Nickel Toxicity Interferes with NO3−/NH4+ Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Results
2.1. Ni-Induced Visible Toxicity Symptoms and Ni Contents in Rice Plants
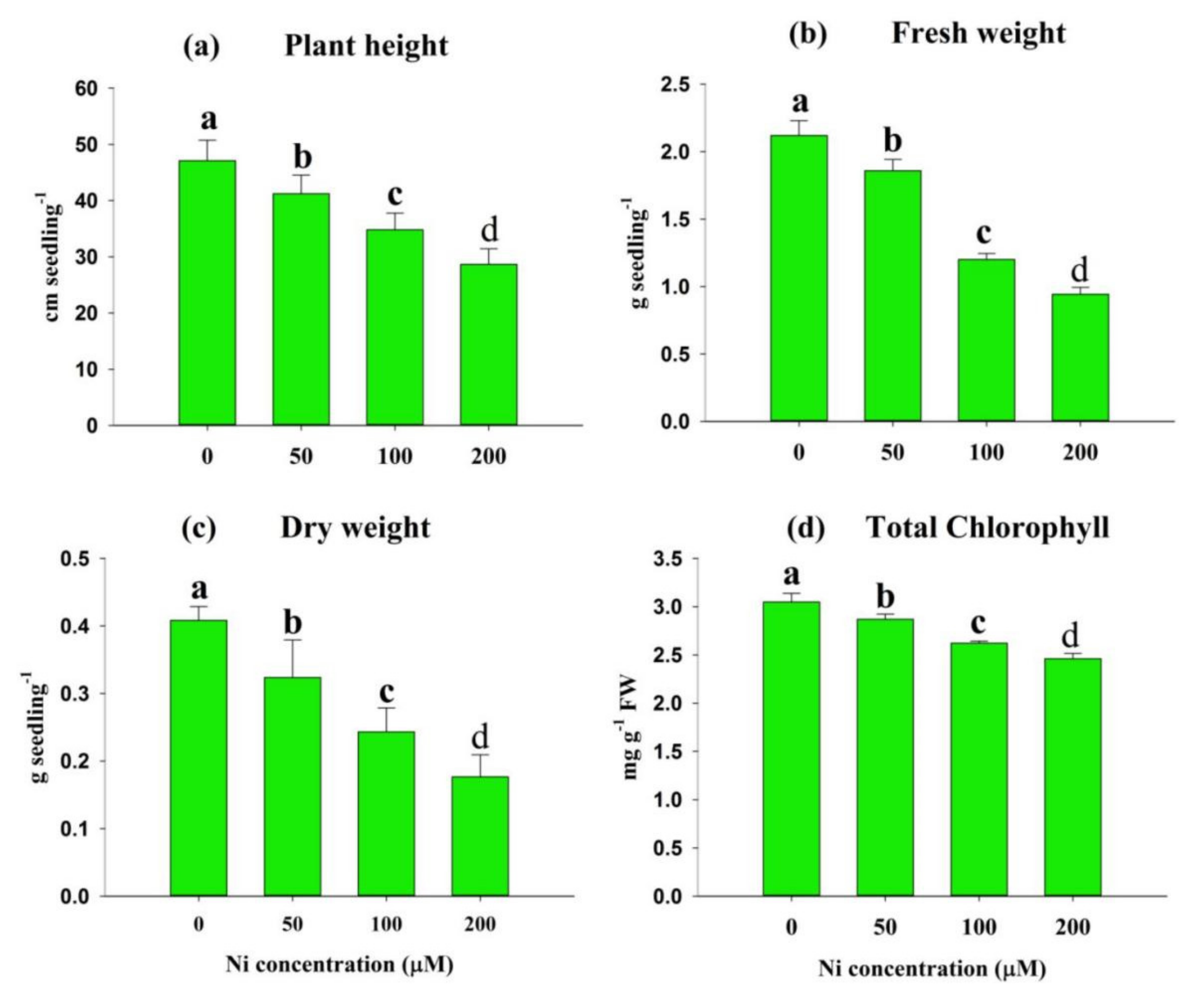
2.2. Impact of Ni Stress on Plant Growth, Biomass, and Photosynthetic Pigments
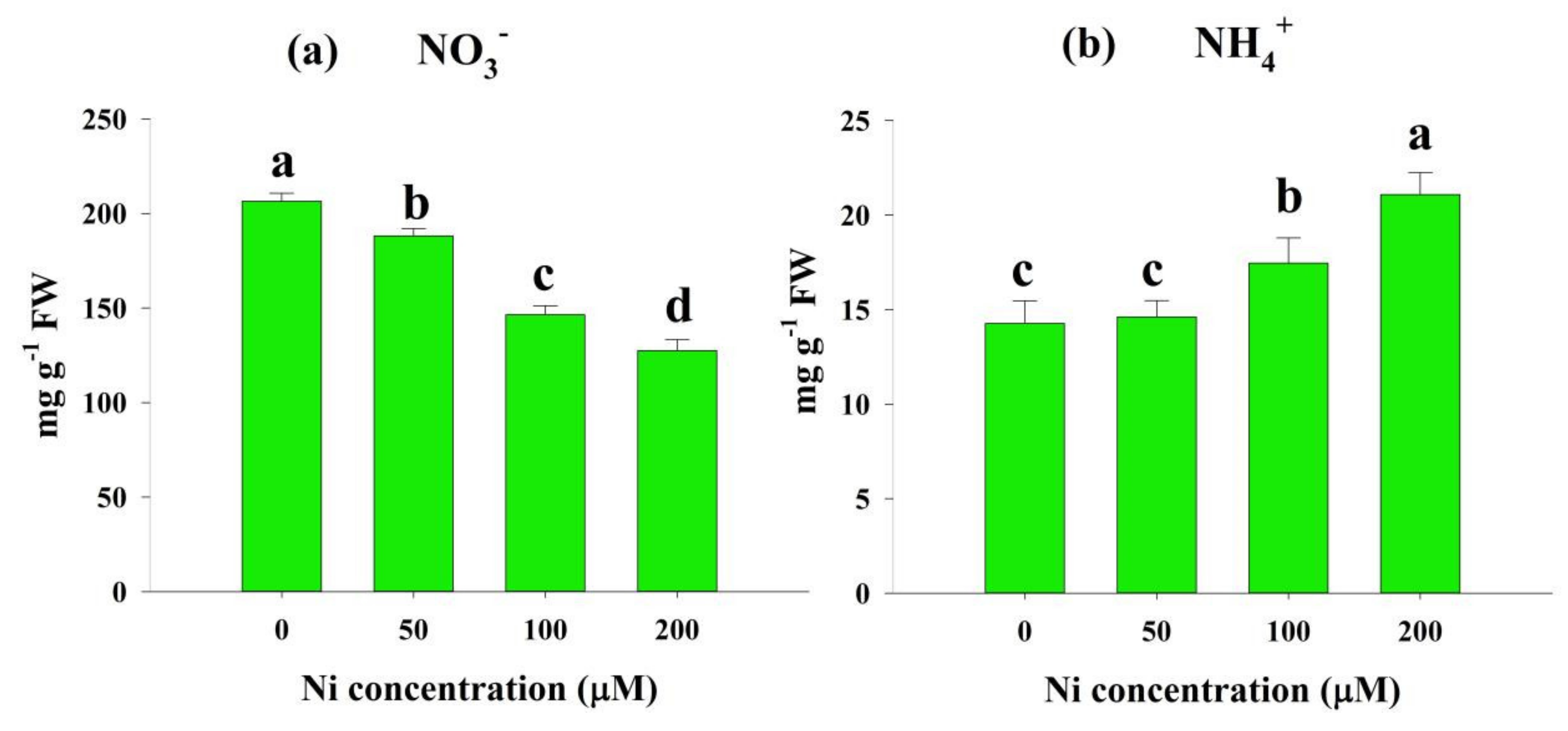
2.3. Effects of Ni Stress on NO3− and NH4+ Concentrations
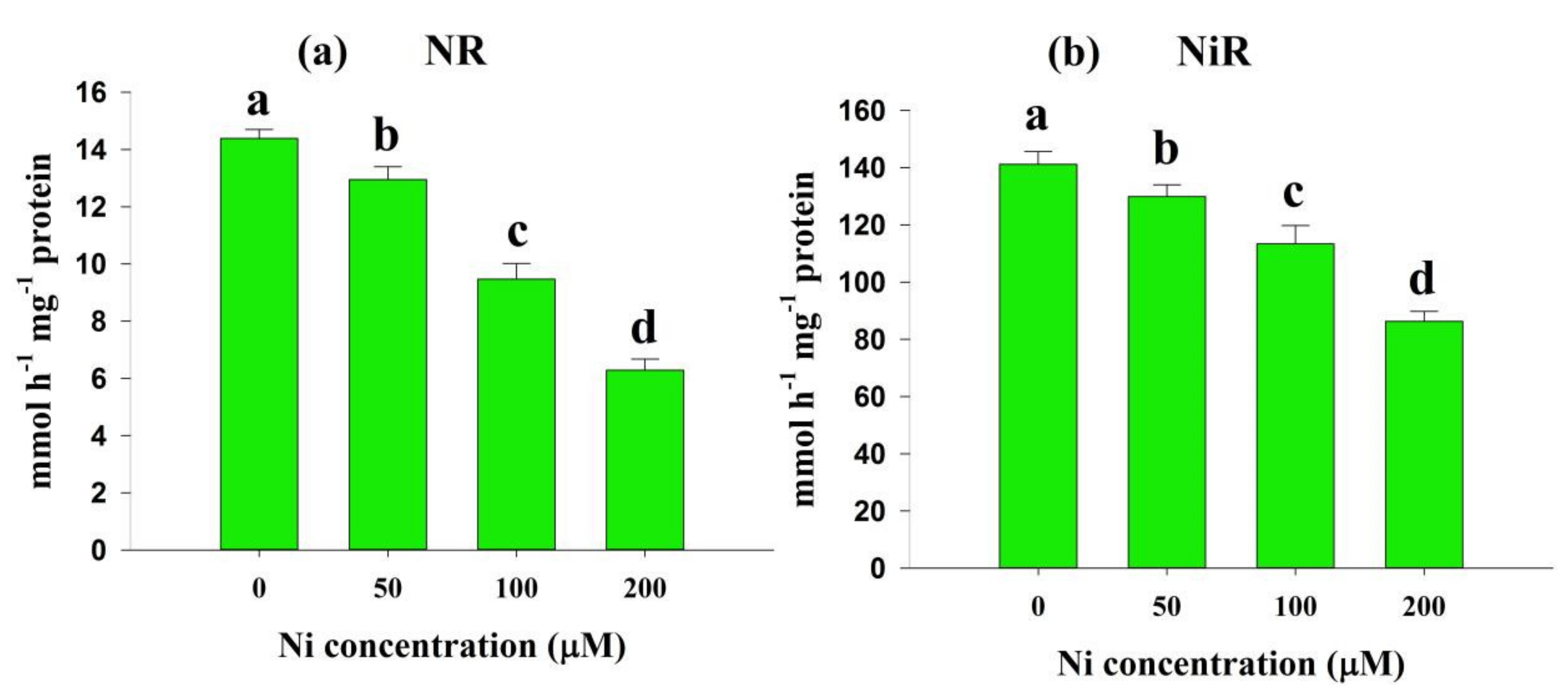
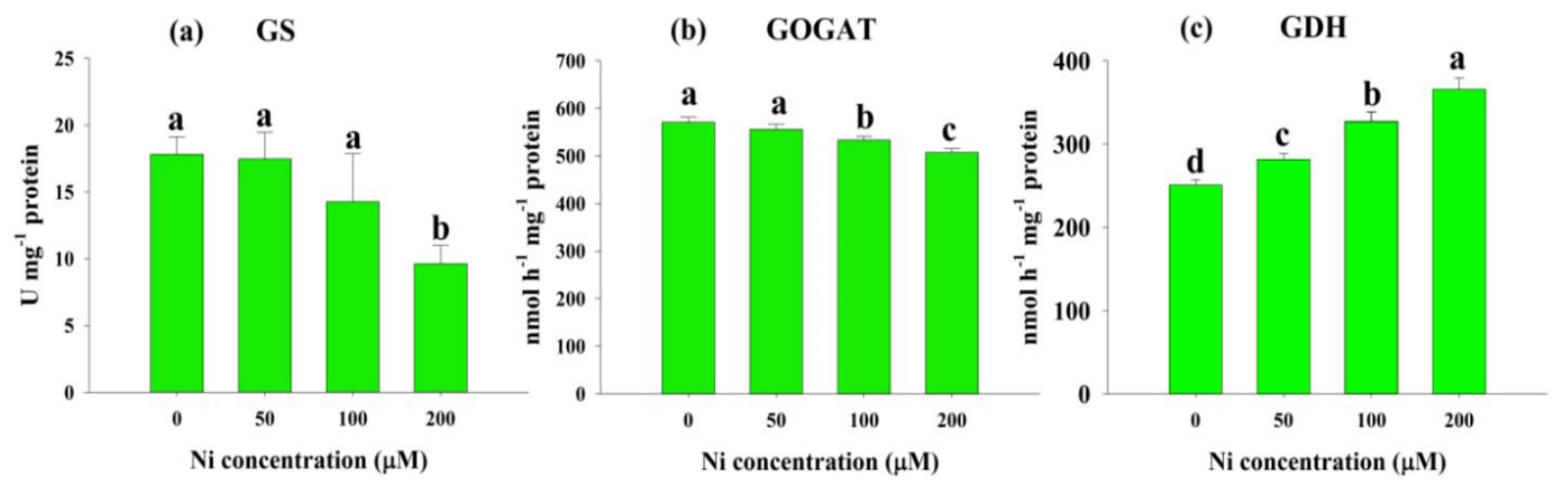
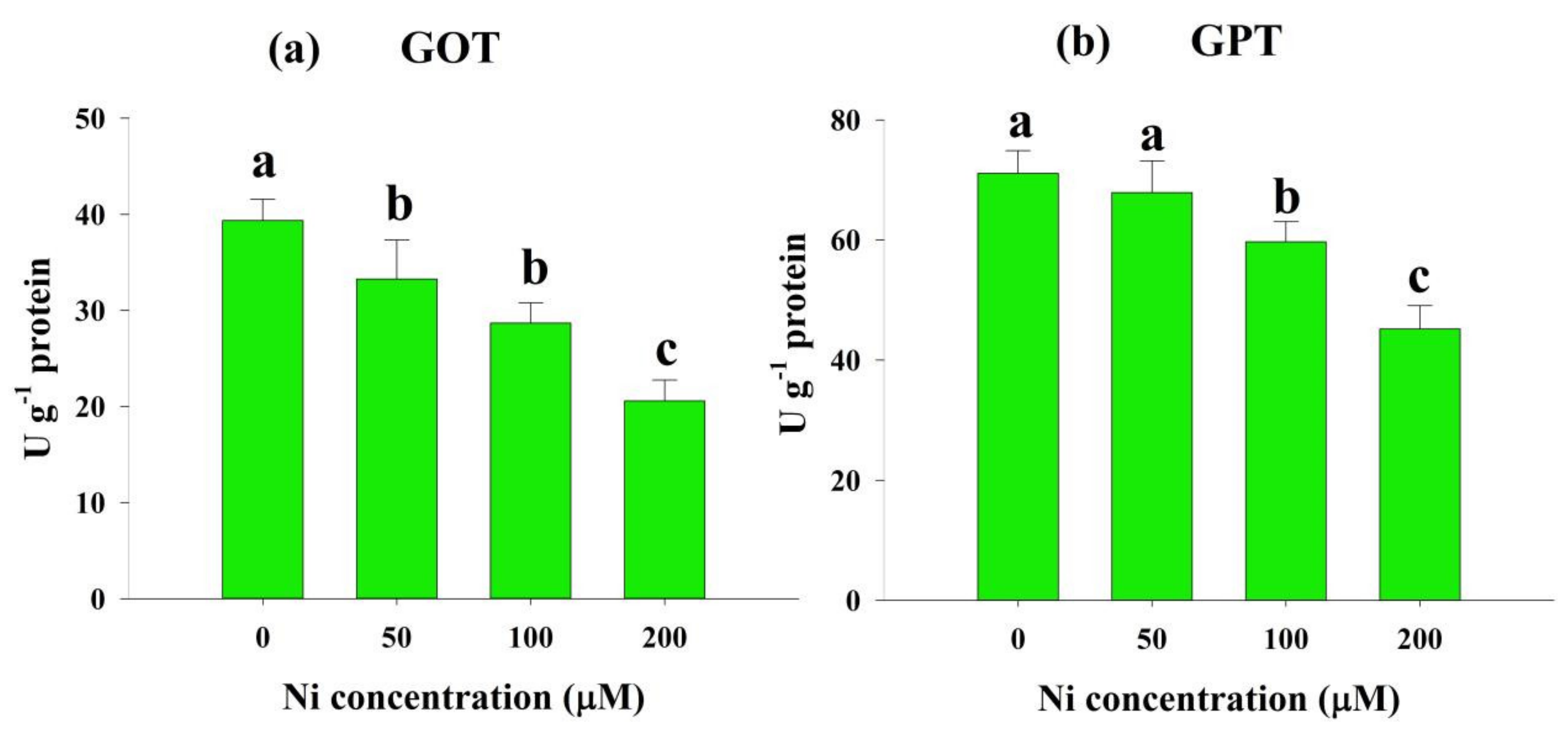
2.4. Effects of Ni Stress on Enzymes Involved in N Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Setup
4.2. Determination of Plant Growth
4.3. Determination of Total Chl Content
4.4. Quantification of Ni Content
4.5. NO3− and NH4+ Content Determination
4.6. Measurement of N Metabolism-Related Enzyme Activities
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The Effect of Excess Copper on Growth and Physiology of Important Food Crops: A Review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.A.; Eqani, S.A.M.A.S.; Bibi, S.; Xu, R.K.; Amna; Monis, M.F.H.; Katsoyiannis, A.; Bokhari, H.; Chaudhary, H.J. Bioaccumulation of Nickel by E. Sativa and Role of Plant Growth Promoting Rhizobacteria (PGPRs) under Nickel Stress. Ecotoxicol. Environ. Saf. 2016, 126, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Imtiaz, M.; Dai, Z.; Mehmood, S.; Adeel, M.; Liu, J.; Tu, S. Nickel Stressed Responses of Rice in Ni Subcellular Distribution, Antioxidant Production, and Osmolyte Accumulation. Environ. Sci. Pollut. Res. 2017, 24, 20587–20598. