Isolation and Characterization of Werneria Chromene and Dihydroxyacidissimol from Burkillanthus malaccensis (Ridl.) Swingle
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Collection
2.2. Preparation of Plant Extracts
2.3. Tested Bacterial Strains
2.4. Broth Microdilution Assay
2.5. Antibiotic-Potentiating Assay
- Zone of combined extract and antibiotic > zone of extract + zone of antibiotic: synergy.
- Zone of combined extract and antibiotic = zone of extract + zone of antibiotic: additive.
- Zone of combined extract and antibiotic < zone of extract + zone of antibiotic: antagonism.
2.6. Cytotoxicity Assay
2.7. Isolation and Identification of Compounds
2.8. In Silico Studies—Auto Dock Vina (Blind Docking Methodology)
2.8.1. Protein Preparation
Main Protease
Spike Protein Receptor-Binding Domain (S-RBD) Bound with the ACE2 Complex
Spike Protein Receptor-Binding Domain (S-RBD)
Cathepsin L
Nsp13 Helicase
2.8.2. Ligand Preparation
Docking
2.9. Statistical Analysis
3. Results
3.1. Plant Extraction
3.2. Broth Microdilution
3.3. Antibiotic-Potentiating Activities
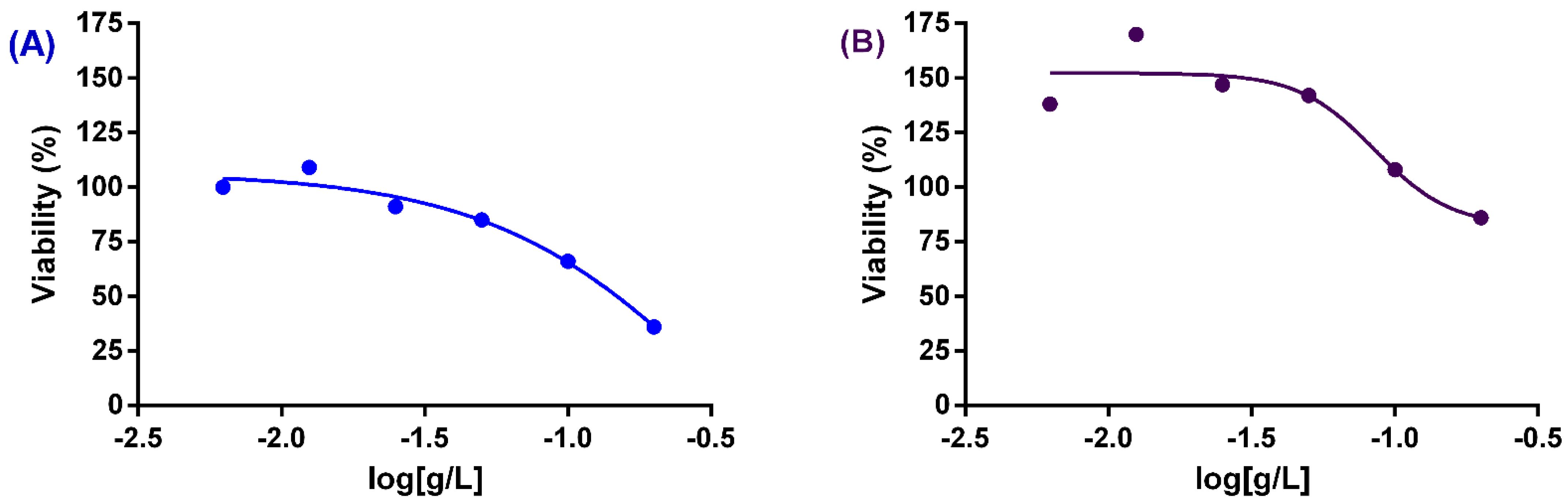
3.4. Cytotoxic Activities
3.5. Isolation of the Main Constituents from Active Extracts and Antibacterial Effects
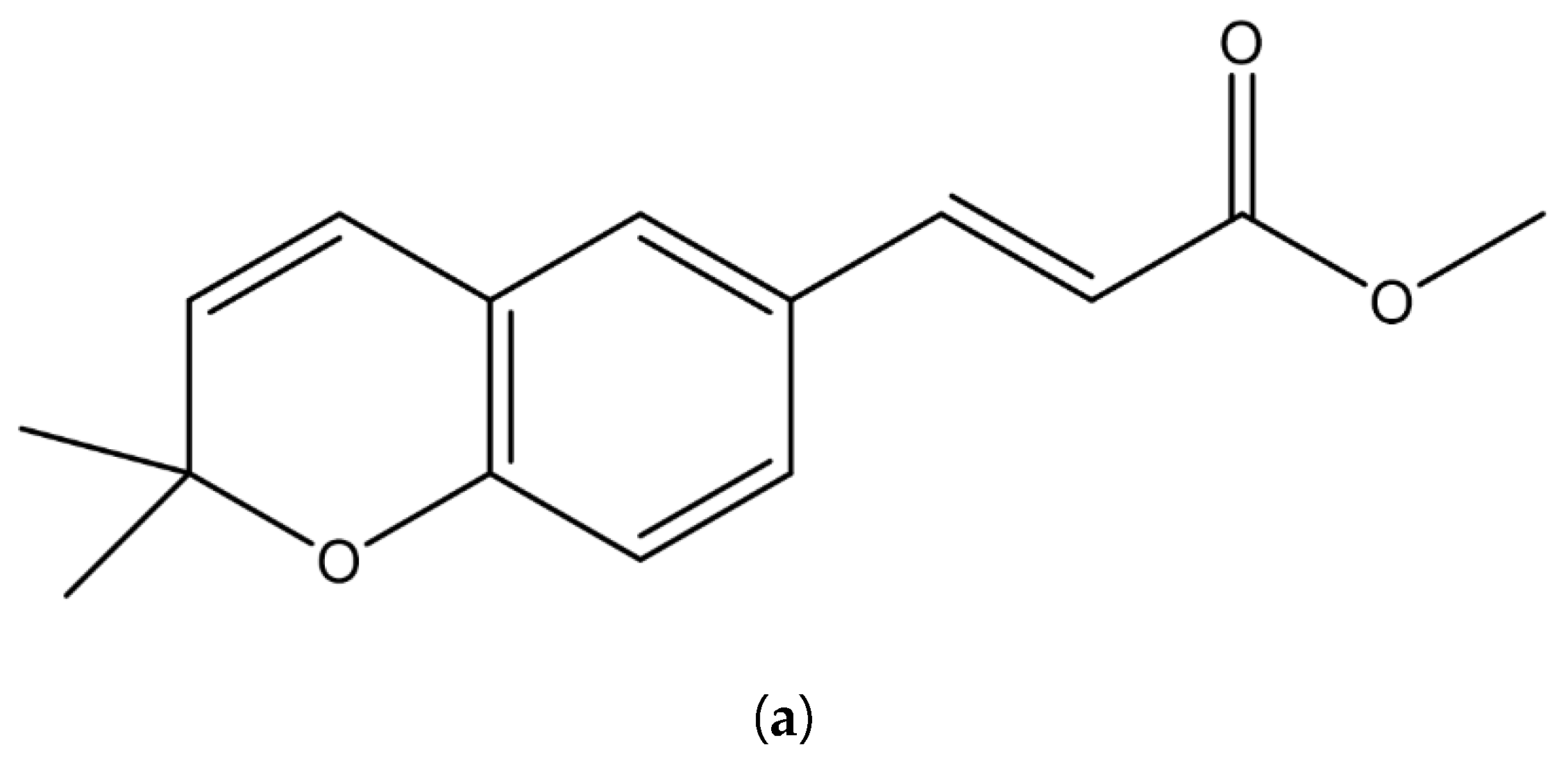
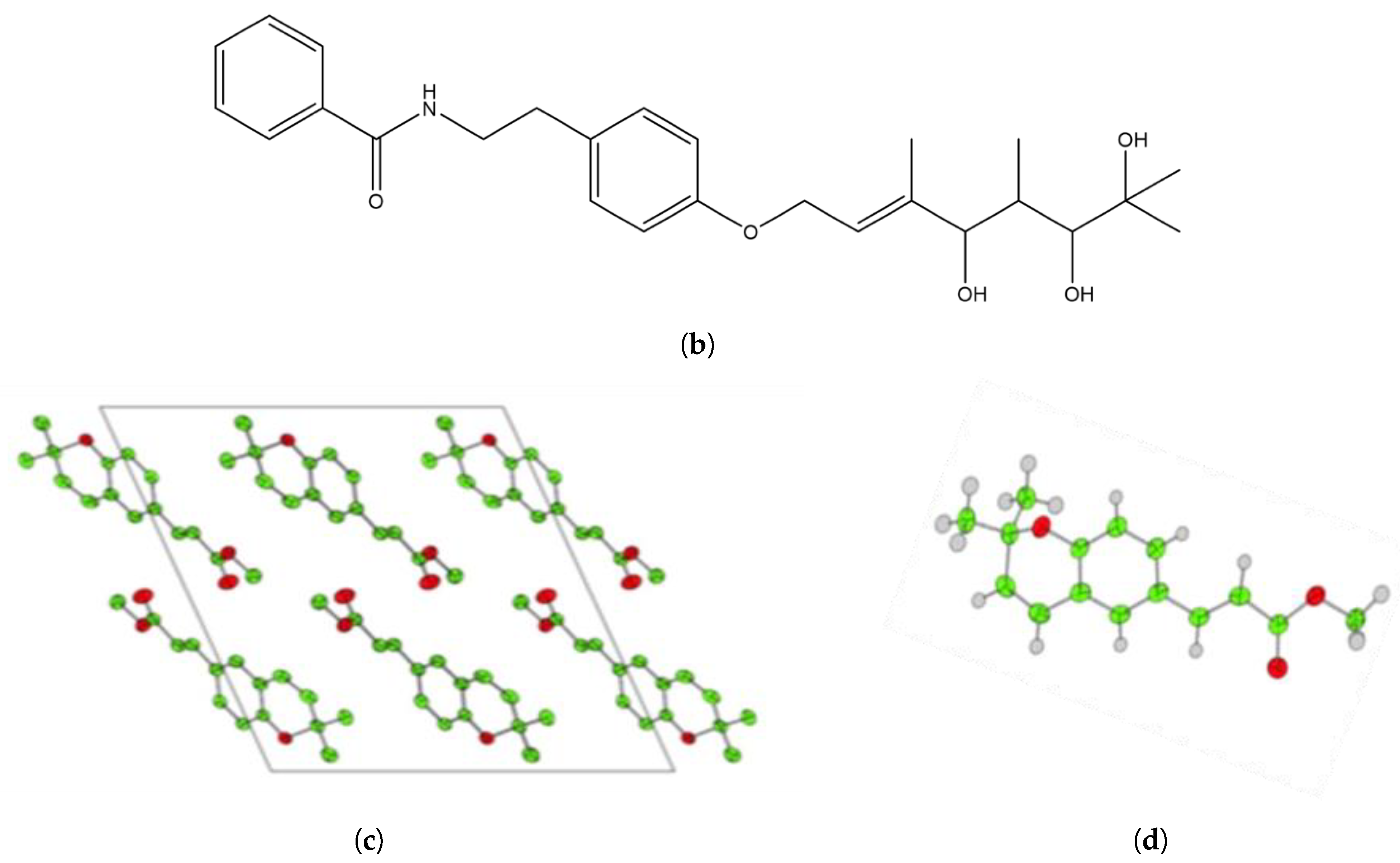
3.6. Crystal Structure of Isolated Methyl (Z)-3-(2,2-dimethyl-2H-chromen-6-yl) Acrylate Werneria Chromene
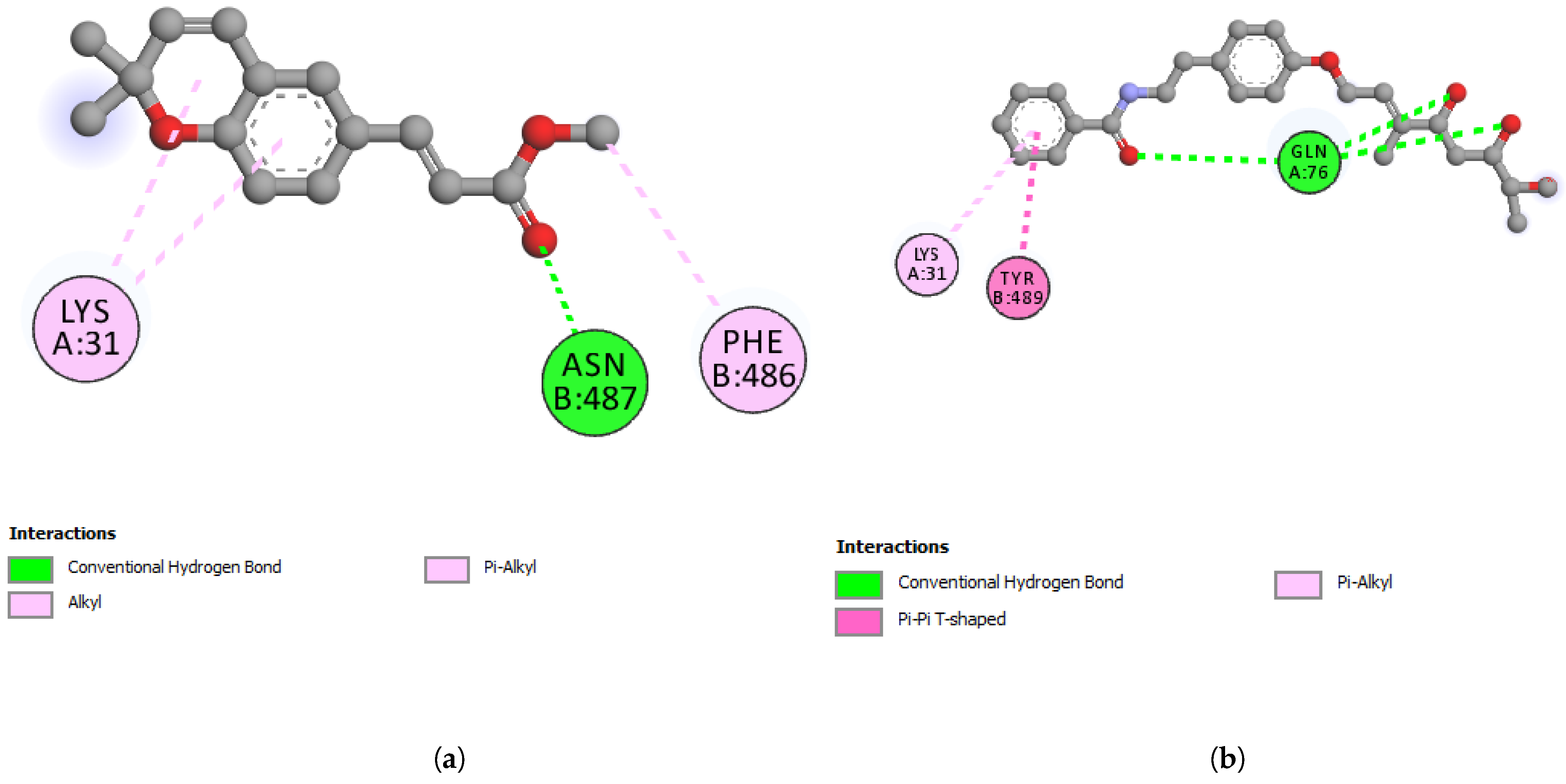
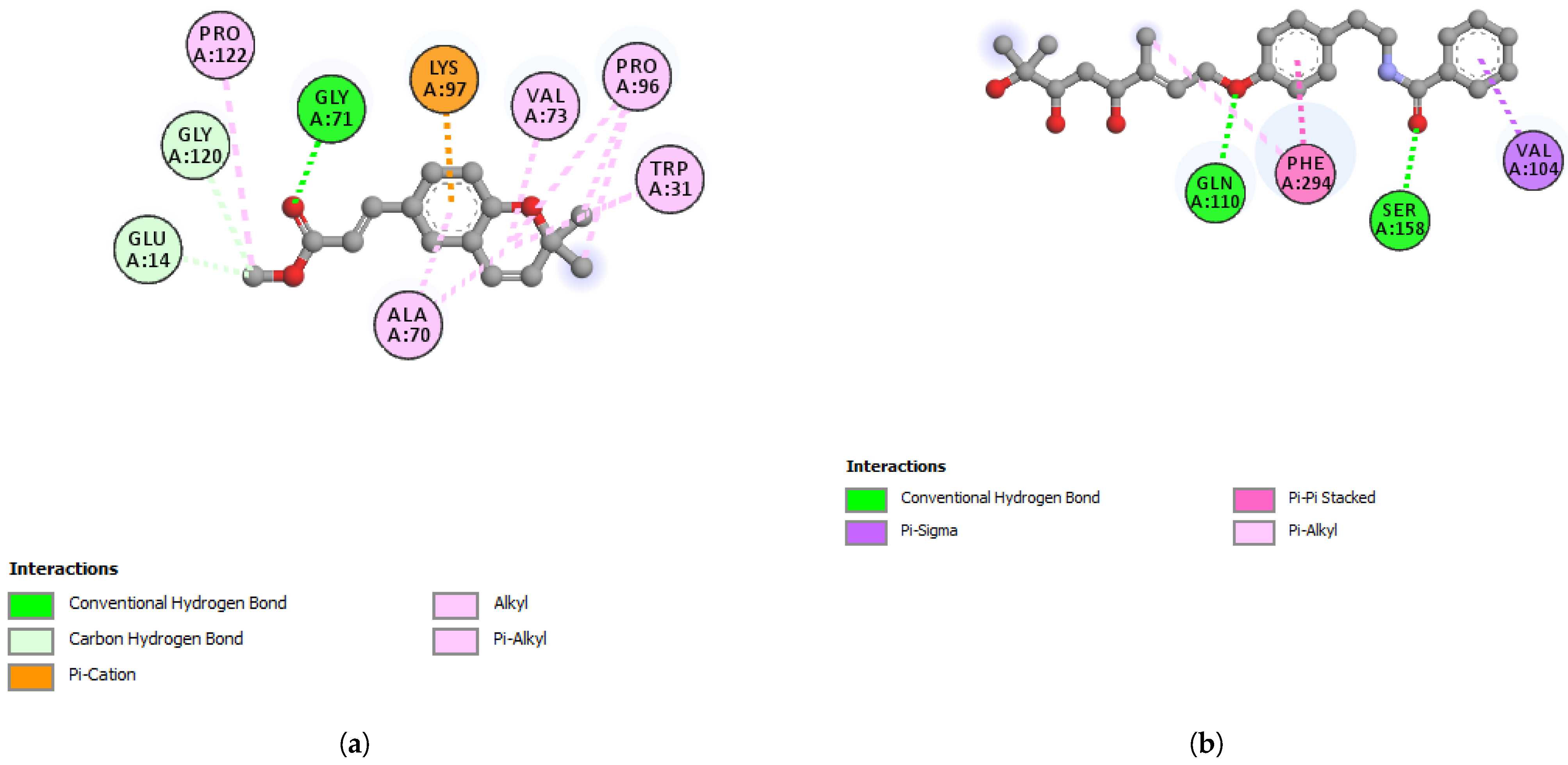
3.7. In Silico Studies with Werneria Chromene and Dihydroxyacidimissinol
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 1 January 2022).
