-History Traits and Climatic Conditions. New Phytol. 2019, 224, 1642–1656. [Google Scholar] [CrossRef]
- Suda, J.; Trávníček, P. Estimation of Relative Nuclear DNA Content in Dehydrated Plant Tissues by Flow Cytometry. Curr. Protoc. Cytom. 2006, 38, 7.30.1–7.30.14. [Google Scholar] [CrossRef]
- Suda, J.; Kron, P.; Husband, B.C.; Trávníček, P. Flow Cytometry and Ploidy: Applications in Plant Systematics, Ecology and Evolutionary Biology. In Flow Cytometry with Plant Cells; Doležel, J., Greilhuber, J., Suda, J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 103–130. ISBN 978-3-527-61092-1. [Google Scholar]
- Greilhuber, J.; Temsch, E.M.; Loureiro, J.C.M. Nuclear DNA Content Measurement. In Flow Cytometry with Plant Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 67–101. ISBN 978-3-527-61092-1. [Google Scholar]
- Piegu, B.; Guyot, R.; Picault, N.; Roulin, A.; Saniyal, A.; Kim, H.; Collura, K.; Brar, D.S.; Jackson, S.; Wing, R.A.; et al. Doubling Genome Size without Polyploidization: Dynamics of Retrotransposition-Driven Genomic Expansions in Oryza australiensis, a Wild Relative of Rice. Genome Res. 2006, 16, 1262–1269. [Google Scholar] [CrossRef] [Green Version]
- Šmarda, P.; Bureš, P. Intraspecific DNA Content Variability in Festuca pallens on Different Geographical Scales and Ploidy Levels. Ann. Bot. 2006, 98, 665–678. [Google Scholar] [CrossRef] [Green Version]
- Šmarda, P.; Bureš, P. Understanding Intraspecific Variation in Genome Size in Plants. Preslia 2010, 82, 41–61. [Google Scholar]
- Sonnleitner, M.; Hülber, K.; Flatscher, R.; García, P.E.; Winkler, M.; Suda, J.; Schönswetter, P.; Schneeweiss, G.M. Ecological Differentiation of Diploid and Polyploid Cytotypes of Senecio carniolicus Sensu Lato (Asteraceae) is Stronger in Areas of Sympatry. Ann. Bot. 2016, 117, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janišová, M.; Skokanová, K.; Hlásny, T. Ecological Differentiation, Speciation, and Rarity: How Do They Match in Tephroseris longifolia Agg. (Asteraceae)? Ecol. Evol. 2018, 8, 2453–2470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doležel, J.; Greilhuber, J.; Lucretti, S.; Meister, A.; Lysák, M.A.; Nardi, L.; Obermayer, R. Plant Genome Size Estimation by Flow Cytometry: Inter-Laboratory Comparison. Ann. Bot. 1998, 82, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.G. When Does Intraspecific C-Value Variation Become Taxonomically Significant? Ann. Bot. 2005, 95, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Bennetzen, J.L. Plant Retrotransposons. Annu. Rev. Genet. 1999, 33, 479–532. [Google Scholar] [CrossRef] [Green Version]
- Bureš, P.; Wang, Y.-F.; Horova, L.; Suda, J. Genome Size Variation in Central European Species of Cirsium (Compositae) and Their Natural Hybrids. Ann. Bot. 2004, 94, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Bennett, M.D.; Leitch, I.J. Genome Size Evolution in Plants. In The Evolution of the Genome; Gregory, T.R., Ed.; Academic Press: Burlington, MA, USA, 2005; pp. 89–162. ISBN 978-0-12-301463-4. [Google Scholar]