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; He, M.; Luo, Q.; Adeel, M.; Jiao, F. Heavy Metals in Agricultural Soils from a Typical Mining City in China: Spatial Distribution, Source Apportionment, and Health Risk Assessment. Polish J. Environ. Stud. 2020, 29, 1379–1390. [Google Scholar] [CrossRef]
- Adeel, M.; Ma, C.; Ullah, S.; Rizwan, M.; Hao, Y.; Chen, C.; Jilani, G.; Shakoor, N.; Li, M.; Wang, L.; et al. Exposure to Nickel Oxide Nanoparticles Insinuates Physiological, Ultrastructural and Oxidative Damage: A Life Cycle Study on Eisenia Fetida. Environ. Pollut. 2019, 254, 113032. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.Z.; Imtiaz, M.; Mehmood, S.; Adeel, M.; Dai, Z.; Li, Z.; Aziz, O.; Zhang, Y.; et al. Nitric Oxide Induces Rice Tolerance to Excessive Nickel by Regulating Nickel Uptake, Reactive Oxygen Species Detoxification and Defense-Related Gene Expression. Chemosphere 2018, 191, 23–35. [Google Scholar] [CrossRef]
- Rehman, M.Z.U.; Rizwan, M.; Ali, S.; Fatima, N.; Yousaf, B.; Naeem, A.; Sabir, M.; Ahmad, H.R.; Ok, Y.S. Contrasting Effects of Biochar, Compost and Farm Manure on Alleviation of Nickel Toxicity in Maize (Zea mays L.) in Relation to Plant Growth, Photosynthesis and Metal Uptake. Ecotoxicol. Environ. Saf. 2016, 133, 218–225. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Ahmad, M.A.Z.; Zhou, Y.; Adeel, M.; Mehmood, S.; Ahmad, M.A.Z.; Javed, R.; Imtiaz, M.; Aziz, O.; et al. Hydrogen Sulfide Enhances Rice Tolerance to Nickel through the Prevention of Chloroplast Damage and the Improvement of Nitrogen Metabolism under Excessive Nickel. Plant Physiol. Biochem. 2019, 138, 100–111. [Google Scholar] [CrossRef]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-Epibrassinolide on ROS Content, Antioxidant System, Lipid Peroxidation and Ni Uptake in Solanum nigrum L. under Ni Stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Nazir, H.; Asghar, H.N.; Zahir, Z.A.; Akhtar, M.J.; Saleem, M. Judicious Use of Kinetin to Improve Growth and Yield of Rice in Nickel Contaminated Soil. Int. J. Phytoremediation 2016, 18, 651–655. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Hayat, S.; Ahmad, A. Nickel: An Overview of Uptake, Essentiality and Toxicity in Plants. Bull. Environ. Contam. Toxicol. 2011, 86, 1–17. [Google Scholar] [CrossRef]
- Magnitskiy, S. Nickel The Last of the Essential Micronutrients. Agron. Colomb. 2011, 29, 49–56. [Google Scholar]
- Singh, M.; Singh, V.P.; Prasad, S.M. Nitrogen Modifies NaCl Toxicity in Eggplant Seedlings: Assessment of Chlorophyll a Fluorescence, Antioxidative Response and Proline Metabolism. Biocatal. Agric. Biotechnol. 2016, 7, 76–86. [Google Scholar] [CrossRef]
- Giagnoni, L.; Pastorelli, R.; Mocali, S.; Arenella, M.; Nannipieri, P.; Renella, G. Availability of Different Nitrogen Forms Changes the Microbial Communities and Enzyme Activities in the Rhizosphere of Maize Lines with Different Nitrogen Use Efficiency. Appl. Soil Ecol. 2016, 98, 30–38. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Mohammad, F.; Khan, M.M.A.; Al-Whaibi, M.H. Cumulative Effect of Nitrogen and Sulphur on Brassica juncea L. Genotypes under NaCl Stress. Protoplasma 2012, 249, 139–153. [Google Scholar] [CrossRef]
- Miflin, B.J.; Habash, D.Z. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Nitrogen Assimilation and Possibilities for Improvement in the Nitrogen Utilization of Crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Dubois, F.; Tercé-Laforgue, T.; Gonzalez-Moro, M.B.; Estavillo, J.M.; Sangwan, R.; Gallais, A.; Hirel, B. Glutamate Dehydrogenase in Plants: Is There a New Story for an Old Enzyme? Plant Physiol. Biochem. 2003, 41, 565–576. [Google Scholar] [CrossRef]
- Bhushan Jha, A.; Shanker Dubey, R. Arsenic Exposure Alters Activity Behaviour of Key Nitrogen Assimilatory Enzymes in Growing Rice Plants. Plant Growth Regul. 2004, 43, 259–268. [Google Scholar] [CrossRef]
- Lancien, M.; Gadal, P.; Hodges, M. Enzyme Redundancy and the Importance of 2-Oxoglutarate in Higher Plant Ammonium Assimilation. Plant Physiol. 2000, 123, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Routine Procedure for Growing Rice Plants in Culture Solution, 3rd ed.; Laboratory Manual for Physiological Studies of Rice; The International Rice Research Institute: Los Baños, Philippines, 1976; pp. 61–66. [Google Scholar]
- Gajewska, E.; Wielanek, M.; Bergier, K.; Skłodowska, M. Nickel-Induced Depression of Nitrogen Assimilation in Wheat Roots. Acta Physiol. Plant. 2009, 31, 1291–1300. [Google Scholar] [CrossRef]