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No eskape. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in eskape pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Herc, E.S.; Kauffman, C.A.; Marini, B.L.; Perissinotti, A.J.; Miceli, M.H. Daptomycin nonsusceptible vancomycin resistant Enterococcus bloodstream infections in patients with hematological malignancies: Risk factors and outcomes. Leuk. Lymphoma 2017, 58, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Mizutani, T.; Hiramatsu, K.; Kikuchi, K. Rapid acquisition of linezolid resistance in methicillin-resistant Staphylococcus aureus: Role of hypermutation and homologous recombination. PLoS ONE 2016, 11, e0155512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, N.R.; Atun, R.; Zhu, N.; Kulasabanathan, K.K.; Silva, S.; Chatterjee, A.; Knight, G.M.; Robotham, J.V. Estimating the burden of antimicrobial resistance: A systematic literature review. Antimicrob. Resist. Infect. Control 2018, 7, 58. [Google Scholar] [CrossRef]
- Wongsrichanalai, C.; Pickard, A.L.; Wernsdorfer, W.H.; Meshnick, S.R. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002, 2, 209–218. [Google Scholar] [CrossRef]
- Cobo, F. Determinants of parasite drug resistance in human lymphatic filariasis. Rev. Esp. Quimioter. 2016, 29, 288–295. [Google Scholar]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel Coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human Coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Jannat, K.; Paul, A.K.; Bondhon, T.A.; Hasan, A.; Nawaz, M.; Jahan, R.; Mahboob, T.; Nissapatorn, V.; Wilairatana, P.; Pereira, M.L.; et al. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics 2021, 13, 1895. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, I.C.; Bosch, B.J.; Li, F.; Li, W.; Lee, K.H.; Ghiran, S.; Vasilieva, N.; Dermody, T.S.; Harrison, S.C.; Dormitzer, P.R.; et al. SARS Coronavirus, but not human Coronavirus nl63, utilizes cathepsin l to infect ACE2-expressing cells. J. Biol. Chem. 2006, 281, 3198–3203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Jomha, F.A. COVID-19 induced superimposed bacterial infection. J. Biomol. Struct. Dyn. 2021, 39, 4185–4191. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Jomha, F.A.; Alhammadi, A.H. Virus-induced secondary bacterial infection: A concise review. Ther. Clin. Risk Manag. 2015, 11, 1265–1271. [Google Scholar] [CrossRef] [Green Version]
- Rusic, D.; Vilovic, M.; Bukic, J.; Leskur, D.; Perisin, A.S.; Kumric, M.; Martinovic, D.; Petric, A.; Modun, D.; Bozic, J. Implications of COVID-19 pandemic on the emergence of antimicrobial resistance: Adjusting the response to future outbreaks. Life 2021, 11, 220. [Google Scholar] [CrossRef]
- Rates, S.M. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Bennet, L. Deforestation and Climate Change; A Publication of the Climate Institute: Washington, DC, USA, 2017. [Google Scholar]
- Faridah-Hanum, I.; Philip, L.; Noor, A.A. Sampling species diversity in a Malaysian rain forest: The case of a logged-over forest. Pak. J. Bot. 2008, 40, 1729–1733. [Google Scholar]
- Koh, L.P.; Wilcove, D.S. Is oil palm agriculture really destroying tropical biodiversity? Conserv. Lett. 2008, 1, 60–64. [Google Scholar] [CrossRef]
- Bryan, J.E.; Shearman, P.L.; Asner, G.P.; Knapp, D.E.; Aoro, G.; Lokes, B. Extreme differences in forest degradation in Borneo: Comparing practices in Sarawak, Sabah, and Brunei. PLoS ONE 2013, 8, e69679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, S.A.; Macdonald, E.A.; Landguth, E.L.; Malhi, Y.; Macdonald, D.W. Multiple-scale prediction of forest loss risk across borneo. Landsc. Ecol. 2017, 32, 1581–1598. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Ramanathan, S.; Sasidharan, S.; Murugaiyah, V.; Mansor, S. Antimicrobial activity evaluation of Cassia spectabilis leaf extracts. Int. J. Pharmacol. 2010, 6, 510–514. [Google Scholar] [CrossRef]
- Salem, M.A.; Ezzat, S.M. The use of aromatic plants and their therapeutic potential as antiviral agents: A hope for finding anti-COVID-19 essential oils. J. Essent. Oil Res. 2021, 33, 105–113. [Google Scholar] [CrossRef]
- Al-Majmaie, S.; Nahar, L.; Rahman, M.M.; Nath, S.; Saha, P.; Talukdar, A.D.; Sharples, G.P.; Sarker, S.D. Anti-MRSA constituents from Ruta chalepensis (rutaceae) grown in Iraq, and in silico studies on two of most active compounds, chalepensin and 6-hydroxy-rutin 3′,7-dimethyl ether. Molecules 2021, 26, 1114. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [Green Version]
- Bohlmann, F.; Zdero, C.; King, R.M.; Robinson, H. Prenylated p-coumarates from Werneria stuebelii. Phytochemistry 1984, 23, 1135–1137. [Google Scholar] [CrossRef]
- Santhi, V.P.; Masilamani, P.; Sriramavaratharajan, V.; Murugan, R.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Therapeutic potential of phytoconstituents of edible fruits in combating emerging viral infections. J. Food Biochem. 2021, 45, e13851. [Google Scholar] [CrossRef]
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The effects of Nigella sativa (ns), Anthemis hyalina (ah) and Citrus sinensis (cs) extracts on the replication of Coronavirus and the expression of trp genes family. Mol. Biol. Rep. 2014, 41, 1703–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clementi, N.; Scagnolari, C.; D’Amore, A.; Palombi, F.; Criscuolo, E.; Frasca, F.; Pierangeli, A.; Mancini, N.; Antonelli, G.; Clementi, M.; et al. Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 2021, 163, 105255. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Prasad, A.S.B.; Mehta, C.H.; Nayak, U.Y. Antimicrobial peptide polymers: No escape to eskape pathogens-A review. World J. Microbiol. Biotechnol. 2020, 36, 131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-M.; Yang, W.-L.; Yang, F.-Y.; Zhang, L.; Huang, W.-J.; Hou, W.; Fan, C.-F.; Jin, R.-H.; Feng, Y.-M.; Wang, Y.-C. Cathepsin l plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Trans. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qiu, Y.; Zhang, D.Y.; et al. SARS-Coronavirus-2 nsp13 possesses NTPase and RNA helicase activities that can be inhibited by bismuth salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.; Farmer, B.D.; Hawke, J.P. Improved method for determining antibiotic susceptibility of Flavobacterium columnare isolates by broth microdilution. J. Aquat. Anim. Health 2008, 20, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Boonyanugomol, W.; Kraisriwattana, K.; Rukseree, K.; Boonsam, K.; Narachai, P. In vitro synergistic antibacterial activity of the essential oil from Zingiber cassumunar Roxb against extensively drug-resistant Acinetobacter baumannii strains. J. Infect. Public Health 2017, 10, 586–592. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ghosh, P.; Ghosh, M.K.; Thakur, S.; Akihisa, T.; Tamura, T.; Kimura, Y. Dihydroxy acidissiminol and acidissiminol epoxide, two tyramine derivatives from Limonia acidissima. Phytochemistry 1994, 37, 757–760. [Google Scholar] [CrossRef]
- Ghosh, P.; Sil, P.; Das, S.; Thakur, S.; Kokke, W.; Akihisa, T.; Shimizu, N.; Tamura, T.; Matsumoto, T. Tyramine derivatives from the fruit of Limonia acidissima. J. Nat. Prod. 1991, 54, 1389–1393. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Jin, Z.; Yang, H.; Rao, Z. The crystal structure of COVID-19 main protease in complex with an inhibitor n3. Nature 2020, 582, 289–293. [Google Scholar]
- Yuan, S.; Chan, H.S.; Hu, Z. Using pymol as a platform for computational drug design. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, e1298. [Google Scholar] [CrossRef]
- Asaad, N.; Bethel, P.A.; Coulson, M.D.; Dawson, J.E.; Ford, S.J.; Gerhardt, S.; Grist, M.; Hamlin, G.A.; James, M.J.; Jones, E.V.; et al. Dipeptidyl nitrile inhibitors of cathepsin L. Bioorg. Med. Chem. Lett. 2009, 19, 4280–4283. [Google Scholar] [CrossRef] [PubMed]
- RCSB pdb-6ZSL: Crystal Structure of the SARS-CoV-2 Helicase at 1.94 Angstrom Resolution. 2021. Available online: https://www.rcsb.org/structure/6ZSL (accessed on 1 January 2022).