- Vidic, T.; Greilhuber, J.; Vilhar, B.; Dermastia, M. Selective Significance of Genome Size in a Plant Community with Heavy Metal Pollution. Ecol. Appl. 2009, 19, 1515–1521. [Google Scholar] [CrossRef]
- Kang, M.; Wang, J.; Huang, H. Nitrogen Limitation as a Driver of Genome Size Evolution in a Group of Karst Plants. Sci. Rep. 2015, 5, 11636. [Google Scholar] [CrossRef] [Green Version]
- Bilinski, P.; Albert, P.S.; Berg, J.J.; Birchler, J.A.; Grote, M.N.; Lorant, A.; Quezada, J.; Swarts, K.; Yang, J.; Ross-Ibarra, J. Parallel Altitudinal Clines Reveal Trends in Adaptive Evolution of Genome Size in Zea mays. PLoS Genet. 2018, 14, e1007162. [Google Scholar] [CrossRef] [PubMed]
- Cacho, N.I.; McIntyre, P.J.; Kliebenstein, D.J.; Strauss, S.Y. Genome Size Evolution Is Associated with Climate Seasonality and Glucosinolates, but Not Life History, Soil Nutrients or Range Size, across a Clade of Mustards. Ann. Bot. 2021, 127, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Bottini, M.C.J.; Greizerstein, E.J.; Aulicino, M.B.; Poggio, L. Relationships among Genome Size, Environmental Conditions and Geographical Distribution in Natural Populations of NW Patagonian Species of Berberis L. (Berberidaceae). Ann. Bot. 2000, 86, 565–573. [Google Scholar] [CrossRef]
- Knight, C.A.; Molinari, N.A.; Petrov, D.A. The Large Genome Constraint Hypothesis: Evolution, Ecology and Phenotype. Ann. Bot. 2005, 95, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Grime, J.P.; Shacklock, J.M.L.; Brand, S.R. Nuclear DNA Contents, Shoot Phenology and Species Co-Existence in a Limestone Grassland Community. New Phytol. 1985, 100, 435–445. [Google Scholar] [CrossRef]
- Leitch, I.J.; Bennett, M.D. Genome Size and Its Uses: The Impact of Flow Cytometry. In Flow Cytometry with Plant Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 153–176. ISBN 978-3-527-61092-1. [Google Scholar]
- Kraaijeveld, K. Genome Size and Species Diversification. Evol. Biol. 2010, 37, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittrich, V. Caryophyllaceae. In Flowering Plants, Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Kubitzki, K., Rohwer, J.G., Eds.; Springer: Berlin/Heidelberg, Germany; London, UK, 1993; Volume 2, pp. 206–236. ISBN 978-3-642-08141-5. [Google Scholar]
- Valente, L.M.; Savolainen, V.; Vargas, P. Unparalleled Rates of Species Diversification in Europe. Proc. R. Soc. B Biol. Sci. 2010, 277, 1489–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernal, M.; Laínz, M.; Munoz Garmendia, F. Dianthus L. In Flora Iberica; Bolibar, S.C., Ed.; Real Jardín Botánico, C.S.I.C.: Madrid, Spain, 1990; Volume 2, pp. 426–462. [Google Scholar]
- Tutin, T.G.; Walters, S.M. Dianthus L. In Flora Europaea; Tutin, T.G., Ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1993; Volume 1, pp. 227–246. ISBN 978-0-521-41007-6. [Google Scholar]
- Bacchetta, G.; Brullo, S.; Casti, M.; del Galdo, G.P.G. Taxonomic Revision of the Dianthus sylvestris Group (Caryophyllaceae) in Central-Southern Italy, Sicily and Sardinia. Nord. J. Bot. 2010, 28, 137–173. [Google Scholar] [CrossRef]