- Sirhindi, G.; Mir, M.A.; Abd-Allah, E.F.; Ahmad, P.; Gucel, S. Jasmonic Acid Modulates the Physio-Biochemical Attributes, Antioxidant Enzyme Activity, and Gene Expression in Glycine Max under Nickel Toxicity. Front. Plant Sci. 2016, 7, 591. [Google Scholar] [CrossRef] [Green Version]
- Gajewska, E.; Skłodowska, M. Nickel-Induced Changes in Nitrogen Metabolism in Wheat Shoots. J. Plant Physiol. 2009, 166, 1034–1044. [Google Scholar] [CrossRef]
- Maheshwari, R.; Dubey, R.S. Nickel Toxicity Inhibits Ribonuclease and Protease Activities in Rice Seedlings: Protective Effects of Proline. Plant Growth Regul. 2007, 51, 231–243. [Google Scholar] [CrossRef]
- Khan, N.; Seshadri, B.; Bolan, N.; Saint, C.P.; Kirkham, M.B.; Chowdhury, S.; Yamaguchi, N.; Lee, D.Y.; Li, G.; Kunhikrishnan, A.; et al. Root Iron Plaque on Wetland Plants as a Dynamic Pool of Nutrients and Contaminants. Adv. Agron. 2016, 138, 1–96. [Google Scholar]
- Hseu, Z.Y.; Lai, Y.J. Nickel Accumulation in Paddy Rice on Serpentine Soils Containing High Geogenic Nickel Contents in Taiwan. Environ. Geochem. Health 2017, 39, 1325–1334. [Google Scholar] [CrossRef]
- Khaliq, A.; Ali, S.; Hameed, A.; Farooq, M.A.; Farid, M.; Shakoor, M.B.; Mahmood, K.; Ishaque, W.; Rizwan, M. Silicon Alleviates Nickel Toxicity in Cotton Seedlings through Enhancing Growth, Photosynthesis, and Suppressing Ni Uptake and Oxidative Stress. Arch. Agron. Soil Sci. 2016, 62, 633–647. [Google Scholar] [CrossRef]
