Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp.
Abstract
:1. Introduction
2. Results
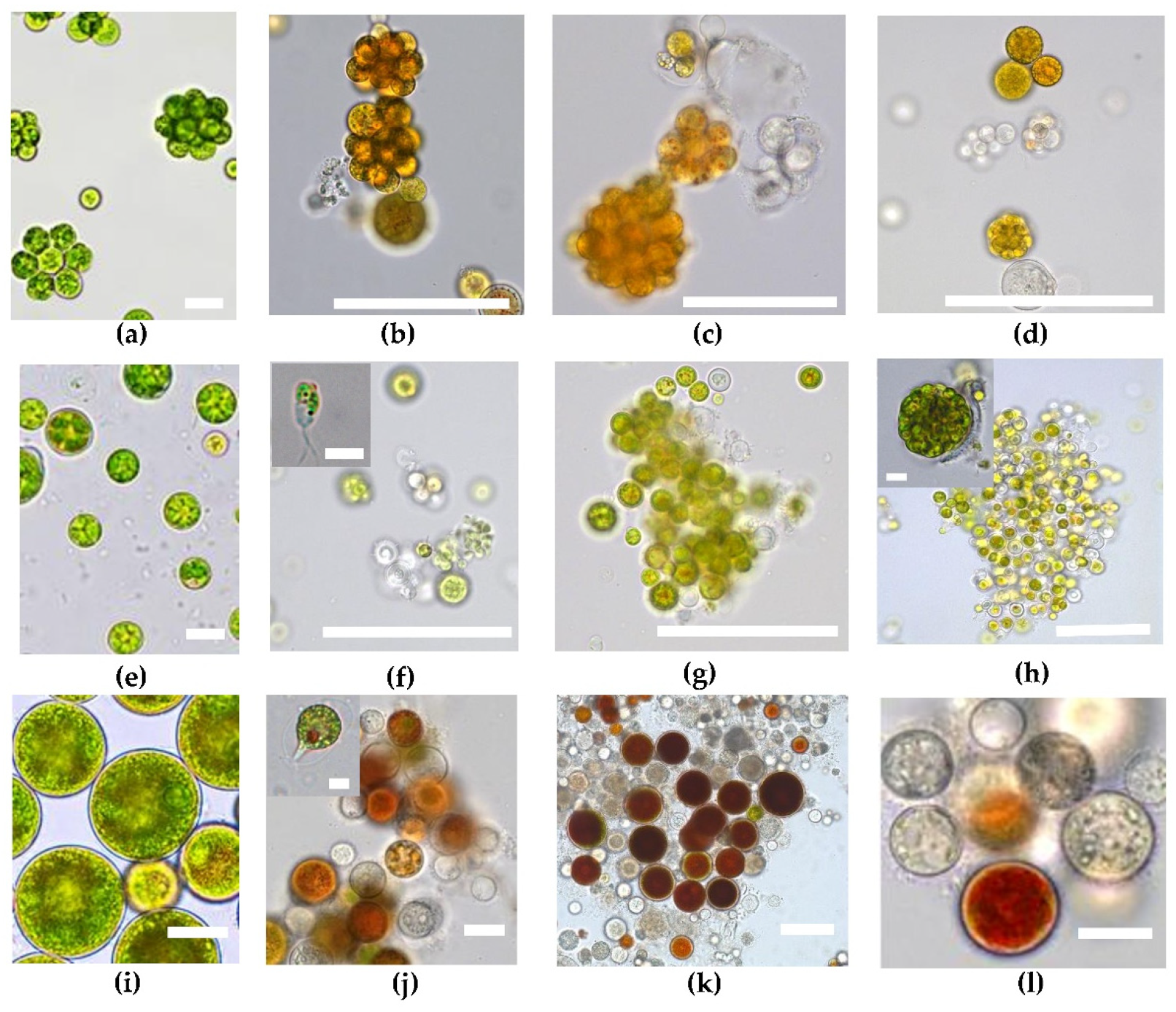
2.1. Microscopic Observations
2.2. Cell Pigment Composition
2.3. The Spectra of Water-Methanol Extracts
2.4. Chlorophyll Fluorescence Induction Curves
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Microscopy
4.3. Absorbance Spectroscopy
4.4. Chlorophyll Fluorescence Analysis
4.5. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosobryukhov, A.A.; Lyubimov, V.Y.; Kreslavski, V.D. Adaptive mechanisms of photosynthetic apparatus to UV radiation. In Stress Responses in Plants; Tripathi, B.N., Müller, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 59–78. [Google Scholar]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Karsten, U.; Holzinger, A. Green algae in alpine biological soil crust communities: Acclimation strategies against ultraviolet radiation and dehydration. Biodivers. Conserv. 2014, 23, 1845–1858. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Mallick, N. Mycosporine-like amino acids: Algal metabolites shaping the safety and sustainability profiles of commercial sunscreens. Algal Res. 2021, 58, 102425. [Google Scholar] [CrossRef]
- Gao, K.; Häder, D.P. Photosynthetic performances of marine microalgae under influences of rising CO2 and solar UV radiation. In Microbial Photosynthesis; Wang, Q., Ed.; Springer: Singapore, 2020; pp. 139–150. [Google Scholar]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Turcsányi, E.; Vass, I. Effect of UV-A radiation on photosynthetic electron transport. Acta Biol. Szeged. 2002, 46, 171–173. [Google Scholar]
- Ivanova, P.I.; Dobrikova, A.G.; Taneva, S.G.; Apostolova, E.L. Sensitivity of the photosynthetic apparatus to UV-A radiation: Role of light-harvesting complex II–photosystem II supercomplex organization. Radiat. Environ. Biophys. 2008, 47, 169–177. [Google Scholar] [CrossRef]
- Forján, E.; Garbayo, I.; Henriques, M.; Rocha, J.; Vega, J.M.; Vílchez, C. UV-A mediated modulation of photosynthetic efficiency, xanthophyll cycle and fatty acid production of Nannochloropsis. Mar. Biotechnol. 2011, 13, 366–375. [Google Scholar] [CrossRef]
- Gao, K.; Wu, Y.; Li, G.; Wu, H.; Villafane, V.E.; Helbling, E.W. Solar UV radiation drives CO2 fixation in marine phytoplankton: A double-edged sword. Plant Physiol. 2007, 144, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Eich, C.; Pont, S.B.; Brussaard, C.P. Effects of UV Radiation on the Chlorophyte Micromonas polaris Host–Virus Interactions and MpoV-45T Virus Infectivity. Microorganisms 2021, 9, 2429. [Google Scholar] [CrossRef]
- Vass, I.; Turcsányi, E.; Touloupakis, E.; Ghanotakis, D.; Petrouleas, V. The mechanism of UV-A radiation-induced inhibition of photosystem II electron transport studied by EPR and chlorophyll fluorescence. Biochemistry 2002, 41, 10200–10208. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Bilger, W.; Křížková, H.; Řezanka, T.; Nedbalová, L. Cysts of the snow alga Chloromonas krienitzii (Chlorophyceae) show increased tolerance to ultraviolet radiation and elevated visible light. Front. Plant Sci. 2020, 11, 617250. [Google Scholar] [CrossRef]