- Domina, G.; Scibetta, S.; Scafidi, F.; Giovino, A. Contribution to the Identification of Dianthus rupicola (Caryophyllaceae) Subspecies Using Morphological and Molecular Approaches. Phytotaxa 2017, 291, 17–32. [Google Scholar] [CrossRef]
- Brullo, S.; Guarino, R. Dianthus. In Flora d’Italia; Pignatti, S., Ed.; Edagricole: Milano, Italy, 2019; Volume 4, ISBN 978-88-506-5245-7. [Google Scholar]
- Hardion, L.; Perrier, A.; Martinez, M.; Navrot, N.; Gaquerel, E.; Tournay, F.; Nguefack, J.; Combroux, I. Integrative Revision of Dianthus superbus Subspecies Reveals Different Degrees of Differentiation, from Plasticity to Species Distinction. Syst. Biodivers. 2020, 18, 255–268. [Google Scholar] [CrossRef]
- Domina, G.; Astuti, G.; Bacchetta, G.; Barone, G.; Rešetnik, I.; Terlević, A.; Thiébaut, M.; Peruzzi, L. Typification of 14 Names in the Dianthus virgineus Group (Caryophyllaceae). PhytoKeys 2021, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Domina, G.; Astuti, G.; Barone, G.; Gargano, D.; Minuto, L.; Varaldo, L.; Peruzzi, L. Lectotypification of the Linnaean Name Dianthus virgineus (Caryophyllaceae) and Its Taxonomic Consequences. Taxon 2021, 70, 1096–1100. [Google Scholar] [CrossRef]
- Gammella, M. Local Adaptation and Gene Flow in Serpentine and Limestone Populations of D. sylvestris. Doctoral dissertation, Università Degli Studi di Napoli Federico II, Naples, Italy, 2016. [Google Scholar]
- Beck-Mannagetta, G. Flora Bosne, Hercegovine i Novopazarskog Sandžaka; Glasnik Zemaljskog muzeja u Bosni i Hercegovini: Sarajevo, Bosnia and Herzegovina, 1909; Volume 21. [Google Scholar]
- Von Hayek, A. Prodromus Florae Peninsulae Balcanicae; Verlag des Repertoriums: Dahlem bei Berlin, Germany, 1924. [Google Scholar]
- Gjurašin, S. Rod Dianthus u Flori Hrvatske i Slavonije, 18th ed.; Prirodoslovna istraživanja Kraljevine Jugoslavije; Jugoslavenska Akademija Znanosti i Umjetnosti: Zagreb, Croatia, 1933. [Google Scholar]
- Mayer, E.; Trpin, D. Dianthus Sylvestris—Kompleks v Jugoslaviji. Biološki Vestnik 1965, 13, 53–59. [Google Scholar]
- Trinajstić, I. Analitička Flora Jugoslavije; Institut za botaniku Sveučilišta u Zagrebu: Zagreb, Croatia, 1979. [Google Scholar]
- Greuter, W.; Burdet, H.M.; Long, G. Med-Checklist. 1; des Conservatoire et Jardin Botaniques de la Ville de Genève: Geneva, Switzerland, 1984; ISBN 978-2-8277-0151-3. [Google Scholar]
- Marhold, K. Caryophyllaceae. Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: https://www.emplantbase.org/home.html (accessed on 20 December 2020).
- Meyer, F.K. Beiträge zur Flora von Albanien; Thüringische Botanische Gesellschaft e.V.: Oberhof, Germany, 2011. [Google Scholar]
- Terlević, A.; Temunović, M.; Bogdanović, S.; Grgurev, M.; Ljubičić, I.; Rešetnik, I. Morphological and Ecological Variability of Dianthus sylvestris Wulfen (Caryophyllaceae) on the Balkan Peninsula. Bot. J. Linn. Soc. 2022, submitted.
- Carolin, R.C. Cytological and Hybridization Studies in the Genus Dianthus. New Phytol. 1957, 56, 81–97. [Google Scholar] [CrossRef]
