Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes
Abstract
:1. Introduction
2. Results
2.1. Determination of the Composition of Essential Oil and n-Hexane Extract
2.2. Antibacterial Assay
2.3. Real-Time Ethidium Bromide Accumulation Assay
2.4. Biofilm Formation Inhibitory Effect
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Solvents and Chemicals
4.3. Preparation of the Extracts for the Bioassays
4.4. Essential Oil Distillation
4.5. Determination of the Composition of Essential Oil by GC and GC-MS
4.6. Bacterial and Fungal Strains and Culture Conditions for Antimicrobial Assay
4.7. Determination of Antibacterial Activity by Disc Diffusion Method
4.8. Determination of MIC Values
4.9. Bacterial Strains for Efflux Pump Inhibitory Assay
4.10. Real-Time Ethidium Bromide Accumulation Assay
4.11. Inhibition of Biofilm Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Zhou, L.; Attia, F.A.-Z.K.K.; Tang, Q.; Wang, M.; Liu, Z.; Waterhouse, G.I.N.; Liu, L.; Kang, W. Origanum majorana L.: A Nutritional Supplement with Immunomodulatory Effects. Front. Nutr. 2021, 8, 748031. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.; Satyanarayana, S.; Khan, K.A.; Raja, K. An Updated Review on Traditional Uses, Taxonomy, Phytochemistry, Pharmacology and Toxicology of Origanum majorana. Int. J. Pharm. Res. Health Sci. 2017, 5, 1717–1723. [Google Scholar]
- Bina, F.; Rahimi, R. Sweet Marjoram: A Review of Ethnopharmacology, Phytochemistry, and Biological Activities. J. Evid. Based Complementary Altern. Med. 2017, 22, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Amor, G.; Caputo, L.; La Storia, A.; De Feo, V.; Mauriello, G.; Fechtali, T. Chemical Composition and Antimicrobial Activity of Artemisia herba-alba and Origanum majorana Essential Oils from Morocco. Molecules 2019, 24, 4021. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatimi, M. Volatile Constituents, Antimicrobial and Antioxidant Activities of the Aerial Parts of Origanum majorana L. from Yemen. J. Pharm. Res. Int. 2018, 23, JPRI.35932. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Nasser, A.; El Gendy, G.; Saleh, I.A.; Esawy, M.A. Chemical Composition and Evaluation of Antimicrobial Activity of the Origanum majorana Essential Oil Extracted by Microwave-Assisted Extraction, Conventional Hydro-distillation and Steam Distillation. J. Essent. Oil Bear. Plants 2019, 22, 563–573. [Google Scholar] [CrossRef]
- Lagha, R.; Ben Abdallah, F.; Al-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S.; Rojas, L.B.; Lucena, M.E.; Meccia, G.; Usubillaga, A. Chemical Composition and Antibacterial Activity of Origanum majorana L. Essential Oil from the Venezuelan Andes. J. Essent. Oil Res. 2011, 23, 45–49. [Google Scholar] [CrossRef]
- Ben Salha, G.; Díaz, R.H.; Lengliz, O.; Abderrabba, M.; Labidi, J. Effect of the Chemical Composition of Free-Terpene Hydrocarbons Essential Oils on Antifungal Activity. Molecules 2019, 24, 3532. [Google Scholar] [CrossRef] [Green Version]
- Zeouk, I.; Lalami, A.E.O.; Bekhti, K. In Vitro Antibacterial Activity of Medicinal Plants in the Central North of Morocco: A Possible Source of Alternative Drugs against Methicillin-Resistant Staphylococcus aureus. Asian J. Pharm. Clin. Res. 2019, 12, 285–292. [Google Scholar] [CrossRef]
- Vasireddy, L.; Lewis, E.H.; Bingle, M.S.D. Antimicrobial Activity of Essential Oils against Multidrug-Resistant Clinical Isolates of the Burkholderia cepacia Complex. PLoS ONE 2018, 13, e0201835. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.S.X.; Lim, S.H.E.; Hu, C.P.; Yiap, B.C. Combination of Essential Oils and Antibiotics Reduce Antibiotic Resistance in Plasmid-Conferred Multidrug Resistant Bacteria. Phytomedicine 2013, 20, 710–713. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-Biofilm Forming and Anti-Quorum Sensing Activity of Selected Essential Oils and their Main Components on Food-Related Microorganisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef] [Green Version]