- Procházková, L.; Leya, T.; Křížková, H.; Nedbalová, L. Sanguina nivaloides and Sanguina aurantia gen. et spp. nov. (Chlorophyta): The taxonomy, phylogeny, biogeography and ecology of two newly recognised algae causing red and orange snow. FEMS Microbiol. Ecol. 2019, 95, fiz064. [Google Scholar] [CrossRef] [Green Version]
- Nishida, Y.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Monthly Variation and Ultraviolet Stability of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Phycology 2021, 1, 119–128. [Google Scholar] [CrossRef]
- Vega, J.; Schneider, G.; Moreira, B.R.; Herrera, C.; Bonomi-Barufi, J.; Figueroa, F.L. Mycosporine-like amino acids from red macroalgae: UV-photoprotectors with potential cosmeceutical applications. Appl. Sci. 2021, 11, 5112. [Google Scholar] [CrossRef]
- Karsten, U.; Lembcke, S.; Schumann, R. The effects of ultraviolet radiation on photosynthetic performance, growth and sunscreen compounds in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Schmitt, F.J.; Keuer, C.; Friedrich, T.; Shirshikova, G.N.; Zharmukhamedov, S.K.; Kosobryukhov, A.A.; Allakhverdiev, S.I. Response of the photosynthetic apparatus to UV-A and red light in the phytochrome B-deficient Arabidopsis thaliana L. hy3 mutant. Photosynthetica 2016, 54, 321–330. [Google Scholar] [CrossRef]
- Zaytseva, A.; Chekanov, K.; Zaytsev, P.; Bakhareva, D.; Gorelova, O.; Kochkin, D.; Lobakova, E. Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation. Plants 2021, 10, 2601. [Google Scholar] [CrossRef]
- Herrmann, H.; Häder, D.P.; Ghetti, F. Inhibition of photosynthesis by solar radiation in Dunaliella salina: Relative efficiencies of UV-B, UV-A and PAR. Plant Cell Environ. 1997, 20, 359–365. [Google Scholar] [CrossRef]
- Roleda, M.Y.; van de Poll, W.H.; Hanelt, D.; Wiencke, C. PAR and UVBR effects on photosynthesis, viability, growth and DNA in different life stages of coexisting Gigartinales: Implications for recruitment and zonation pattern. Mar. Ecol. Prog. Ser. 2004, 281, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.Y.; Kong, Z.Y.; Ye, M.W.; Zhang, Y.F.; Xu, J.L.; Zhou, C.X.; Liao, K.; Yan, X.J. Metabolomic and transcriptomic analyses reveal the effects of ultraviolet radiation deprivation on Isochrysis galbana at high temperature. Algal Res. 2019, 38, 101424. [Google Scholar] [CrossRef]
- Přibyl, P. Light is a crucial signal for zoosporogenesis and gametogenesis in some green microalgae. Eur. J. Phycol. 2013, 48, 106–115. [Google Scholar] [CrossRef]
- Mine, I.; Okuda, K.; Tatewaki, M. Gamete discharge by Bryopsis plumosa (Codiales, Chlorophyta) induced by blue and UV-A light. Phycol. Res. 1996, 44, 185–191. [Google Scholar] [CrossRef]
- Holzinger, A.; Karsten, U. Desiccation stress and tolerance in green algae: Consequences for ultrastructure, physiological and molecular mechanisms. Front. Plant Sci. 2013, 4, 327. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, L.J.; Borowitzka, M.A. Commercial production of β-carotene by Dunaliella salina in open ponds. Bull. Mar. Sci. 1990, 47, 244–252. [Google Scholar]
- Ben-Amotz, A. Industrial production of microalgal cell-mass and secondary products-major industrial species. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2004; pp. 273–280. [Google Scholar]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Biores. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2020, 96, fiz183. [Google Scholar] [CrossRef]