- Mosa, A.; El-Banna, M.F.; Gao, B. Biochar Filters Reduced the Toxic Effects of Nickel on Tomato (Lycopersicon esculentum L.) Grown in Nutrient Film Technique Hydroponic System. Chemosphere 2016, 149, 254–262. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and Toxicity of Nickel in Plants: Recent Advances and Future Prospects. Clean—Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Mishra, P.; Dubey, R.S. Nickel and Al-Excess Inhibit Nitrate Reductase but Upregulate Activities of Aminating Glutamate Dehydrogenase and Aminotransferases in Growing Rice Seedlings. Plant Growth Regul. 2011, 64, 251–261. [Google Scholar] [CrossRef]
- Boussama, N.; Ouariti, O.; Ghorbal, M.H. Changes in Growth and Nitrogen Assimilation in Barley Seedlings under Cadmium Stress. J. Plant Nutr. 1999, 22, 731–752. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at High Dose Perturbs Growth, Photosynthesis and Nitrogen Metabolism While at Low Dose It up Regulates Sulfur Assimilation and Antioxidant Machinery in Garden Cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Sharma, P.; Shanker Dubey, R. Modulation of Nitrate Reductase Activity in Rice Seedlings under Aluminium Toxicity and Water Stress: Role of Osmolytes as Enzyme Protectant. J. Plant Physiol. 2005, 162, 854–864. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Yan, B.; Li, B.; Sun, J.; Guo, S.; Tezuka, T. Bottle Gourd Rootstock-Grafting Affects Nitrogen Metabolism in NaCl-Stressed Watermelon Leaves and Enhances Short-Term Salt Tolerance. J. Plant Physiol. 2013, 170, 653–661. [Google Scholar] [CrossRef]