- Khadhri, A.; Bouali, I.; Aouadhi, C.; Lagel, M.-C.; Masson, E.; Pizzi, A. Determination of Phenolic Compounds by MALDI–TOF and Essential Oil Composition by GC–MS during Three Development Stages of Origanum majorana L. Biomed. Chromatogr. 2019, 33, e4665. [Google Scholar] [CrossRef]
- Tabanca, N.; Özek, T.; Baser, K.H.C. Comparison of the Essential Oils of Origanum majorana L. and Origanum × majoricum Cambess. J. Essent. Oil Res. 2004, 16, 248–252. [Google Scholar] [CrossRef]
- Kincses, A.; Szabó, S.; Rácz, B.; Szemerédi, N.; Watanabe, G.; Saijo, R.; Sekiya, H.; Tamai, E.; Molnár, J.; Kawase, M.; et al. Benzoxazole-Based Metal Complexes to Reverse Multidrug Resistance in Bacteria. Antibiotics 2020, 9, 649. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Donadu, M.G.; Usai, M.; Marchetti, M.; Ferrari, M.; Mazzarello, V.; Zanetti, S.; Nagy, F.; Kovács, R. Evaluation of the Antimicrobial and Antivirulent Potential of Essential Oils Isolated from Juniperus oxycedrus L. ssp. macrocarpa Aerial Parts. Microorganisms 2022, 10, 758. [Google Scholar] [CrossRef]
- Guerra-Boone, L.; Alvarez-Roman, R.; Salazar-Aranda, R.; Cirio, A.T.; Rivas-Galindo, V.M.; de Torres, N.W.; Gonzalez, G.; Lopez, L.A.P. Antimicrobial and Antioxidant Activities and Chemical Characterization of Essential Oils of Thymus vulgaris, Rosmarinus officinalis, and Origanum majorana from Northeastern Mexico. Pak. J. Pharm. Sci. 2015, 28, 363–369. [Google Scholar]
- Nové, M.; Kincses, A.; Szalontai, B.; Rácz, B.; Blair, J.M.A.; González-Prádena, A.; Benito-Lama, M.; Domínguez-Álvarez, E.; Spengler, G. Biofilm Eradication by Symmetrical Selenoesters for Food-Borne Pathogens. Microorganisms 2020, 8, 566. [Google Scholar] [CrossRef] [Green Version]
- Carson, C.F.; Riley, T.V. Antimicrobial Activity of the Major Components of the Essential Oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Szemerédi, N.; Kincses, A.; Rehorova, K.; Hoang, L.; Salardón-Jiménez, N.; Sevilla-Hernández, C.; Viktorová, J.; Domínguez-Álvarez, E.; Spengler, G. Ketone- and Cyano-Selenoesters to Overcome Efflux Pump, Quorum-Sensing, and Biofilm-Mediated Resistance. Antibiotics 2020, 9, 896. [Google Scholar] [CrossRef]
- Yoshida, N.; Koizumi, M.; Adachi, I.; Kawakami, J. Inhibition of P-Glycoprotein-Mediated Transport by Terpenoids Contained in Herbal Medicines and Natural Products. Food Chem. Toxicol. 2006, 44, 2033–2039. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and Mechanisms of Essential Oils for Biofilm Control on Food-Contact Surfaces: An Updated Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Aradski, A.A.; Kolarević, S.; Vuković-Gačić, B.; Oalđe, M.; Živković, J.; Šavikin, K.; Marin, P.D. Antineurodegenerative, Antioxidant and Antibacterial Activities and Phenolic Components of Origanum majorana L. (Lamiaceae) Extracts. J. Appl. Bot. Food Qual. 2018, 91, 126–134. [Google Scholar]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic Acid Interaction with Planktonic and Biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1747–1750. [Google Scholar] [CrossRef] [Green Version]
- Karunanidhi, A.; Thomas, R.; van Belkum, A.; Neela, V. In Vitro Antibacterial and Antibiofilm Activities of Chlorogenic Acid against Clinical Isolates of Stenotrophomonas maltophilia including the Trimethoprim/Sulfamethoxazole Resistant Strain. Biomed. Res. Int. 2013, 2013, 392058. [Google Scholar] [CrossRef] [Green Version]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Flavor and Fragrance Natural and Synthetic Compounds Library, Shimadzu Europa GmbH, Duisburg, Germany. Available online: https://www.shimadzu.fr/flavour-and-fragrance-natural-and-synthetic-compounds-library (accessed on 8 February 2022).