- Minyuk, G.; Chelebieva, E.; Chubchikova, I.; Dantsyuk, N.; Drobetskaya, I.; Sakhon, E.; Chekanov, K.; Solovchenko, A. Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids. Algae 2017, 32, 245–259. [Google Scholar] [CrossRef]
- Kawasaki, S.; Yoshida, R.; Ohkoshi, K.; Toyoshima, H. Coelastrella astaxanthina sp. nov. (Sphaeropleales, Chlorophyceae), a novel microalga isolated from an asphalt surface in midsummer in Japan. Phycol. Res. 2020, 68, 107–114. [Google Scholar] [CrossRef]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef]
- Mulders, K.J.; Janssen, J.H.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Effect of biomass concentration on secondary carotenoids and triacylglycerol (TAG) accumulation in nitrogen-depleted Chlorella zofingiensis. Algal Res. 2014, 6, 8–16. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodriguez, H.; Moreno, J.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of nitrogen source on the growth, lipid, and valuable carotenoid production in the green microalga Chromochloris zofingiensis. J. Appl. Phycol. 2020, 32, 923–935. [Google Scholar] [CrossRef]
- Shibzukhova, K.A.; Gavrilova, O.V.; Chivkunova, O.B.; Sidorov, R.A. Solovchenko, A.E.; Lobakova, E.S. Estimation of biotechnological potential and clarification of taxonomic status of Parietochloris genus microalgae (Trebouxiophyceae) from the NAMSU collection. Mosc. Univ. Biol. Sci. 2017, 72, 137–141. [Google Scholar] [CrossRef]
- Doppler, P.; Kornpointner, C.; Halbwirth, H.; Remias, D.; Spadiut, O. Tetraedron minimum, first reported member of hydrodictyaceae to accumulate secondary carotenoids. Life 2021, 11, 107. [Google Scholar] [CrossRef]
- Přibyl, P.; Pilný, J.; Cepák, V.; Kaštánek, P. The role of light and nitrogen in growth and carotenoid accumulation in Scenedesmus sp. Algal Res. 2016, 16, 69–75. [Google Scholar] [CrossRef]
- Rajput, A.; Singh, D.P.; Khattar, J.S.; Swatch, G.K.; Singh, Y. Evaluation of growth and carotenoid production by a green microalga Scenedesmus quadricauda PUMCC 4.1. 40. under optimized culture conditions. J. Basic Microbiol. 2021, in press. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Moreno, J.; Rodrıguez, H.; Vargas, M.A.; Rivas, J.; Guerrero, M.G. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp.(Chlorophyta). J. Biotechnol. 2000, 76, 51–59. [Google Scholar] [CrossRef]
- Han, D.; Li, Y.; Hu, Q. Astaxanthin in microalgae: Pathways, functions and biotechnological implications. Algae 2013, 28, 131–147. [Google Scholar] [CrossRef]
- Kostikov, I.J.; Romanenko, P.O.; Demchenko, E.M.; Darienko, T.M.; Mikhayljuk, T.I.; Rybchnnskiy, O.V.; Solonenko, A.M. Soil Algae of Ukraine (Vodorosti Gruntiv Ukrajiny); Phytosotsiologichniy Center: Kiev, Ukraine, 2001. (In Ukrainian) [Google Scholar]
- Wong, C.Y.; Chu, W.L.; Marchant, H.; Phang, S.M. Comparing the response of Antarctic, tropical and temperate microalgae to ultraviolet radiation (UVR) stress. J. Appl. Phycol. 2007, 19, 689–699. [Google Scholar] [CrossRef]