- Hähnke, V.D.; Kim, S.; Bolton, E.E. Pubchem chemical structure standardization. J. Cheminform. 2018, 10, 36. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. Autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Studio, D. Dassault Systemes Biovia, Discovery Studio Modelling Environment, version 4.5; Accelrys Softw Inc.: San Diego, CA, USA, 2015; pp. 98–104. [Google Scholar]
- Baby, K.; Maity, S.; Mehta, C.H.; Suresh, A.; Nayak, U.Y.; Nayak, Y. SARS-CoV-2 entry inhibitors by dual targeting tmprss2 and ace2: An in silico drug repurposing study. Eur. J. Pharmacol. 2021, 896, 173922. [Google Scholar] [CrossRef]
- Madadlou, A. Food proteins are a potential resource for mining cathepsin l inhibitory drugs to combat SARS-CoV-2. Eur. J. Pharmacol. 2020, 885, 173499. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Krishnaswamy, S.; Samal, A. Potential phytochemical inhibitors of SARS-CoV-2 helicase nsp13: A molecular docking and dynamic simulation study. Mol. Divers. 2022, 26, 429–442. [Google Scholar] [CrossRef]
- Choudhary, S.; Malik, Y.S.; Tomar, S. Identification of SARS-CoV-2 cell entry inhibitors by drug repurposing using in silico structure-based virtual screening approach. Front. Immunol. 2020, 11, 1664. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 23, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Mishra, P.; Arora, N.K. Linkages between environmental issues and zoonotic diseases: With reference to COVID-19 pandemic. Environ. Sustain. 2021, 4, 455–467. [Google Scholar] [CrossRef]
- Bayer, R.J.; Mabberley, D.J.; Morton, C.; Miller, C.H.; Sharma, I.K.; Pfeil, B.E.; Rich, S.; Hitchcock, R.; Sykes, S. A molecular phylogeny of the orange subfamily (Rutaceae: Aurantioideae) using nine cpDNA sequences. Am. J. Bot. 2009, 96, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Parthasarathy, V.; Chempakam, B.; Zachariah, T. Chemistry of Spices; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Fernández-Hinojosa, E.; Aldabó-Pallás, T.; Cayuela, A.; Marquez-Vácaro, J.A.; Garcia-Curiel, A.; Jiménez-Jiménez, F.J. Acinetobacter baumannii ventilator-associated pneumonia: Epidemiological and clinical findings. Intensiv. Care Med. 2005, 31, 649–655. [Google Scholar] [CrossRef]
- Richet, H.M.; Mohammed, J.; McDonald, L.C.; Jarvis, W.R. Building communication networks: International network for the study and prevention of emerging antimicrobial resistance. Emerg. Infect. Dis. 2001, 7, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Arias, M.E.; Gomez, J.D.; Cudmani, N.M.; Vattuone, M.A.; Isla, M.I. Antibacterial activity of ethanolic and aqueous extracts of Acacia aroma gill. Ex hook et arn. Life Sci. 2004, 75, 191–202. [Google Scholar] [CrossRef]
- Scherrer, R.; Gerhardt, P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 1971, 107, 718–735. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. Pseudomonas aeruginosa: Resistance to the max. Front. Microbiol. 2011, 2, 65. [Google Scholar] [CrossRef] [Green Version]