- Moore, D.M. Flora Europaea Check-List and Chromosome Index; Cambridge University Press: Cambridge UK; New York, NY, USA, 1982; ISBN 978-0-521-23759-8. [Google Scholar]
- Rice, A.; Glick, L.; Abadi, S.; Einhorn, M.; Kopelman, N.M.; Salman-Minkov, A.; Mayzel, J.; Chay, O.; Mayrose, I. The Chromosome Counts Database (CCDB)—A Community Resource of Plant Chromosome Numbers. New Phytol. 2015, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Leitch, I.J. The Plant DNA C-Values Database (Release 7.1): An Updated Online Repository of Plant Genome Size Data for Comparative Studies. New Phytol. 2020, 226, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Balao, F.; Casimiro-Soriguer, R.; Talavera, M.; Herrera, J.; Talavera, S. Distribution and Diversity of Cytotypes in Dianthus broteri as Evidenced by Genome Size Variations. Ann. Bot. 2009, 104, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Behroozian, M.; Vaezi, J.; Joharchi, M.R. A Karyological Study of Some Dianthus L. Species (Caryophyllaceae) in Northeast of Iran. Feddes Repert. 2012, 123, 265–272. [Google Scholar] [CrossRef]
- Şahin, E.; Eroğlu, H.E.; Hamzaoğlu, E.; Koç, M. Karyotype Analysis of Four Species of Dianthus Section Fimbriati (Caryophyllaceae, Sileneae). Caryologia 2016, 69, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Gatt, M.K.; Hammett, K.R.W.; Markham, K.R.; Murray, B.G. Yellow Pinks: Interspecific Hybridization between Dianthus plumarius and Related Species with Yellow Flowers. Sci. Hortic. 1998, 77, 207–218. [Google Scholar] [CrossRef]
- Siljak-Yakovlev, S.; Pustahija, F.; Šolić, E.M.; Bogunić, F.; Muratović, E.; Bašić, N.; Catrice, O.; Brown, S.C. Towards a Genome Size and Chromosome Number Database of Balkan Flora: C-Values in 343 Taxa with Novel Values for 242. Adv. Sci. Lett. 2010, 3, 190–213. [Google Scholar] [CrossRef]
- Löve, Á. IOPB Chromosome Number Reports. XVIII. Taxon 1968, 17, 419–422. [Google Scholar] [CrossRef]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of Nuclear DNA Content in Plants Using Flow Cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Barow, M.; Jovtchev, G. Endopolyploidy in Plants and Its Analysis by Flow Cytometry. In Flow Cytometry with Plant Cells; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 349–372. ISBN 978-3-527-61092-1. [Google Scholar]
- Záveská, E.; Maylandt, C.; Paun, O.; Bertel, C.; Frajman, B.; The STEPPE Consortium; Schönswetter, P. Multiple Auto- and Allopolyploidisations Marked the Pleistocene History of the Widespread Eurasian Steppe Plant Astragalus onobrychis (Fabaceae). Mol. Phylogenet. Evol. 2019, 139, 106572. [Google Scholar] [CrossRef] [PubMed]
- Niketić, M.; Đurović, S.Z.; Tomović, G.; Schönswetter, P.; Frajman, B. Diversification within Ploidy-Variable Balkan Endemic Cerastium decalvans (Caryophyllaceae) Reconstructed Based on Genetic, Morphological and Ecological Evidence. Bot. J. Linn. Soc. 2021, 199, 578–608. [Google Scholar] [CrossRef]
- Balao, F.; Valente, L.M.; Vargas, P.; Herrera, J.; Talavera, S. Radiative Evolution of Polyploid Races of the Iberian Carnation Dianthus broteri (Caryophyllaceae). New Phytol. 2010, 187, 542–551. [Google Scholar] [CrossRef]
