Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity
Abstract
:1. Introduction
2. Results
2.1. Growth Media pH and EC Levels
2.2. Seedlings Growth
2.3. Shoot Number Affected by Treatments
2.4. Leaves and Inflorescences Number under the Influence of Treatments
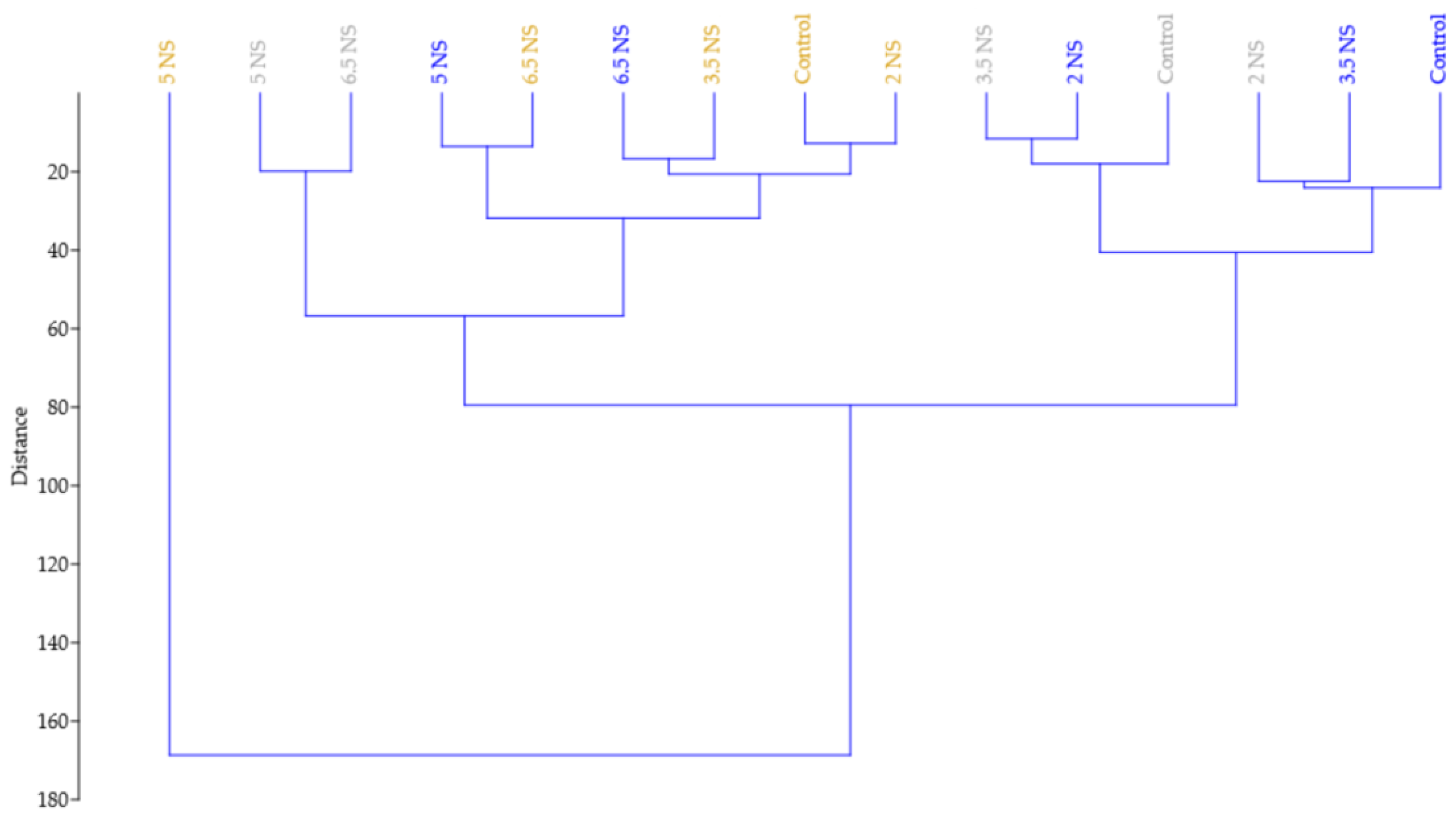
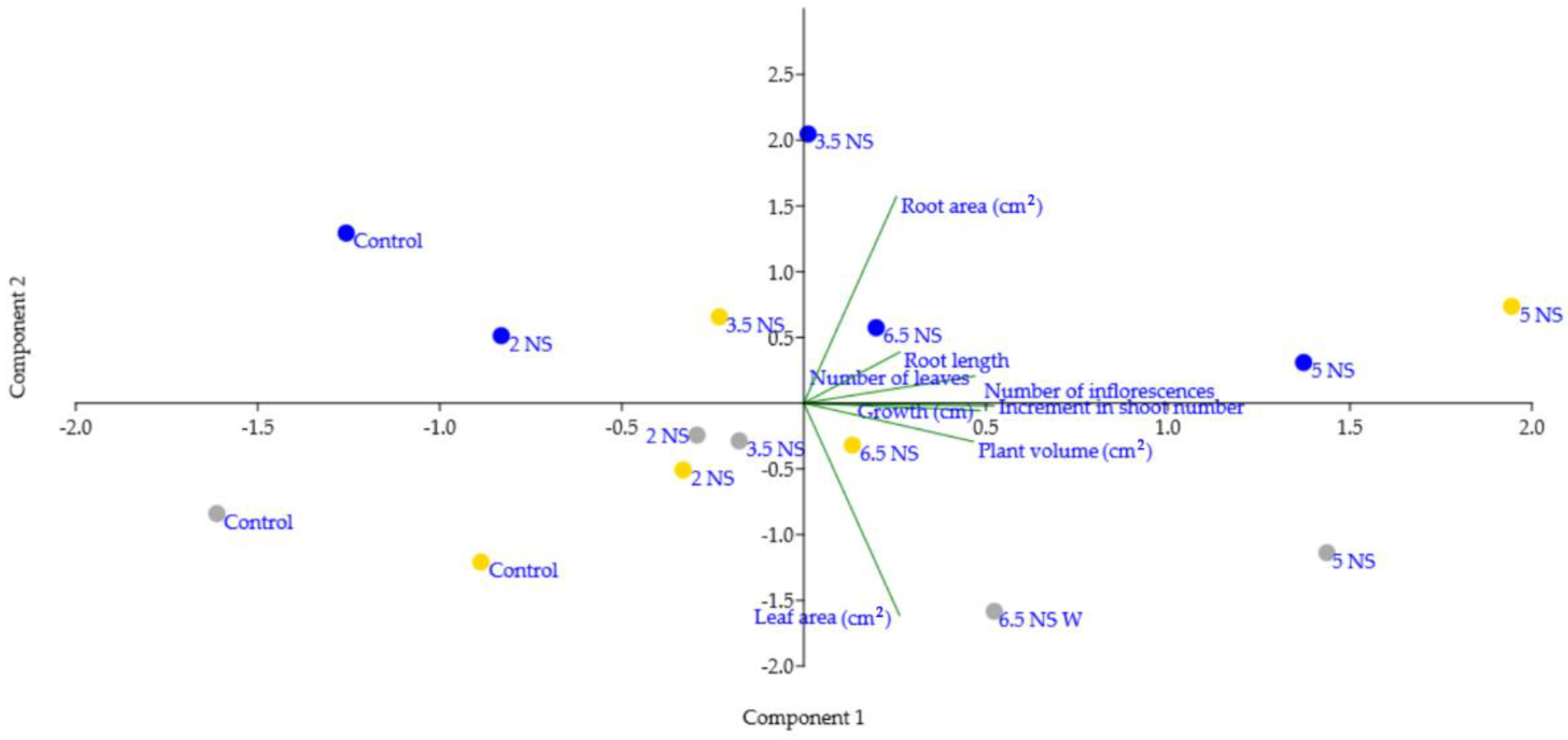
2.5. Relationship between the Vegetative Parts and Number of Inflorescences Appearance under the Influence of Different EC Nutrient Solutions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Site
4.2. Experimental Design
4.3. Data Evaluation/Plant Growth Parameters
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volckaert, E.; Gobin, B. Ornamental plants and floriculture. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publications: Paris, France, 2010. [Google Scholar]
- Uffelen, R.L.V.; Groot, N.S.D. Floriculture World Wide; Production, Trade and Consumption Patterns Show Market Opportunities and Challenges; Wageningen University and Agricultural Economics Institute: Hague, The Netherlands, 2005. [Google Scholar]
- Traversari, S.; Cacini, S.; Galieni, A.; Nesi, B.; Nicastro, N.; Pane, C. Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants. Sustainability 2021, 13, 3707. [Google Scholar] [CrossRef]
- Xia, Y.; Deng, X.; Zhou, P.; Shima, K.; Silva, J.A.T.D. The world floriculture industry: Dynamics of production and markets. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues; Silva, J.A.T.D., Ed.; Global Science Books: Isleworth, UK, 2006; pp. 336–347. [Google Scholar]
- Wani, M.A.; Nazki, I.T.; Din, A.; Iqbal, S.; Wani, S.A.; Khan, F.U. Floriculture sustainability initiative: The dawn of new era. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2018; pp. 91–127. [Google Scholar] [CrossRef]
- Manikas, I.; Malindretos, G.; Abeliotis, K. Sustainable Cities through alternative urban farming: The case of floriculture. J. Int. Food Agribus. Mark. 2020, 32, 295–311. [Google Scholar] [CrossRef]
- The Plant List, Version 1.1. Published on the Internet. Available online: http://www.theplantlist.org/ (accessed on 27 April 2022).
