A Novel High-Affinity Potassium Transporter IbHKT-like Gene Enhances Low–Potassium Tolerance in Transgenic Roots of Sweet Potato (Ipomoea batatas (L.) Lam.)
Abstract
:1. Introduction
2. Results
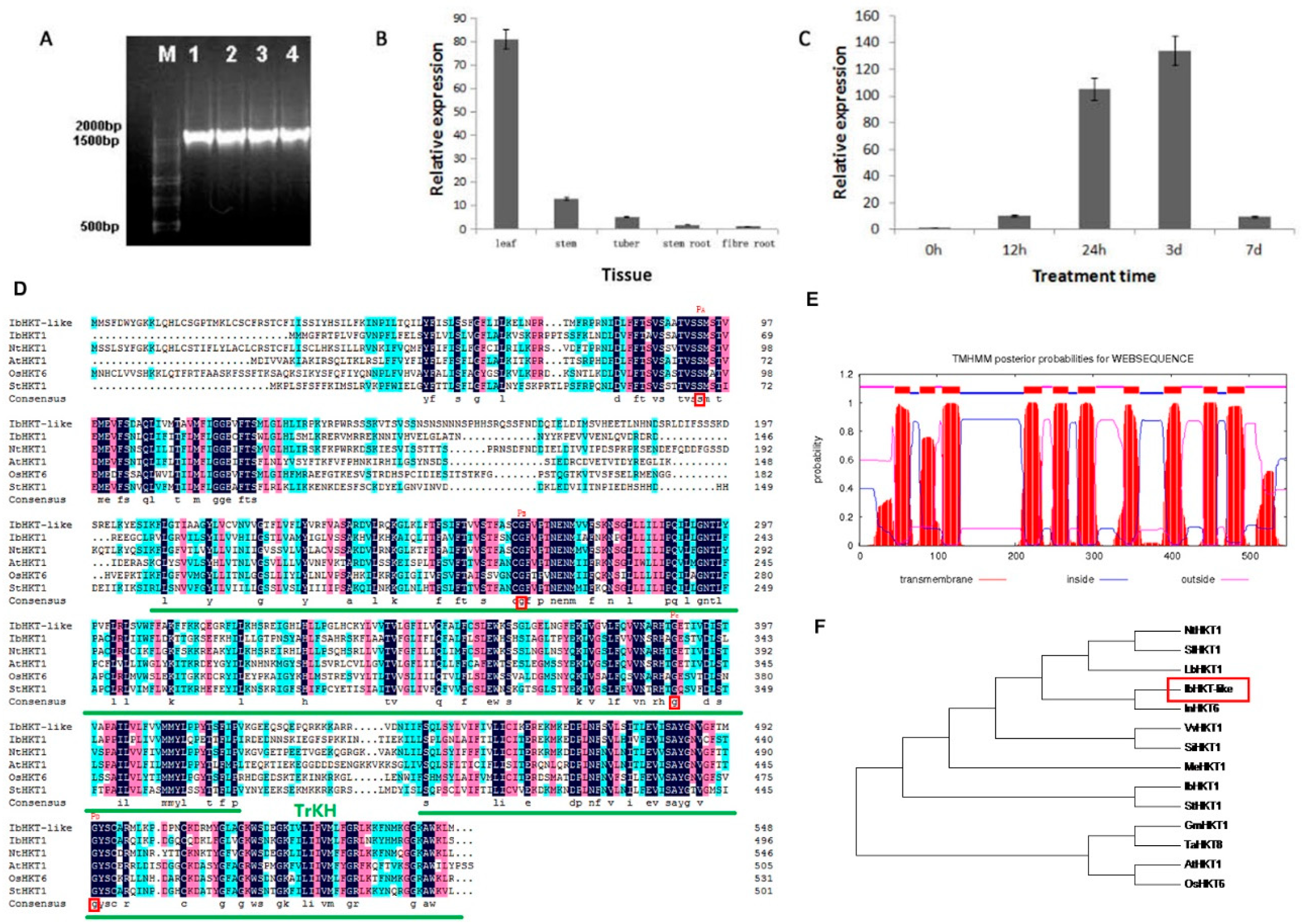
2.1. Identification of IbHKT-like Gene from Sweet Potato
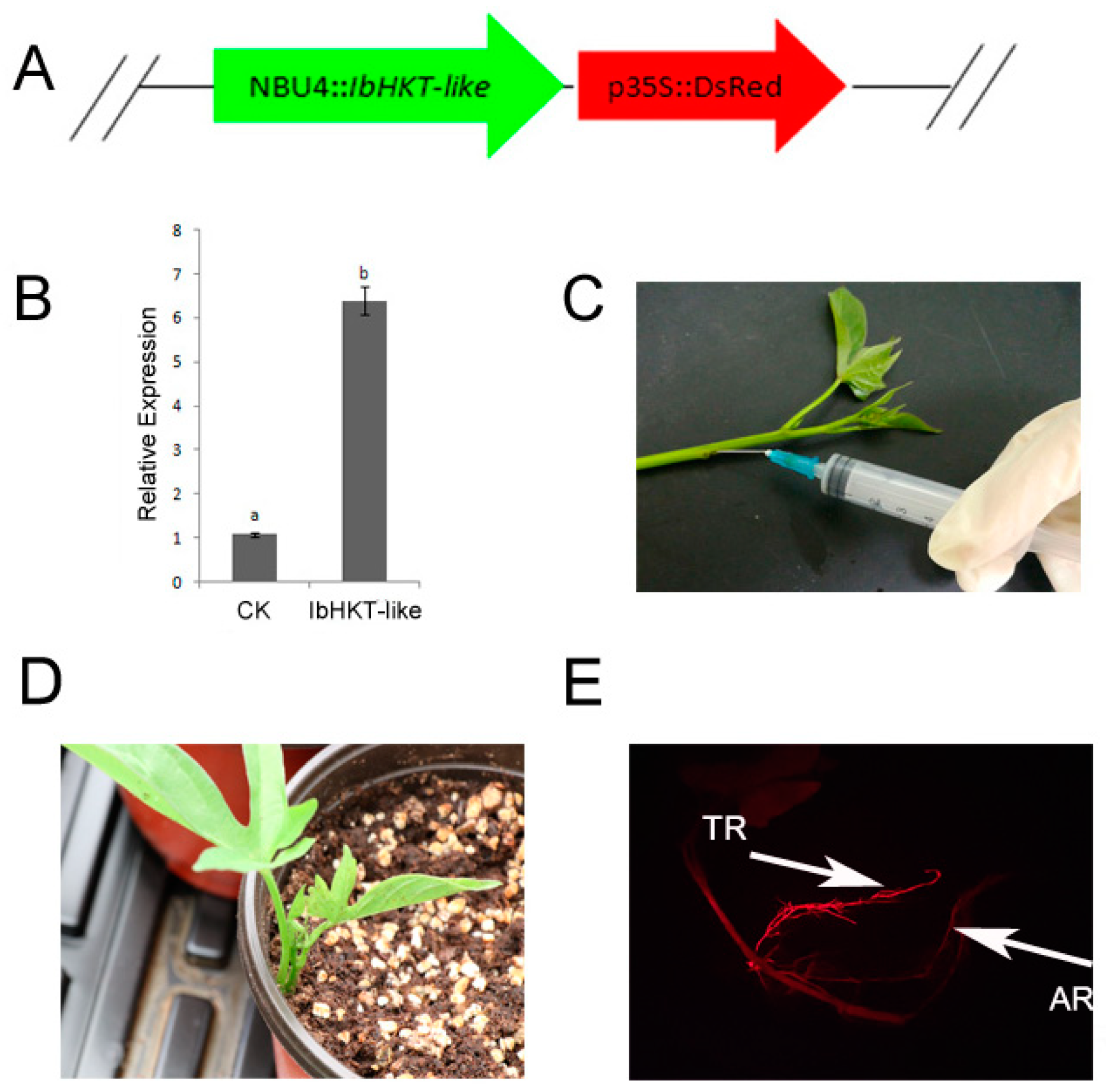
2.2. Generation of IbHKT-like Gene Overexpressing Roots in Sweet Potato