- Kovats, E. Gas chromatographic characterization of organic substances in the retention index system. In Advances in Chromatography; Giddings, J.C., Keller, R.A., Eds.; Marcel Dekker: New York, NY, USA, 1965; Volume 1, pp. 229–247. [Google Scholar]
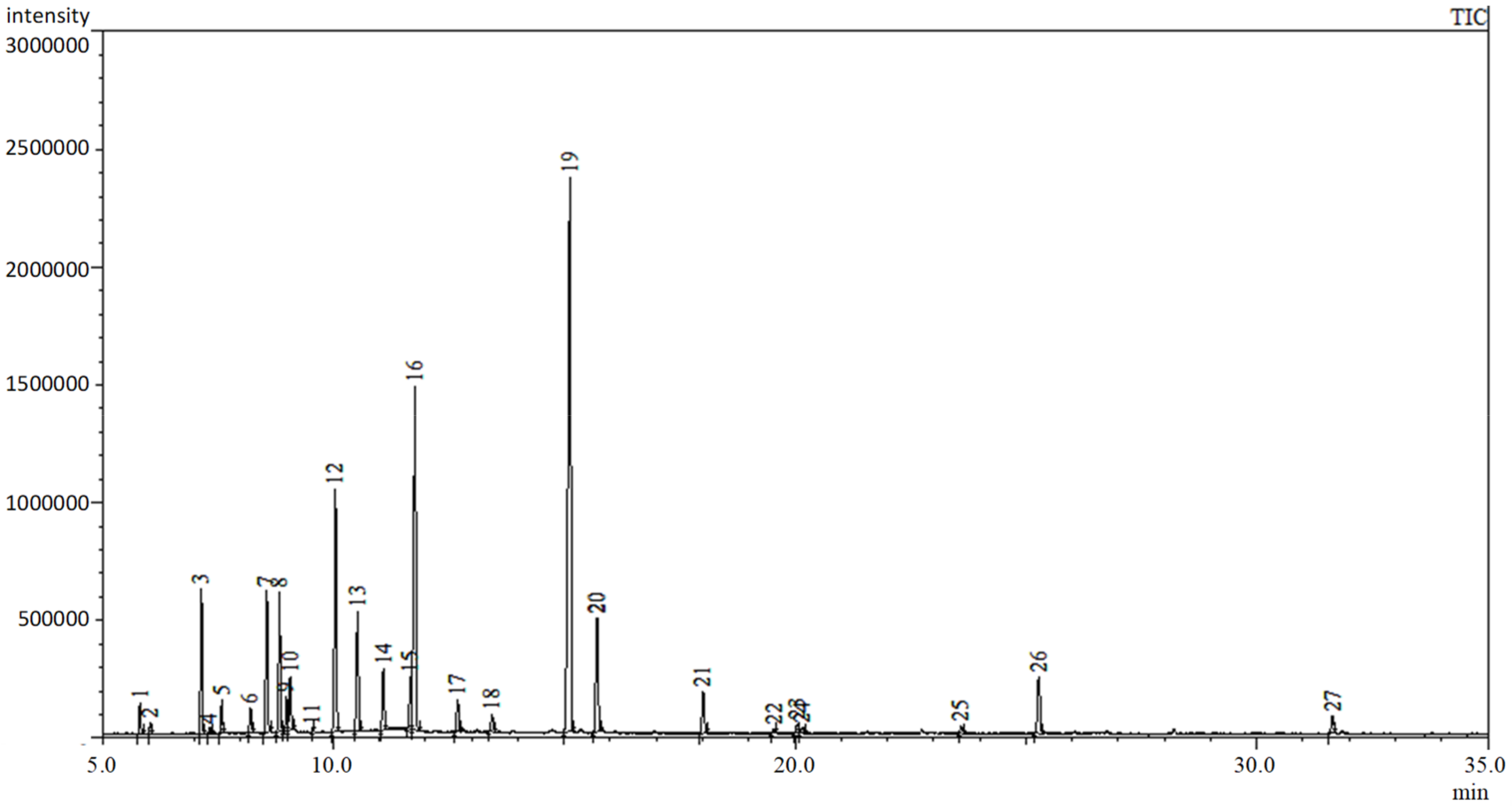
| Compounds a | RI b | % in Sample | |
|---|---|---|---|
| 1 | α-thujene | 924 | 0.51 |
| 2 | α-pinene | 932 | 0.43 |
| 3 | sabinene | 972 | 4.53 |
| 4 | β-pinene | 978 | 0.26 |
| 5 | myrcene | 987 | 1.23 |
| 6 | α-phelladrene | 1007 | 0.14 |
| 7 | α-terpinene | 1017 | 3.00 |
| 8 | p-cymene | 1024 | 4.72 |
| 9 | limonene | 1028 | 0.20 |
| 10 | β-phelladrene | 1030 | 2.14 |
| 11 | β-ocimene | 1044 | 0.10 |
| 12 | γ-terpinene | 1057 | 6.48 |
| 13 | cis-sabinene hydrate | 1070 | 5.44 |
| 14 | terpinolene | 1085 | 1.62 |
| 15 | linalool | 1101 | 0.10 |
| 16 | trans-sabinene hydrate | 1103 | 25.18 |
| 17 | cis-p-menth-2-en-1-ol | 1125 | 2.35 |
| 18 | trans-p-menth-2-en-1-ol | 1142 | 1.34 |
| 19 | terpinen-4-ol | 1182 | 24.92 |
| 20 | α-terpineol | 1196 | 4.43 |
| 21 | linalyl acetate | 1249 | 1.92 |
| 22 | bornyl acetate | 1284 | 0.27 |
| 23 | terpin-1-en-4-yl acetate | 1295 | 0.36 |
| 24 | carvacrol | 1298 | 0.27 |
| 25 | trans-geranyl acetate | 1377 | 0.28 |
| 26 | β-caryophyllene | 1417 | 1.98 |
| 27 | spatulenol | 1574 | 0.47 |
| Total | 94.67 |
| Sample | S. aureus ATCC 29213 | S. aureus MRSA ATCC 43300 | S. epidermidis ATCC 12228 | E. faecalis ATCC 29212 | E. coli ATCC 35218 | E. coli AG-100 | K. pneumoniae ATCC 700603 | P. aeruginosa ATCC 27853 | C. albicans ATCC 10231 |
|---|---|---|---|---|---|---|---|---|---|