- Agnieszka, R.; Holubowicz, R. Effect of Pansy (Viola × wittrockiana Gams) Seeds Colour and Size on their Germination. Not. Bot. Horti Agrobo. 2008, 36, 47–50. [Google Scholar] [CrossRef]
- Sharma, R.; Dilta, B.S.; Baweja, H.S.; Kumari, P.; Thaneshwari, T.; Sahare, H.A. Effect of Seed Priming and Planting Dates on Growth, Flowering and Seed Production of Pansy (Viola × wittrockiana Gams.). Ann. Biol. 2018, 34, 176–180. [Google Scholar]
- Biswas, A.; Mandal, T.; Khan, S.A.; Thakur, B. Assessing the Impact of Weather Parameters on Growth and Flowering of Pansy (Viola × wittrockiana Gams.) under West Bengal Condition. Adv. Floric. Urb. Hortic. 2018, 209–212. [Google Scholar]
- Pušić, M.G.; Mladenović, E.M.; Čukanović, J.D.; Lakićević, M.D.; Pavlović, L.M. Influence of salinity on the growth and development of pansies (Viola × wittrockiana Gams.). Zbornik Matice Srpske za Prirodne Nauke 2019, 137, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Vukics, V.; Kery, A.; Guttman, A. Analysis of polar antioxidants in heartsease (Viola tricolor L.) and garden pansy (Viola × wittrockiana Gams.). J. Chromatogr. Sci. 2008, 46, 823–827. [Google Scholar] [CrossRef] [Green Version]
- Khalaj, M.A.; Kanani, M. Flower longevity and quality attributes of gerbera cut flower affected by different nutrient solutions. J. Appl. Hortic. 2018, 20, 247–252. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaâl, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol. Plant. 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of Foliar Fertilization: A Review of Current Status and Future Perspectives. J. Soil Sci. Plant Nutr. 2020, 21, 104–118. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoegberg, P.; Fan, H.; Quist, M.; Binkley, D.A.N.; Tamm, C.O. Tree growth and soil acidification in response to 30 years of experimental nitrogen loading on boreal forest. Glob. Change Biol. 2006, 12, 489–499. [Google Scholar] [CrossRef]
- Ni, K.; Shi, Y.Z.; Yi, X.Y.; Zhang, Q.F.; Fang, L.; Ma, L.F.; Ruan, J. Effects of long-term nitrogen application on soil acidification and solution chemistry of a tea plantation in China. Agric. Ecosyst. Environ. 2018, 252, 74–82. [Google Scholar] [CrossRef]
- Kaudal, B.B.; Chen, D.; Madhavan, D.B.; Downie, A.; Weatherley, A. An examination of physical and chemical properties of urban biochar for use as growing media substrate. Biomass Bioenergy 2016, 84, 49–58. [Google Scholar] [CrossRef]
- Nobile, C.; Denier, J.; Houben, D. Linking biochar properties to biomass of basil, lettuce and pansy cultivated in growing media. Sci. Hortic. 2020, 261, 109001. [Google Scholar] [CrossRef] [Green Version]
- Nemali, K.S.; Iersel, M.W.V. Light intensity and fertilizer concentration: I. Estimating optimal fertilizer concentrations from water-use efficiency of wax begonia. HortScience 2004, 39, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.K.L.; Yeom, M.S.; Oh, M.M. Effect of a Newly-Developed Nutrient Solution and Electrical Conductivity on Growth and Bioactive Compounds in Perilla frutescens var. crispa. Agronomy 2021, 11, 932. [Google Scholar] [CrossRef]
- Javadi, F.; Kalatejari, S.; Diyanat, M. Effect of foliar or soil application of selenium on some morphological and physiological traits of garden pansy (Viola × wittrockiana Gams) grown under salinity stress. Acta Agric. Slov. 2020, 115, 357–368. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Larbi, A.; Kchaou, H.; Gaaliche, B.; Gargouri, K.; Boulal, H.; Morales, F. Supplementary potassium and calcium improves salt tolerance in olive plants. Sci. Hortic. 2020, 260, 108912. [Google Scholar] [CrossRef]