- Kevrešan, S.; Petrović, N.; Popović, M.; Kandrač, J. Effect of Heavy Metals on Nitrate and Protein Metabolism in Sugar Beet. Biol. Plant. 1998, 41, 235–240. [Google Scholar] [CrossRef]
- Erdal, S.; Turk, H. Cysteine-Induced Upregulation of Nitrogen Metabolism-Related Genes and Enzyme Activities Enhance Tolerance of Maize Seedlings to Cadmium Stress. Environ. Exp. Bot. 2016, 132, 92–99. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Operative Photo Assimilation Associated Proteome Modulations Are Critical for Iron-Dependent Cadmium Tolerance in Oryza sativa L. Protoplasma 2015, 252, 1375–1386. [Google Scholar] [CrossRef]
- Du, J.; Shu, S.; An, Y.; Zhou, H.; Guo, S.; Sun, J. Influence of Exogenous Spermidine on Carbon–Nitrogen Metabolism under Ca(NO3)2 Stress in Cucumber Root. Plant Growth Regul. 2017, 81, 103–115. [Google Scholar] [CrossRef]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic Stress Generates ROS That Signal Expression of Anionic Glutamate Dehydrogenases to Form Glutamate for Proline Synthesis in Tobacco and Grapevine. Plant Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.G.; Chen, L.P.; Wang, Y.; Liu, J.; Xu, G.L.; Li, T. High Temperature at Grain-Filling Stage Affects Nitrogen Metabolism Enzyme Activities in Grains and Grain Nutritional Quality in Rice. Rice Sci. 2011, 18, 210–216. [Google Scholar] [CrossRef]