- López-Jurado, J.; Mateos-Naranjo, E.; Balao, F. Niche Divergence and Limits to Expansion in the High Polyploid Dianthus broteri Complex. New Phytol. 2019, 222, 1076–1087. [Google Scholar] [CrossRef]
- Soltis, P.S.; Marchant, D.B.; Van de Peer, Y.; Soltis, D.E. Polyploidy and Genome Evolution in Plants. Curr. Opin. Genet. Dev. 2015, 35, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Soltis, D.E.; Visger, C.J.; Marchant, D.B.; Soltis, P.S. Polyploidy: Pitfalls and Paths to a Paradigm. Am. J. Bot. 2016, 103, 1146–1166. [Google Scholar] [CrossRef] [Green Version]
- Leitch, I.J.; Bennett, M.D. Genome Downsizing in Polyploid Plants. Biol. J. Linn. Soc. 2004, 82, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Weiss-Schneeweiss, H.; Greilhuber, J.; Schneeweiss, G.M. Genome Size Evolution in Holoparasitic Orobanche (Orobanchaceae) and Related Genera. Am. J. Bot. 2006, 93, 148–156. [Google Scholar] [CrossRef]
- Tsukaya, H. Controlling Size in Multicellular Organs: Focus on the Leaf. PLoS Biol. 2008, 6, e174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flatscher, R.; García, P.E.; Hülber, K.; Sonnleitner, M.; Winkler, M.; Saukel, J.; Schneeweiss, G.M.; Schönswetter, P. Underestimated Diversity in One of the World’s Best Studied Mountain Ranges: The Polyploid Complex of Senecio carniolicus (Asteraceae) Contains Four Species in the European Alps. Phytotaxa 2015, 213, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, M.; Flatscher, R.; García, P.E.; Rauchová, J.; Suda, J.; Schneeweiss, G.M.; Hülber, K.; Schönswetter, P. Distribution and Habitat Segregation on Different Spatial Scales among Diploid, Tetraploid and Hexaploid Cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps. Ann. Bot. 2010, 106, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Duchoslav, M.; Jandová, M.; Kobrlová, L.; Šafářová, L.; Brus, J.; Vojtěchová, K. Intricate Distribution Patterns of Six Cytotypes of Allium oleraceum at a Continental Scale: Niche Expansion and Innovation Followed by Niche Contraction With Increasing Ploidy Level. Front. Plant Sci. 2020, 11, 1885. [Google Scholar] [CrossRef]
- Weiss-Schneeweiss, H.; Emadzade, K.; Jang, T.-S.; Schneeweiss, G.M. Evolutionary Consequences, Constraints and Potential of Polyploidy in Plants. Cytogenet. Genome Res. 2013, 140, 137–150. [Google Scholar] [CrossRef]
- Parisod, C.; Besnard, G. Glacial in Situ Survival in the Western Alps and Polytopic Autopolyploidy in Biscutella laevigata L. (Brassicaceae). Mol. Ecol. 2007, 16, 2755–2767. [Google Scholar] [CrossRef] [Green Version]
- Kolář, F.; Lučanová, M.; Vít, P.; Urfus, T.; Chrtek, J.; Fér, T.; Ehrendorfer, F.; Suda, J. Diversity and Endemism in Deglaciated Areas: Ploidy, Relative Genome Size and Niche Differentiation in the Galium pusillum Complex (Rubiaceae) in Northern and Central Europe. Ann. Bot. 2013, 111, 1095–1108. [Google Scholar] [CrossRef] [Green Version]