- Szekely-Varga, Z.; González-Orenga, S.; Cantor, M.; Jucan, D.; Boscaiu, M.; Vicente, O. Effects of Drought and Salinity on Two Commercial Varieties of Lavandula angustifolia Mill. Plants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.J.; Park, H.J.; Kwon, S.J.; Lee, J.K.; Park, J.H. Early detection of plant stress using the internal electrical conductivity of Capsicum annuum in response to temperature and salinity stress. Plant Growth Regul. 2021, 95, 371–380. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Almeida, H.J.; Pancelli, M.A.; Prado, R.M.; Cavalcante, V.S.; Cruz, F.J.R. Effect of potassium on nutritional status and productivity of peanuts in succession with sugar cane. J. Soil Sci. Plant Nutr. 2015, 15, 1–10. [Google Scholar] [CrossRef]
- Yang, B.M.; Yao, L.X.; Li, G.L.; He, Z.H.; Zhou, C.M. Dynamic changes of nutrition in litchi foliar and effects of potassium-nitrogen fertilization ratio. J. Soil Sci. Plant Nutr. 2015, 15, 98–110. [Google Scholar] [CrossRef]
- Hussain, Z.; Khattak, R.A.; Irshad, M.; Mahmood, Q.; An, P. Effect of saline irrigation water on the leachability of salts, growth and chemical composition of wheat (Triticum aestivum L.) in saline-sodic soil supplemented with phosphorus and potassium. J. Soil Sci. Plant Nutr. 2016, 16, 604–620. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H.; Emami, S.; Alikhani, H.A. Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects A review. J. Soil Sci. Plant Nutr. 2017, 17, 897–911. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Nahar, K.; Hossain, M.; Mahmud, J.A.; Hossen, M.; Masud, A.A.C.; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.F.; Mateus, N.S.; Rosario, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol. Plant. 2021, 172, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Barrow, N.J. The effects of pH on phosphate uptake from the soil. Plant Soil 2017, 410, 401–410. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Liu, X.; Wang, Z.; Liang, Z.; Wang, M.; Liu, M.; Suarez, D.L. Interactive effects of pH, EC and nitrogen on yields and nutrient absorption of rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Nieto, A.; Gasco, G.; Paz-Ferreiro, J.; Fernández, J.M.; Plaza, C.; Mendez, A. The effect of pruning waste and biochar addition on brown peat based growing media properties. Sci. Hortic. 2016, 199, 142–148. [Google Scholar] [CrossRef]
- Park, S.Y.; Oh, S.B.; Kim, S.M.; Cho, Y.Y.; Oh, M.M. Evaluating the effects of a newly developed nutrient solution on growth, antioxidants, and chicoric acid contents in Crepidiastrum denticulatum. Hortic. Environ. Biotechnol. 2016, 57, 478–486. [Google Scholar] [CrossRef]
- Plaza, B.M.; Maggini, R.; Borghesi, E.; Pardossi, A.; Lao, M.T.; Jiménez-Becker, S. Nutrient Extraction in Pansy Fertigated with Pure, Diluted, Depurated and Phytodepurated Leachates from Municipal Solid Waste. Agronomy 2020, 10, 1911. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Dilta, B.S.; Kanwar, H.S.; Sharma, B.P.; Kumar, P. Effect of nitrogen and potassium on growth and flowering of pansy (Viola× wittrockiana Gams). Int. J. Farm Sci. 2017, 7, 127–130. [Google Scholar]
- Currey, C.J.; Walters, K.J.; Flax, N.J. Nutrient solution strength does not interact with the daily light integral to affect hydroponic cilantro, dill, and parsley growth and tissue mineral nutrient concentrations. Agronomy 2019, 9, 389. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Stanghellini, C. Analysis of the effect of EC and potential transpiration on vegetative growth of tomato. Sci. Hortic. 2001, 89, 9–21. [Google Scholar] [CrossRef]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klamkowski, K.; Treder, W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J. Fruit Orn. Plant Res. 2008, 16, 179–188. [Google Scholar]