- Gangwar, S.; Singh, V.P. Indole Acetic Acid Differently Changes Growth and Nitrogen Metabolism in Pisum sativum L. Seedlings under Chromium (VI) Phytotoxicity: Implication of Oxidative Stress. Sci. Hortic. (Amsterdam) 2011, 129, 321–328. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Montes, M.; De La Rosa, G.; Corral-Diaz, B. Bioaccumulation of Cadmium, Chromium and Copper by Convolvulus arvensis L.: Impact on Plant Growth and Uptake of Nutritional Elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizwan, M.; Usman, K.; Alsafran, M.; Jabri, H.A.; Samreen, T.; Saleem, M.H.; Tu, S. Nickel Toxicity Interferes with NO3−/NH4+ Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.). Plants 2022, 11, 1401. https://doi.org/10.3390/plants11111401
Rizwan M, Usman K, Alsafran M, Jabri HA, Samreen T, Saleem MH, Tu S. Nickel Toxicity Interferes with NO3−/NH4+ Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.). Plants. 2022; 11(11):1401. https://doi.org/10.3390/plants11111401
Chicago/Turabian StyleRizwan, Muhammad, Kamal Usman, Mohammed Alsafran, Hareb Al Jabri, Tayyaba Samreen, Muhammad Hamzah Saleem, and Shuxin Tu. 2022. "Nickel Toxicity Interferes with NO3−/NH4+ Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.)" Plants 11, no. 11: 1401. https://doi.org/10.3390/plants11111401
APA StyleRizwan, M., Usman, K., Alsafran, M., Jabri, H. A., Samreen, T., Saleem, M. H., & Tu, S. (2022). Nickel Toxicity Interferes with NO3−/NH4+ Uptake and Nitrogen Metabolic Enzyme Activity in Rice (Oryza sativa L.). Plants, 11(11), 1401. https://doi.org/10.3390/plants11111401