- Wong, C.Y.; Teoh, M.L.; Phang, S.M.; Lim, P.E.; Beardall, J. Interactive effects of temperature and UV radiation on photosynthesis of Chlorella strains from polar, temperate and tropical environments: Differential impacts on damage and repair. PLoS ONE 2015, 10, e0139469. [Google Scholar] [CrossRef] [Green Version]
- White, A.L.; Jahnke, L.S. Contrasting effects of UV-A and UV-B on photosynthesis and photoprotection of β-carotene in two Dunaliella spp. Plant Cell Physiol. 2002, 43, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Helbling, E.W.; Li, W.; Villafañe, V.E.; Gao, K. Motility and photosynthetic responses of the green microalga Tetraselmis subcordiformis to visible and UV light levels. J. Appl. Phycol. 2012, 24, 1613–1621. [Google Scholar] [CrossRef]
- Beardall, J.; Berman, T.; Markager, S.; Martinez, R.; Montecino, V. The effects of ultraviolet radiation on respiration and photosynthesis in two species of microalgae. Can. J. Fish. Aquat. Sci. 1997, 54, 687–696. [Google Scholar] [CrossRef]
- Salguero, A.; León, R.; Mariotti, A.; de la Morena, B.; Vega, J.M.; Vílchez, C. UV-A mediated induction of carotenoid accumulation in Dunaliella bardawil with retention of cell viability. Appl. Microbiol. Biotechnol. 2005, 66, 506–511. [Google Scholar] [CrossRef]
- Chen, S.; Gao, K. Solar ultraviolet radiation and CO2-induced ocean acidification interacts to influence the photosynthetic performance of the red tide alga Phaeocystis globosa (Prymnesiophyceae). Hydrobiologia 2011, 675, 105–117. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Cohen, Z.; Merzlyak, M.N. Carotenoid-to-chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J. Appl. Phycol. 2009, 21, 361–366. [Google Scholar] [CrossRef]
- Mogedas, B.; Casal, C.; Forján, E.; Vílchez, C. β-Carotene production enhancement by UV-A radiation in Dunaliella bardawil cultivated in laboratory reactors. J. Biosci. Bioeng. 2009, 108, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaichi, S. Carotenoids in algae: Distributions, biosyntheses and functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Turcsányi, E.; Vass, I. Inhibition of photosynthetic electron transport by UV-A radiation targets the photosystem II complex. Photochem. Photobiol. 2000, 72, 513–520. [Google Scholar] [CrossRef]
- Döhler, G.; Drebes, G.; Lohmann, M. Effect of UV-A and UV-B radiation on pigments, free amino acids and adenylate content of Dunaliella tertiolecta Butcher (Chlorophyta). J. Photochem. Photobiol. B Biol. 1997, 40, 126–131. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, Germany, 2007; pp. 321–362. [Google Scholar]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Chekanov, K.; Lukyanov, A.; Boussiba, S.; Aflalo, C.; Solovchenko, A. Modulation of photosynthetic activity and photoprotection in Haematococcus pluvialis cells during their conversion into haematocysts and back. Photosynth. Res. 2016, 128, 313–323. [Google Scholar] [CrossRef]
- Chekanov, K.; Schastnaya, E.; Neverov, K.; Leu, S.; Boussiba, S.; Zarka, A.; Solovchenko, A. Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress. Biochim. Biophys.–Gen. Subj. 2019, 1863, 1429–1442. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalized sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [Green Version]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; de Paepe, D.; Baart, G.J.E.; de Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Triki, A.; Maillard, P.; Gudin, C. Gametogenesis in Haematococcus pluvialis Flotow (Volvocales, Chlorophyta). Phycologia 1997, 36, 190–194. [Google Scholar] [CrossRef]