- Kang, J.G.; Iersel, M.W.V. Nutrient solution concentration affects shoot: Root ratio, leaf area ratio, and growth of subirrigated salvia (Salvia splendens). HortScience 2004, 39, 49–54. [Google Scholar] [CrossRef]
- Gallace, N.; Boonen, M.; Lieten, P.; Bylemans, D. Electrical conductivity of the nutrient solution: Implications for flowering and yield in day-neutral cultivars. In Proceedings of the VIII International Strawberry Symposium, Quebec City, QC, Canada, 13–17 August 2016; pp. 223–228. [Google Scholar] [CrossRef]
- Naik, S.K.; Barman, D.; Medhi, R.P. Evaluation of electrical conductivity of the fertiliser solution on growth and flowering of a Cymbidium hybrid. S. Afr. J. Plant Soil 2013, 30, 33–39. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]







| Sample | Seedlings Growth (cm) | Leaf Area (cm2) | Increment in Shoot Number | Number of Leaves | Plant Volume (cm2) |
|---|---|---|---|---|---|
| White | |||||
| Control | 6.92 ± 1.2 a | 18.176 ± 2.5 c | 1.41 ± 0.3 a | 8.91 ± 2.1 a | 115.164 ± 23.4 a |
| 2 EC | 8.71 ± 0.8 bc | 20.541 ± 3.1 cd | 2.22 ± 0.5 b | 15.11 ± 2.5 b | 145.14 ± 15.8 c |
| 3.5 EC | 9.07 ± 0.9 c | 19.339 ± 2.3 c | 2.56 ± 0.2 b | 18.67 ± 3.1 cd | 105.473 ± 16.7 a |
| 5 EC | 9.65 ± 1.1 d | 28.436 ± 3.3 f | 4.52 ± 0.9 c | 19.5 ± 4.1 d | 250.007 ± 22.5 g |
| 6.5 EC | 8.89 ± 1.2 c | 31.115 ± 1.8 g | 2.78 ± 0.3 b | 18.4 ± 3.8 cd | 231.757 ± 23.4 f |
| Blue | |||||
| Control | 6.99 ± 1.8 a | 14.729 ± 1.2 b | 1.23 ± 0.2 a | 8.7 ± 2.7 a | 128.16 ± 13.3 b |
| 2 EC | 8.59 ± 1.7 b | 11.294 ± 0.9 a | 2.33 ± 0.4 b | 14.1 ± 3.3 b | 99.38 ± 16.2 a |
| 3.5 EC | 8.95 ± 1.9 c | 11.084 ± 2.1 a | 2.34 ± 0.5 b | 17.6 ± 2.9 c | 148.602 ± 12.1 c |
| 5 EC | 9.83 ± 1.2 d | 20.974 ± 1.0 cd | 4.61 ± 1.0 c | 19.7 ± 3.4 d | 201.441 ± 14.9 e |
| 6.5 EC | 8.58 ± 1.1 b | 22.627 ± 2.6 d | 2.39 ± 0.4 b | 17.9 ± 2.9 c | 179.231 ± 15.7 d |
| Yellow | |||||
| Control | 8.64 ± 0.7 b | 26.966 ± 2.7 f | 1.11 ± 0.1 a | 8.8 ± 1.5 a | 168.847 ± 16.6 d |
| 2 EC | 8.61 ± 0.5 b | 20.74 ± 2.2 c | 2.15 ± 0.1 b | 14.6 ± 2.3 b | 176.371 ± 12.9 d |
| 3.5 EC | 8.97 ± 0.4 c | 13.357± 0.6 b | 2.35 ± 0.7 b | 17.7 ± 4.0 c | 185.978 ± 20.5 d |
| 5 EC | 9.84 ± 0.6 d | 24.036 ± 3.0 be | 4.42 ± 0.8 c | 19.6 ± 2.8 d | 334.38 ± 25.5 h |
| 6.5 EC | 8.64 ± 0.5 b | 23.209 ± 2.1 de | 2.76 ± 0.3 b | 17.4 ± 3.1 c | 212.128 ± 17.8 f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kentelky, E.; Szekely-Varga, Z.; Morar, I.M.; Cornea-Cipcigan, M. Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity. Plants 2022, 11, 1433. https://doi.org/10.3390/plants11111433
Kentelky E, Szekely-Varga Z, Morar IM, Cornea-Cipcigan M. Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity. Plants. 2022; 11(11):1433. https://doi.org/10.3390/plants11111433
Chicago/Turabian StyleKentelky, Endre, Zsolt Szekely-Varga, Irina M. Morar, and Mihaiela Cornea-Cipcigan. 2022. "Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity" Plants 11, no. 11: 1433. https://doi.org/10.3390/plants11111433
APA StyleKentelky, E., Szekely-Varga, Z., Morar, I. M., & Cornea-Cipcigan, M. (2022). Morphological Responses of Viola Accessions to Nutrient Solution Application and Electrical Conductivity. Plants, 11(11), 1433. https://doi.org/10.3390/plants11111433