| MeOH extract | 7 ± 0.3 | 7 ± 0.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| n-hexane extract | 7 ± 0.5 | 7 ± 0.3 | 7 ± 0 | 0 | 8 ± 0.2 | 7 ± 0 | 8 ± 0 | 7 ± 0.2 | 7 ± 0 |
| Essential oil | 0 | 0 | 0 | 0 | 7 ± 0.2 | 0 | 7 ± 0.1 | 0 | 0 |
| Essential oil c | 16 ± 0.5 | 14 ± 0.3 | 14 ± 0 | 7 ± 0.3 | 14 ± 0.4 | 14 ± 0.4 | 10 ± 0.3 | 8 ± 0 | 13 ± 0.3 |
| DMSO | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Samples | S. aureus ATCC 25923 | S. aureus MRSA ATCC 43300 | E. coli ATCC 25922 | E. coli AG100 |
|---|---|---|---|---|
| MeOH extract | >100 µg/mL | >100 µg/mL | >100 µg/mL | >100 µg/mL |
| n-hexane extract | >100 µg/mL | >100 µg/mL | >100 µg/mL | >100 µg/mL |
| Essential oil | 0.125% | 0.125% | 0.125% | 0.250% |
| Compounds | S. aureus ATCC 29213 | S. aureus MRSA ATCC 43300 | E. coli ATCC 35218 | E. coli AG100 |
|---|---|---|---|---|
| linalool | >10 µL/mL >56 mM | >10 µL/mL >56 mM | 10 µL/mL 56 mM | 10 µL/mL 56 mM |
| sabinene | 10 µL/mL >62 mM | 10 µL/mL >62 mM | >10 µL/mL >62 mM | >10 µL/mL >62 mM |
| sabinene-hydrate * | >0.154 mg/mL >62 mM | >0.154 mg/mL >62 mM | >0.154 mg/mL >62 mM | >0.154 mg/mL >62 mM |
| α-terpinene | 10 µL/mL 61 mM | 10 µL/mL 61 mM | 10 µL/mL 61 mM | 10 µL/mL 61 mM |
| γ-terpinene | >10 µL/mL >62 mM | >10 µL/mL >62 mM | >10 µL/mL >62 mM | >10 µL/mL >62 mM |
| terpinen-4-ol | 10 µL/mL 60 mM | 10 µL/mL 60 mM | 5 µL/mL 30 mM | 5 µL/mL 30 mM |
| Relative Fluorescence Index (RFI) 1 | ||||
|---|---|---|---|---|
| E. coli ATCC 25922 | E. coli AG100 | S. aureus ATCC 25923 | S. aureus MRSA ATCC 43300 | |
| 0.0625% | 0.125% | 0.0625% | 0.0625% | |
| MeOH extract | −0.14 | −0.17 | −0.17 | −0.52 |
| n-hexane extract | 0.04 | −0.12 | −0.06 | 0.39 |