- Jean, S.S.; Hsueh, P.R. High burden of antimicrobial resistance in asia. Int. J. Antimicrob. Agents 2011, 37, 291–295. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Fabry, W.; Okemo, P.O.; Ansorg, R. Antibacterial activity of east African medicinal plants. J. Ethnopharmacol. 1998, 60, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuete, V.; Betrandteponno, R.; Mbaveng, A.T.; Tapondjou, L.A.; Meyer, J.J.; Barboni, L.; Lall, N. Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement. Altern. Med. 2012, 12, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, H.; Katsuta, Y.; Nagashima, M.; Kamei, T.; Yano, M. Expression of the Arthrobacter viscosus penicillin G acylase gene in Escherichia coli and Bacillus subtilis. Appl. Environ. Microbiol. 1989, 55, 1351–1356. [Google Scholar] [CrossRef] [Green Version]
- Lehtinen, J.; Lilius, E.M. Promethazine renders Escherichia coli susceptible to penicillin g: Real-time measurement of bacterial susceptibility by fluoro-luminometry. Int. J. Antimicrob. Agents 2007, 30, 44–51. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef] [Green Version]
- Ogbole, O.O.; Segun, P.A.; Adeniji, A.J. In vitro cytotoxic activity of medicinal plants from Nigeria ethnomedicine on rhabdomyosarcoma cancer cell line and hplc analysis of active extracts. BMC Complement. Altern. Med. 2017, 17, 494. [Google Scholar] [CrossRef]
- Keum, Y.S.; Jeong, Y.J. Development of chemical inhibitors of the SARS Coronavirus: Viral helicase as a potential target. Biochem. Pharmacol. 2012, 84, 1351–1358. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin l prevent Severe Acute Respiratory Syndrome Coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Cardoza, C.G.; Vique-Sánchez, J.L. Potential inhibitors of the interaction between ACE2 and SARS-CoV-2 (RBD), to develop a drug. Life Sci. 2020, 256, 117970. [Google Scholar] [CrossRef] [PubMed]
- Ton, A.T.; Gentile, F.; Hsing, M.; Ban, F.; Cherkasov, A. Rapid identification of potential inhibitors of SARS-CoV-2 main protease by deep docking of 1.3 billion compounds. Mol. Inform. 2020, 39, e2000028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Part Extracted | Plant Extracts Yield (%) | ||
|---|---|---|---|
| Hexane | Chloroform | Methanol | |
| Leaves | 1.8 | 3.3 | 1.9 |
| Bark | 3.8 | 5.4 | 1.2 |
| Wood | 1.0 | 3.4 | 4.2 |
| Fruit pericarp | 5.1 | 2.9 | 2.1 |
| Fruit endocarp | 3.4 | 9.5 | 1.0 |
| Seeds | 1.5 | 5.1 | 3.6 |
| Average yields | 2.7 | 4.9 | 2.3 |
| Plant Part | Solvent | S. aureus (ATCC 11632) | B. subtilis (ATCC 6633) | E. coli (ATCC 8379) | P. aeruginosa (ATCC 10145) | A. baumannii (Imipenem-Resistant) |
|---|---|---|---|---|---|---|
| Leaves | Hexane | 1000 | - | - | - | - |
| Leaves | Chloroform | 250 (>1000) | 250 (>1000) | - | - | - |
| Leaves | Methanol | 500 | 1000 | - | - | - |
| Bark | Hexane | - | - | - | 1000 | - |
| Bark | Chloroform | 1000 | - | - | - | - |
| Bark | Methanol | 250 (>1000) | - | 500 | 250 (>1000) | - |
| Wood | Hexane | 625 | 1250 | 5000 | 2500 | - |
| Wood | Chloroform | 2500 | 2500 | 2500 | 625 | - |
| Wood | Methanol | - | 2500 | 2500 | 2500 | - |
| Endocarp | Chloroform | - | - | - | 1000 | - |
| Endocarp | Methanol | - | - | - | 1000 | - |
| Seeds | Hexane | - | - | - | 1000 | - |
| Chloramphenicol | 0.03 | 0.02 | Nt | Nt | Nt | |
| Tetracycline | Nt | Nt | 0.02 | 0.01 | - | |
| Imipenem Negative control | Nt Fg | Nt Fg | Nt Fg | Nt Fg | 12.0 Fg | |
| Treatment with | S. aureus (ATCC 11632) | B. subtilis (ATCC 6633) | E. coli (ATCC 8379) | P. aeruginosa (ATCC 10145) | A. baumannii (Imipenem-Resistant) |