- Woodwell, G.M.; Whittaker, R.H. Effects of chronic gamma irradiation on plant communities. Q. Rev. Biol. 1968, 43, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.C.B.G.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lazár, D.; Nauš, J. Statistical properties of chlorophyll fluorescence induction parameters. Photosynthetica 1998, 35, 121–127. [Google Scholar] [CrossRef]
- Chekanov, K.; Feoktistov, A.; Lobakova, E. Spatial organization of the three-component lichen Peltigera aphthosa in functional terms. Physiol. Plant. 2017, 160, 328–338. [Google Scholar] [CrossRef]
- Baruffo, L.; Piccotto, M.; Tretiach, M. Intrathalline variation of chlorophyll a fluorescence emission in the epiphytic lichen Flavoparmelia caperata. Bryologist 2008, 111, 455–462. [Google Scholar] [CrossRef]





| Time | Fv/Fm | Ψ0 | ABS/RC, μs−1 | NPQ |
|---|---|---|---|---|
| Haematococcus rubicundus BM7/13 | ||||
| 0 d | 0.58 (0.58–0.58) a | 0.53 (0.53–0.53) a | 1.36 (1.36–1.36) a | 0.17 (.17–0.17) a |
| 7 d | 0.24 (0.13–0.33) b | 0.24 (0.22–0.27) b | 0.50 (0.34–0.54) b | 0.11 (0.08–0.15) a,b |
| 14 d | 0.33 (0.08–0.41) b | 0.20 (0.20–0.21) b | 0.31 (0.23–0.38) b | 0.07 (0.07–0.07) b |
| 21 d | 0.09 (0.05–0.10) c | 0.18 (0.14–0.20) b | 0.34 (0.19–0.40) b | 0.10 (0.08–0.12) a,b |
| Bracteacoccus aggregatus BM5/15 | ||||
| 0 d | 0.41 (0.41–0.41) a | 0.27 (0.27–0.27) a | 0.86 (0.86–0.86) a | 0.07 (0.07–0.07) a |
| 7 d | 0.39 (0.35–0.42) a | 0.23 (0.22–0.24) a | 0.57 (0.19–0.68) b | 0.20 (0.02–0.40) b |
| 14 d | 0.25 (0.17–0.30) b | 0.25 (0.22–0.28) a | 0.75 (0.54–0.81) a,b | 0.42 (0.38–0.82) b,c |
| 21 d | 0.29 (0.19–0.35) a,b | 0.31 (0.29–0.34) a | 0.55 (0.42–0.60) b | 0.38 (0.28–0.44) b,c |
| Deasonia sp. NAMSU 934/2 | ||||
| 0 d | 0.70 (0.70–0.70) a | 0.44 (0.44–0.44) a | 0.54 (0.54–0.54) a | 0.10 (0.10–0.10) a |
| 7 d | 0.12 (0.09–0.15) b | 0.10 (0.08–0.16) b | 1.38 (1.22–1.40) b | 0.13 (0.10–0.15) a |
| 14 d | 0.08 (0.08–0.09) b | 0.11 (0.05–0.13) b | 0.86 (0.42–0.91) a,b | 0.03 (0.02–0.03) b |
| 21 d | 0.06 (0.00–0.06) b | 0.02 (0.01–0.02) c | 0.18 (0.14–0.24) d | 0.05 (0.00–0.08) a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chekanov, K.; Shibzukhova, K.; Lobakova, E.; Solovchenko, A. Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. Plants 2022, 11, 1431. https://doi.org/10.3390/plants11111431
Chekanov K, Shibzukhova K, Lobakova E, Solovchenko A. Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. Plants. 2022; 11(11):1431. https://doi.org/10.3390/plants11111431
Chicago/Turabian StyleChekanov, Konstantin, Karina Shibzukhova, Elena Lobakova, and Alexei Solovchenko. 2022. "Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp." Plants 11, no. 11: 1431. https://doi.org/10.3390/plants11111431
APA StyleChekanov, K., Shibzukhova, K., Lobakova, E., & Solovchenko, A. (2022). Differential Responses to UV-A Stress Recorded in Carotenogenic Microalgae Haematococcus rubicundus, Bracteacoccus aggregatus, and Deasonia sp. Plants, 11(11), 1431. https://doi.org/10.3390/plants11111431