- Castro, M.; Loureiro, J.; Serrano, M.; Tavares, D.; Husband, B.C.; Siopa, C.; Castro, S. Mosaic Distribution of Cytotypes in a Mixed-Ploidy Plant Species, Jasione montana: Nested Environmental Niches but Low Geographical Overlap. Bot. J. Linn. Soc. 2019, 190, 51–66. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömöry, D.; Latałowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A New Scenario for the Quaternary History of European Beech Populations: Palaeobotanical Evidence and Genetic Consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial Refugia Influence Plant Diversity Patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of Three DNA Fluorochromes for Flow Cytometric Estimation of Nuclear DNA Content in Plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Schönswetter, P.; Lachmayer, M.; Lettner, C.; Prehsler, D.; Rechnitzer, S.; Reich, D.S.; Sonnleitner, M.; Wagner, I.; Hülber, K.; Schneeweiss, G.M.; et al. Sympatric Diploid and Hexaploid Cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps Are Separated along an Altitudinal Gradient. J. Plant Res. 2007, 120, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Caković, D.; Stešević, D.; Schönswetter, P.; Frajman, B. How Many Taxa? Spatiotemporal Evolution and Taxonomy of Amphoricarpos (Asteraceae, Carduoideae) on the Balkan Peninsula. Org. Divers. Evol. 2015, 15, 429–445. [Google Scholar] [CrossRef]
- Falch, M.; Schönswetter, P.; Frajman, B. Both Vicariance and Dispersal Have Shaped the Genetic Structure of Eastern Mediterranean Euphorbia myrsinites (Euphorbiaceae). Perspect. Plant Ecol. 2019, 39, 125459. [Google Scholar] [CrossRef]
- Đurović, S.; Schönswetter, P.; Niketić, M.; Tomović, G.; Frajman, B. Disentangling Relationships among the Members of the Silene saxifraga Alliance (Caryophyllaceae): Phylogenetic Structure Is Geographically Rather than Taxonomically Segregated. Taxon 2017, 66, 343–364. [Google Scholar] [CrossRef]
- Schönswetter, P.; Stehlik, I.; Holderegger, R.; Tribsch, A. Molecular Evidence for Glacial Refugia of Mountain Plants in the European Alps. Mol. Ecol. 2005, 14, 3547–3555. [Google Scholar] [CrossRef]
- Qiu, F.; Baack, E.J.; Whitney, K.D.; Bock, D.G.; Tetreault, H.M.; Rieseberg, L.H.; Ungerer, M.C. Phylogenetic Trends and Environmental Correlates of Nuclear Genome Size Variation in Helianthus Sunflowers. New Phytol. 2019, 221, 1609–1618. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.A.; Ackerly, D.D. Variation in Nuclear DNA Content across Environmental Gradients: A Quantile Regression Analysis. Ecol. Lett. 2002, 5, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Micevski, K. Flora na SR Makedonija; Makedonska Akademija na Naukite i Umetnostite: Skopje, North Macedonia, 1993; Volume 1. [Google Scholar]
- Martinčič, A. Mala Flora Slovenije, 4th ed.; Tehniška Založba Slovenije: Ljubljana, Slovenia, 2007; ISBN 978-961-251-026-8. [Google Scholar]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. (Eds.) Vascular Plants of Greece: An Annotated Checklist; Englera; Botanic Garden and Botanical Museum Berlin-Dahlem, Berlin and Hellenic botanical society: Athens, Greek, 2013; ISBN 978-3-921800-88-1. [Google Scholar]