|---|---|---|---|---|---|
| Extracts | - | - | - | ||
| I | - | 7.0 ± 1.4 | - | - | - |
| II | 12 ± 0.0 | - | - | - | - |
| III | - | - | - | - | - |
| IV | - | - | - | - | - |
| V | - | - | - | - | - |
| VI | - | - | - | - | - |
| VII | - | - | - | - | - |
| Amoxicillin | 16 ± 0.0 | 14.3 ± 0.5 | - | - | - |
| Ampicillin | 41 ± 0.3 | 20 ± 0.1 | - | - | - |
| Ciprofloxacin | - | 38 ± 0.0 | - | 35 ± 0.02 | - |
| Gentamicin | - | - | 25 ± 0.01 | - | - |
| Levofloxacin | - | 35 ± 1.0 | 38 ± 0.2 | 28 ± 0.3 | - |
| Penicillin G | - | - | - | - | - |
| Imipenem | - | - | - | - | 10 ± 0.04 |
| Amoxicillin + I | - | 26.7 ± 0.0 | - | - | - |
| Amoxicillin + II | 22.5 ± 0.5 | - | - | - | - |
| Amoxicillin + III | 23.7 ± 0.5 | - | - | - | - |
| Ampicillin + II | 40.7 ± 0.8 | - | - | - | - |
| Ampicillin + III | 42.0 ± 0.5 | - | - | - | - |
| Ampicillin + IV | - | 22 ± 0.3 | - | - | - |
| Ciprofloxacin + IV | - | 39 ± 0.1 | - | 38 ± 1.1 | - |
| Ciprofloxacin + V | - | 38.5 ± 0.0 | - | 36 ± 0.1 | - |
| Gentamicin + I | - | - | 34.3 ± 1.7 | - | - |
| Gentamicin + II | - | - | 35.3 ± 1.3 | - | - |
| Levofloxacin + IV | - | - | - | 30.7 ± 0.6 | - |
| Levofloxacin + V | - | 38.5 ± 0.3 | - | 30 ± 1.0 | - |
| Penicillin G + VI | - | - | 6.5 ± 0.02 | - | - |
| Imipenem + V | - | - | - | - | 11 ± 1.2 |
| Position | δ-H [31] | δ-H Werneria Chromene | Integration | Position | δ-C [31] | δ-C Werneria Chromene |
|---|---|---|---|---|---|---|
| 3 | 5.62 d | 5,65 d | 1 | 2 | 77.1 | 78 |
| 4 | 6.28 d | 6.30 d | 1 | 3 | 131.3 | 132 |
| 5 | 7.12 d | 7.18 d | 1 | 4 | 121.7 | 122 |
| 7 | 7.26 dd | 7.25 dd | 1 | 5 | 134.3 | 134 |
| 8 | 6.74 d | 6.78 d | 1 | 6 | 127.1 | 128 |
| 9 | 7.58 d | 7.60 d | 1 | 7 | 129.4 | 129 |
| 10 | 6.26 d | 6.20 d | 1 | 8 | 116.7 | 116 |
| 12,13 | 7.58 d | 7.57 d | 2,2 | 10 | 121.3 | 122 |
| O-CH3 | 3.76 d | 3.80 s | 3 | 11 | 115 | 115 |
| 12 | 144.6 | 145 |
| Position | δ-H [42] | δ-H Dihydroxyacidissiminol | Integration | δ-C [42] | δ-C Dihydroxyacidissiminol |
|---|---|---|---|---|---|
| 1′ | - | - | - | 134.60 | 135.00 |
| 2′,6′ | 7.69 d | 7.70 d | 1,1 | 126.80 | 126.50 |
| 3′,5′ | 7.41 t | 7.40 t | 1,1 | 128.60 | 128.90 |
| 4′ | 7.49 t | 7.49 t | 1 | 131.40 | 130.00 |
| CO-NH | 6.10 m | 6.15 m | 1 | 167.60 | 167.50 |
| N-CH2 | 3.70 q | 3.70 m | 2 | 41.30 | 41.50 |
| Ar-CH2 | 2.88 t | 2.80 t | 2 | 34.80 | 35.00 |
| 1″ | - | - | - | 157.30 | 157.00 |
| 2″, 6″ | 6.87 d | 6.87 d | 1,1 | 114.90 | 115.00 |
| 3″, 5″ | 7.18 d | 7.16 d | 1,1 | 129.80 | 129.90 |
| 4″ | - | - | - | 131.00 | 131.50 |
| 1 | 4.58 d | 4.60 d | 2 | 64.50 | 64.50 |
| 2 | 5.80 d | 5.78 d | 1 | 121.10 | 121.50 |
| 3 | - | - | - | 142.00 | 140.50 |
| 3-Me | 1.76 s | 1.75 s | 3 | 12.40 | 12.90 |
| 4 | 4.35 dd | 4.35 dd | 1 | 77.40 | 79.00 |
| 5 | - | - | - | - | - |
| 6 | 3.64 m | 3.65 m | 1 | 78.70 | 77.50 |
| 7 | - | - | - | 72.60 | 74.00 |
| 7-Me | 1.18 s | 1.17 s | 3 | 23.70 | 24.00 |
| 4-OH | 1.55 s | 1.75 s | - | - | - |
| 6,7 OH | 1.55 s | 1.65 s | - | - | - |
| Phytochemical | Spike Protein RBD Bound with ACE2 PDB: 6LZG | Cathepsin L PDB: 3HHA | Nsp13 Helicase PDB: 6ZSL | Mpro PDB: 6LU7 | Spike Protein RBD PDB: 6M0J * |
|---|---|---|---|---|---|
| Dihydroxyacidissiminol | −5.8 | −8.1 | −7.6 | −7.0 | −7.5 |