| Essential oil | 4.27 | 1.27 | 0.19 | 0.35 |
| 0.0312% | 0.0625% | |||
| MeOH extract | −0.10 | −0.18 | ||
| n-hexane extract | −0.09 | −0.13 | ||
| Essential oil | 3.21 | 0.64 | ||
| 100 µM | 100 µM | 100 µM | 100 µM | |
| α-terpinene | 0.07 | −0.00 | 0.06 | 0.00 |
| γ-terpinene | 0.01 | −0.03 | 0.06 | −0.01 |
| terpinen-4-ol | 0.07 | −0.00 | 0.02 | −0.01 |
| sabinene | 0.25 | −0.02 | 0.06 | 0.10 |
| sabinene hydrate | 0.02 | −0.04 | 0.00 | 0.27 |
| linalool | −0.01 | −0.05 | 0.03 | −0.05 |
| 50 µM | 50 µM | |||
| α-terpinene | 0.02 | 0.01 | ||
| γ-terpinene | 0.05 | −0.01 | ||
| terpinen-4-ol | 0.07 | −0.02 | ||
| sabinene | 0.04 | −0.04 | ||
| sabinene hydrate | −0.02 | −0.05 | ||
| linalool | −0.02 | −0.08 | ||
| CCCP 2 | 2.00 | 0.70 | ||
| RES 3 | − | 1.55 | 1.06 | |
| Sample | Inhibition % | |||
|---|---|---|---|---|
| E. coli ATCC 25922 | E. coli AG100 | |||
| 0.0625% | 0.0312% | 0.125% | 0.0625% | |
| MeOH extract | −5.77 ** | 55.61 * | −59.60 ns | −56.68 ns |
| n-hexane extract | 57.79 * | 64.43 ns | −13.74 * | −41.58 ** |
| Essential oil | −104.64 | – | 104.16 | – |
| 100 µM/ | 50 µM | 100 µM | 50 µM | |
| α-terpinene | 1.95 * | 17.68 ** | −53.71 ns | −35.98 * |
| γ-terpinene | 34.34 ** | 37.80 * | −33.37 ns | −13.62 ns |
| terpinene 4-ol | −2.09 *** | 42.36ns | −28.19 ns | 8.51 ns |
| sabinene | 36.35 ** | 48.57ns | −59.78 ns | 15.40 ns |
| sabinene hydrate | 37.93 *** | 55.97 * | −42.47 ns | 12.13 * |
| linalool | 28.98 *** | 49.68ns | −0.89 * | −11.78 * |
| CCCP | 63.37 | 50.14 | ||
| Sample | Inhibition % | |||
|---|---|---|---|---|
| S. aureus ATCC 25923 | S. aureus MRSA ATCC 43300 | |||
| 0.0625% | 0.0312% | 0.0625% | 0.0312% | |