- Stešević, D.; Caković, D. Katalog Vaskularne Flore Crne Gore; Crnogorska Akademija Nauka i Umjetnosti: Podgorica, Montenegro, 2013; Volume 1. [Google Scholar]
- Vangjeli, J. Excursion Flora of Albania; Koeltz Scientific Books: Oberreifenberg, Germany, 2015; ISBN 978-3-87429-477-5. [Google Scholar]
- Barina, Z.; Somogyi, G.; Pifkó, D.; Rakaj, M. Checklist of vascular plants of Albania. Phytotaxa 2018, 378, 1–339. [Google Scholar] [CrossRef]
- Nikolić, T. Flora Croatica Database. Prirodoslovno-Matematički Fakultet, Sveučilište u Zagrebu. Available online: http://hirc.botanic.hr/fcd (accessed on 7 June 2020).
- Suda, J.; Trávníček, P. Reliable DNA Ploidy Determination in Dehydrated Tissues of Vascular Plants by DAPI Flow Cytometry—New Prospects for Plant Research. Cytom. Part A 2006, 69A, 273–280. [Google Scholar] [CrossRef]
- Suda, J.; Krahulcová, A.; Trávníek, P.; Krahulec, F. Ploidy Level versus DNA Ploidy Level: An Appeal for Consistent Terminology. Taxon 2006, 55, 447–450. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at High Resolution for the Earth’s Land Surface Areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karger, D.N.; Schmatz, D.R.; Dettling, G.; Zimmermann, N.E. High-Resolution Monthly Precipitation and Temperature Time Series from 2006 to 2100. Sci. Data 2020, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.M.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global Gridded Soil Information Based on Machine Learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [Green Version]
- Amatulli, G.; Domisch, S.; Tuanmu, M.-N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. A Suite of Global, Cross-Scale Topographic Variables for Environmental and Biodiversity Modeling. Sci. Data 2018, 5, 180040. [Google Scholar] [CrossRef] [Green Version]
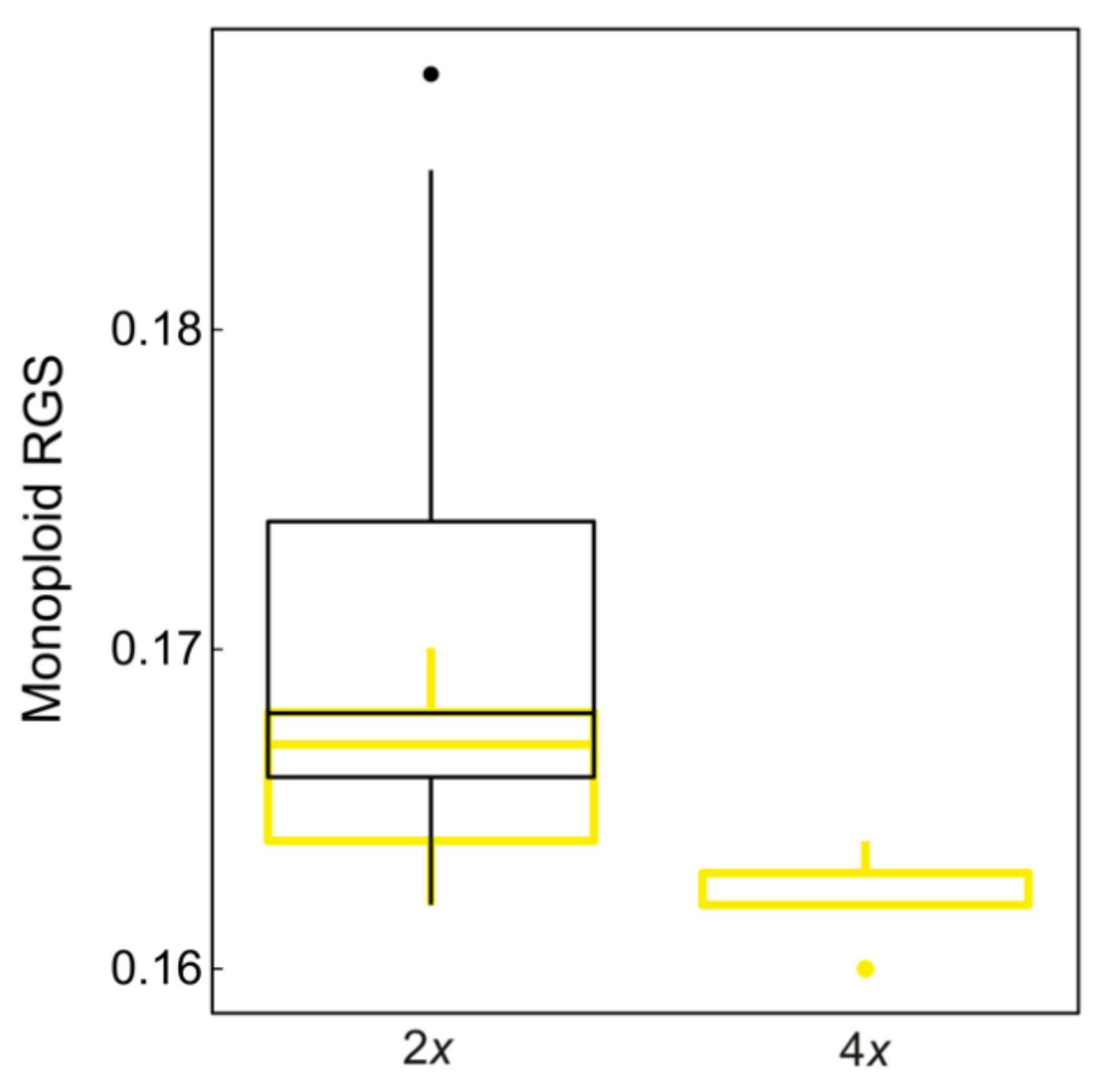
| Ploidy | No. Measurements | Populations | Individuals | Min. No. Individuals | Max. No. Individuals | Mean No. Individuals | Mean RGS | SD RGS | Min RGS | Max RGS | Mean mRGS | SD mRGS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2x | 536 | 152 | 657 | 3 | 12 | 4.3 | 0.341 | 0.011 | 0.324 | 0.376 | 0.17 | 0.005 |
| 4x | 45 | 10 | 50 | 3 | 8 | 5 | 0.649 | 0.006 | 0.64 | 0.657 | 0.162 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terlević, A.; Bogdanović, S.; Frajman, B.; Rešetnik, I. Genome Size Variation in Dianthus sylvestris Wulfen sensu lato (Caryophyllaceae). Plants 2022, 11, 1481. https://doi.org/10.3390/plants11111481
Terlević A, Bogdanović S, Frajman B, Rešetnik I. Genome Size Variation in Dianthus sylvestris Wulfen sensu lato (Caryophyllaceae). Plants. 2022; 11(11):1481. https://doi.org/10.3390/plants11111481
Chicago/Turabian StyleTerlević, Ana, Sandro Bogdanović, Božo Frajman, and Ivana Rešetnik. 2022. "Genome Size Variation in Dianthus sylvestris Wulfen sensu lato (Caryophyllaceae)" Plants 11, no. 11: 1481. https://doi.org/10.3390/plants11111481
APA StyleTerlević, A., Bogdanović, S., Frajman, B., & Rešetnik, I. (2022). Genome Size Variation in Dianthus sylvestris Wulfen sensu lato (Caryophyllaceae). Plants, 11(11), 1481. https://doi.org/10.3390/plants11111481