| Werneria chromene | −6.6 | −6.4 | −6.4 | −5.9 | −6.0 |
| Residue (Cathepsin L) | Distance | Category | Type |
| Dihydroxyacidissiminol | |||
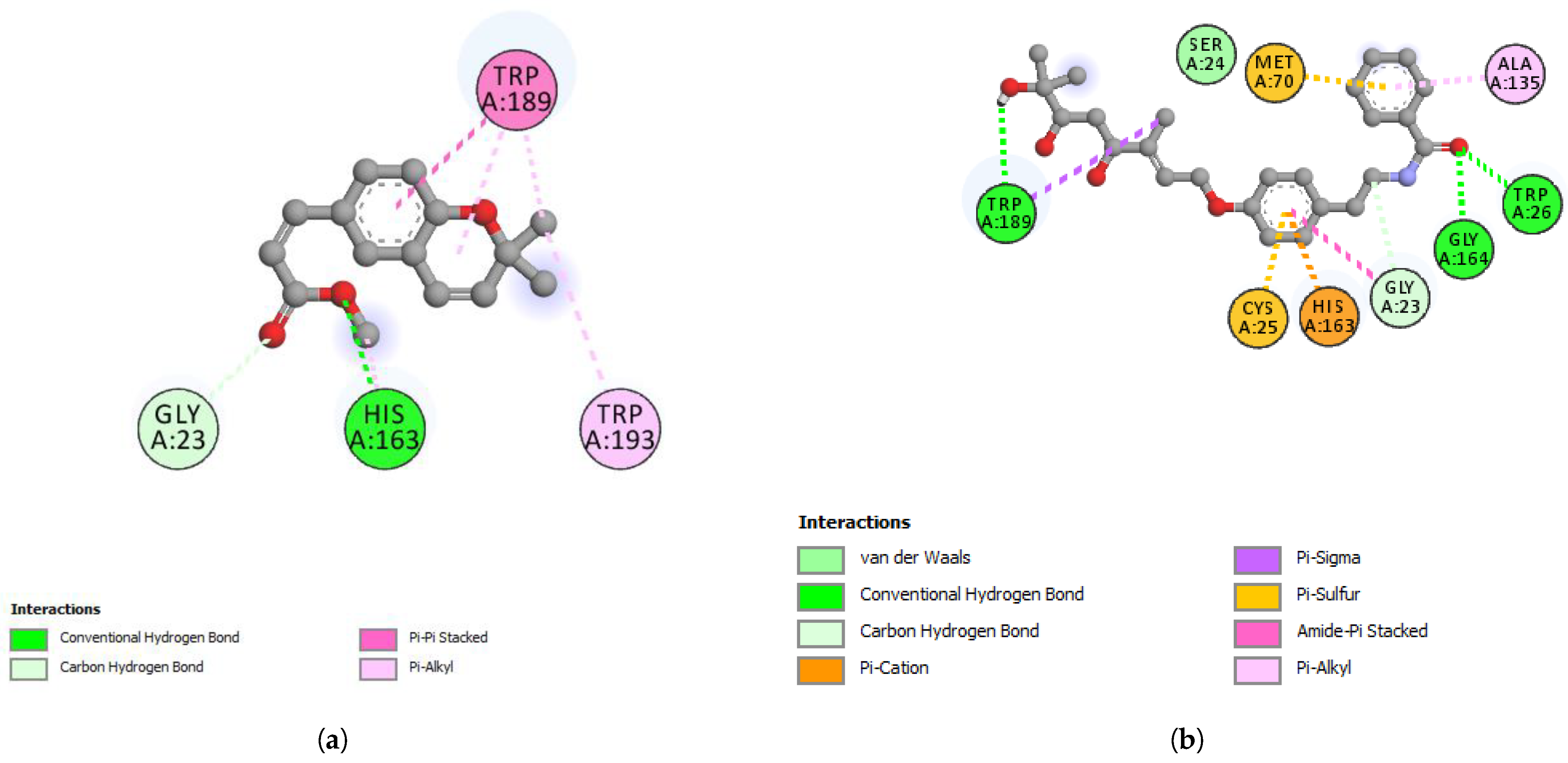
| TRP26 | 2.68 | Hydrogen Bond | Conventional Hydrogen Bond |
| GLY164 | 2.06 | Hydrogen Bond | Conventional Hydrogen Bond |
| TRP189 | 2.10 | Hydrogen Bond | Conventional Hydrogen Bond |
| GLY23 | 3.60 | Hydrogen Bond | Carbon Hydrogen Bond |
| HIS163 | 3.64 | Electrostatic | Pi-Cation |
| TRP189 | 3.67 | Hydrophobic | Pi-Sigma |
| TRP189 | 3.99 | Hydrophobic | Pi-Sigma |
| CYS25 | 4.81 | Other | Pi-Sulfur |
| MET70 | 5.04 | Other | Pi-Sulfur |
| GLY23, SER24 | 4.38 | Hydrophobic | Amide-Pi Stacked |
| ALA135 | 4.15 | Hydrophobic | Pi-Alkyl |
| Residue (Nsp13 helicase) | Distance | Category | Type |
| Dihydroxyacidissiminol | |||
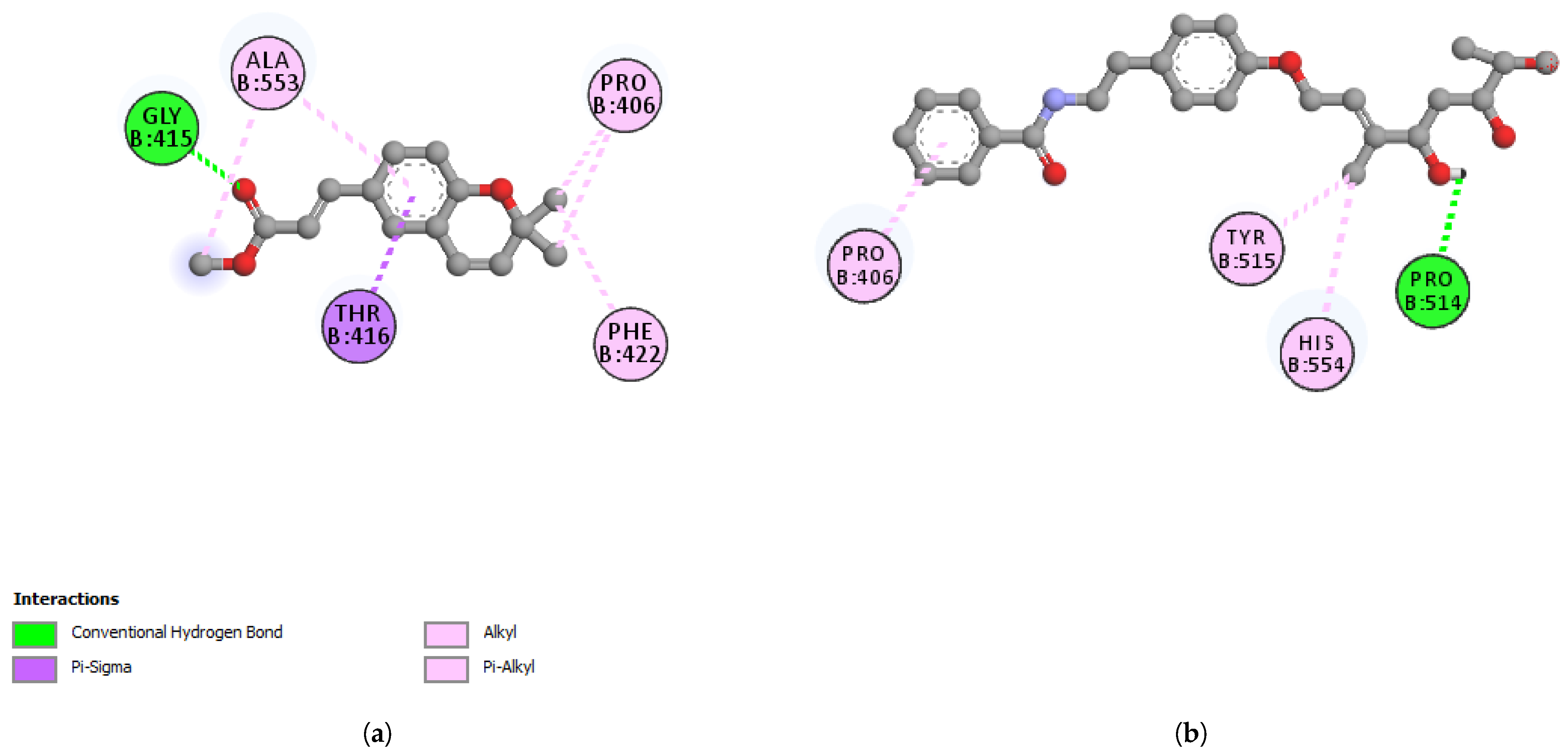
| PRO514 | 2.29 | Hydrogen Bond | Conventional Hydrogen Bond |
| TYR515 | 4.64 | Hydrophobic | Pi-Alkyl |
| HIS554 | 4.06 | Hydrophobic | Pi-Alkyl |
| PRO406 | 4.13 | Hydrophobic | Pi-Alkyl |
| Residue (S-RBD) | Distance | Category | Type |
| Dihydroxyacidissiminol | |||
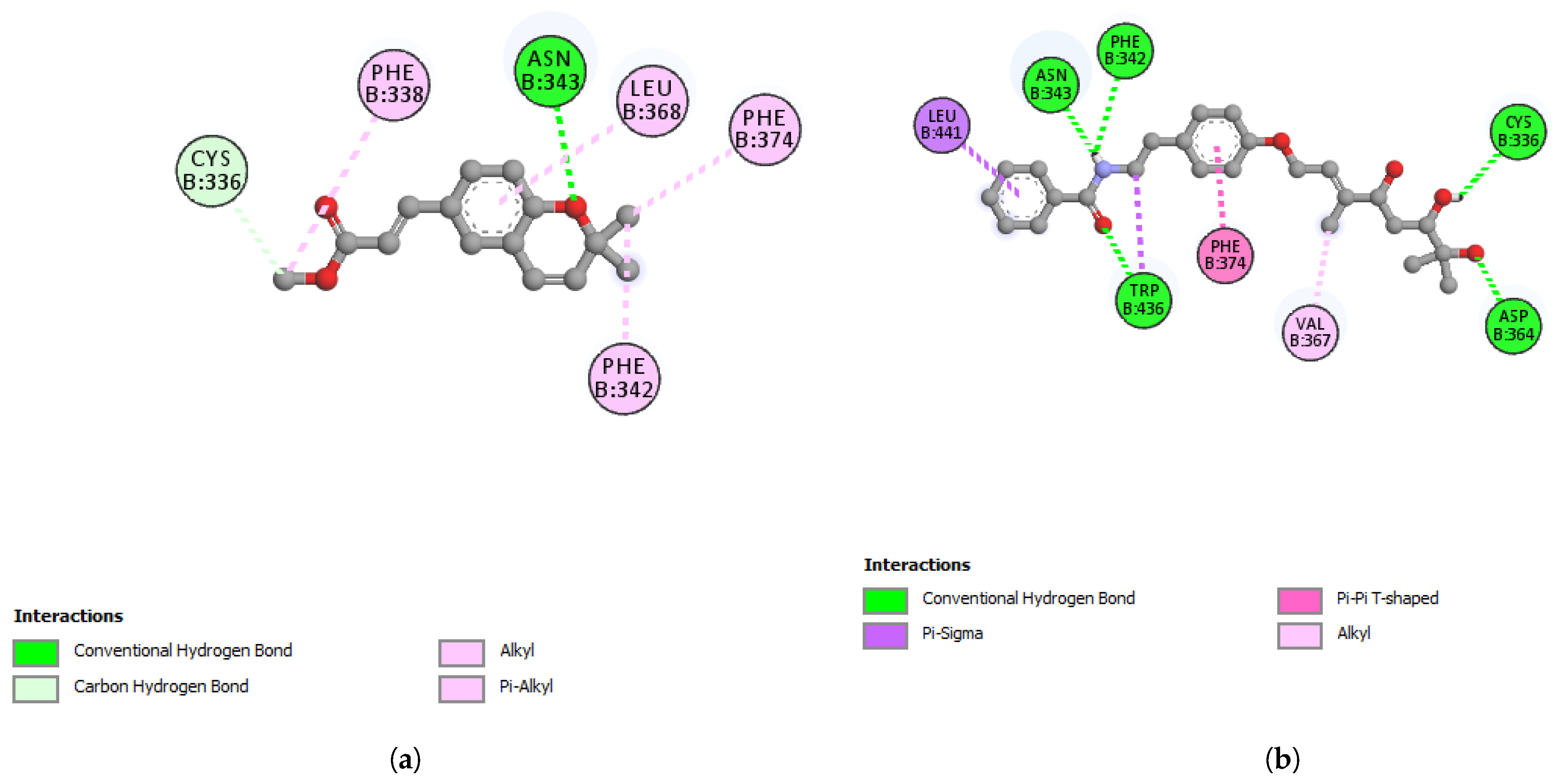
| ASP364 | 1.89 | Hydrogen Bond | Conventional Hydrogen Bond |
| B:TRP436 | 2.29 | Hydrogen Bond | Conventional Hydrogen Bond |
| CYS336 | 2.38 | Hydrogen Bond | Conventional Hydrogen Bond |
| PHE342 | 2.70 | Hydrogen Bond | Conventional Hydrogen Bond |
| ASN343 | 1.94 | Hydrogen Bond | Conventional Hydrogen Bond |
| LEU441 | 3.58 | Hydrophobic | Pi-Sigma |
| TRP436 | 3.98 | Hydrophobic | Pi-Sigma |
| PHE374 | 4.75 | Hydrophobic | Pi-Pi T-shaped |
| VAL367 | 4.58 | Hydrophobic | Alkyl |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zulkipli, M.; Mahbub, N.; Fatima, A.; Wan-Lin, S.L.; Khoo, T.-J.; Mahboob, T.; Rajagopal, M.; Samudi, C.; Kathirvalu, G.; Abdullah, N.H.; et al. Isolation and Characterization of Werneria Chromene and Dihydroxyacidissimol from Burkillanthus malaccensis (Ridl.) Swingle. Plants 2022, 11, 1388. https://doi.org/10.3390/plants11111388
Zulkipli M, Mahbub N, Fatima A, Wan-Lin SL, Khoo T-J, Mahboob T, Rajagopal M, Samudi C, Kathirvalu G, Abdullah NH, et al. Isolation and Characterization of Werneria Chromene and Dihydroxyacidissimol from Burkillanthus malaccensis (Ridl.) Swingle. Plants. 2022; 11(11):1388. https://doi.org/10.3390/plants11111388
Chicago/Turabian StyleZulkipli, Masyitah, Nuzum Mahbub, Ayesha Fatima, Stefanie Lim Wan-Lin, Teng-Jin Khoo, Tooba Mahboob, Mogana Rajagopal, Chandramathi Samudi, Gheetanjali Kathirvalu, Nor Hayati Abdullah, and et al. 2022. "Isolation and Characterization of Werneria Chromene and Dihydroxyacidissimol from Burkillanthus malaccensis (Ridl.) Swingle" Plants 11, no. 11: 1388. https://doi.org/10.3390/plants11111388
APA StyleZulkipli, M., Mahbub, N., Fatima, A., Wan-Lin, S. L., Khoo, T.-J., Mahboob, T., Rajagopal, M., Samudi, C., Kathirvalu, G., Abdullah, N. H., Pinho, A. R., Oliveira, S. M. R., Pereira, M. d. L., Rahmatullah, M., Hasan, A., Paul, A. K., Butler, M. S., Nawaz, M., Wilairatana, P., ... Wiart, C. (2022). Isolation and Characterization of Werneria Chromene and Dihydroxyacidissimol from Burkillanthus malaccensis (Ridl.) Swingle. Plants, 11(11), 1388. https://doi.org/10.3390/plants11111388