| MeOH extract | 0.93 ** | −1.93 ** | 44.99 ** | 39.69 * |
| n-hexane extract | 1.22 ** | 0.46 ** | −53.03 ns | 18.37 ** |
| Essential oil | 69.24 | – | 4.38 | – |
| 100 µM | 50 µM | 100 µM | 50 µM | |
| α-terpinene | 1.47 ** | −1.13 ** | −64.81 ns | 26.82 ** |
| γ-terpinene | 0.76 ** | −1.91 *** | −125.22 * | −53.03 * |
| terpinene 4-ol | −0.64 ** | −1.51 ** | 66.31 ** | 53.71 ** |
| sabinene | 0.61 ** | −1.83 *** | 53.53 ** | 86.26 * |
| sabinene hydrate | −0.80 ** | −2.84 ** | 60.56 ** | 69.48 ** |
| linalool | 0.21 ** | −2.58 ** | 34.44 ** | 28.87 * |
| DMSO | 0.56 | −64.81 | ||
| TZ | − | 97.07 | 94.66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghazal, T.S.A.; Schelz, Z.; Vidács, L.; Szemerédi, N.; Veres, K.; Spengler, G.; Hohmann, J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants 2022, 11, 1432. https://doi.org/10.3390/plants11111432
Ghazal TSA, Schelz Z, Vidács L, Szemerédi N, Veres K, Spengler G, Hohmann J. Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants. 2022; 11(11):1432. https://doi.org/10.3390/plants11111432
Chicago/Turabian StyleGhazal, Tasneem Sultan Abu, Zsuzsanna Schelz, Lívia Vidács, Nikoletta Szemerédi, Katalin Veres, Gabriella Spengler, and Judit Hohmann. 2022. "Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes" Plants 11, no. 11: 1432. https://doi.org/10.3390/plants11111432
APA StyleGhazal, T. S. A., Schelz, Z., Vidács, L., Szemerédi, N., Veres, K., Spengler, G., & Hohmann, J. (2022). Antimicrobial, Multidrug Resistance Reversal and Biofilm Formation Inhibitory Effect of Origanum majorana Extracts, Essential Oil and Monoterpenes. Plants, 11(11), 1432. https://doi.org/10.3390/plants11111432