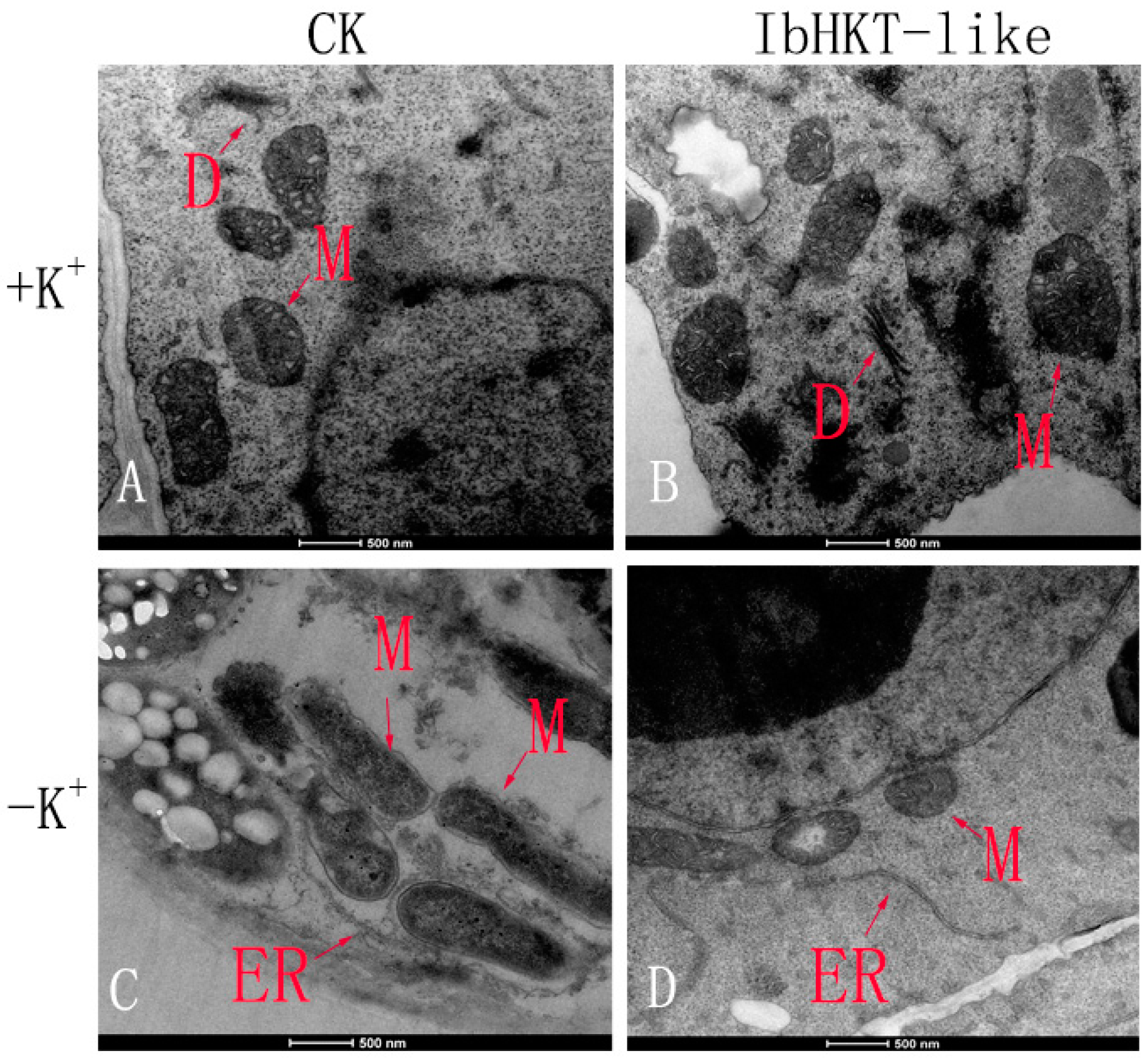
2.3. Protection of Cell Ultrastructure in IbHKT-like Gene Overexpressing Roots
2.4. Increase in K+ Absorption in IbHKT-like Gene Overexpressing Roots
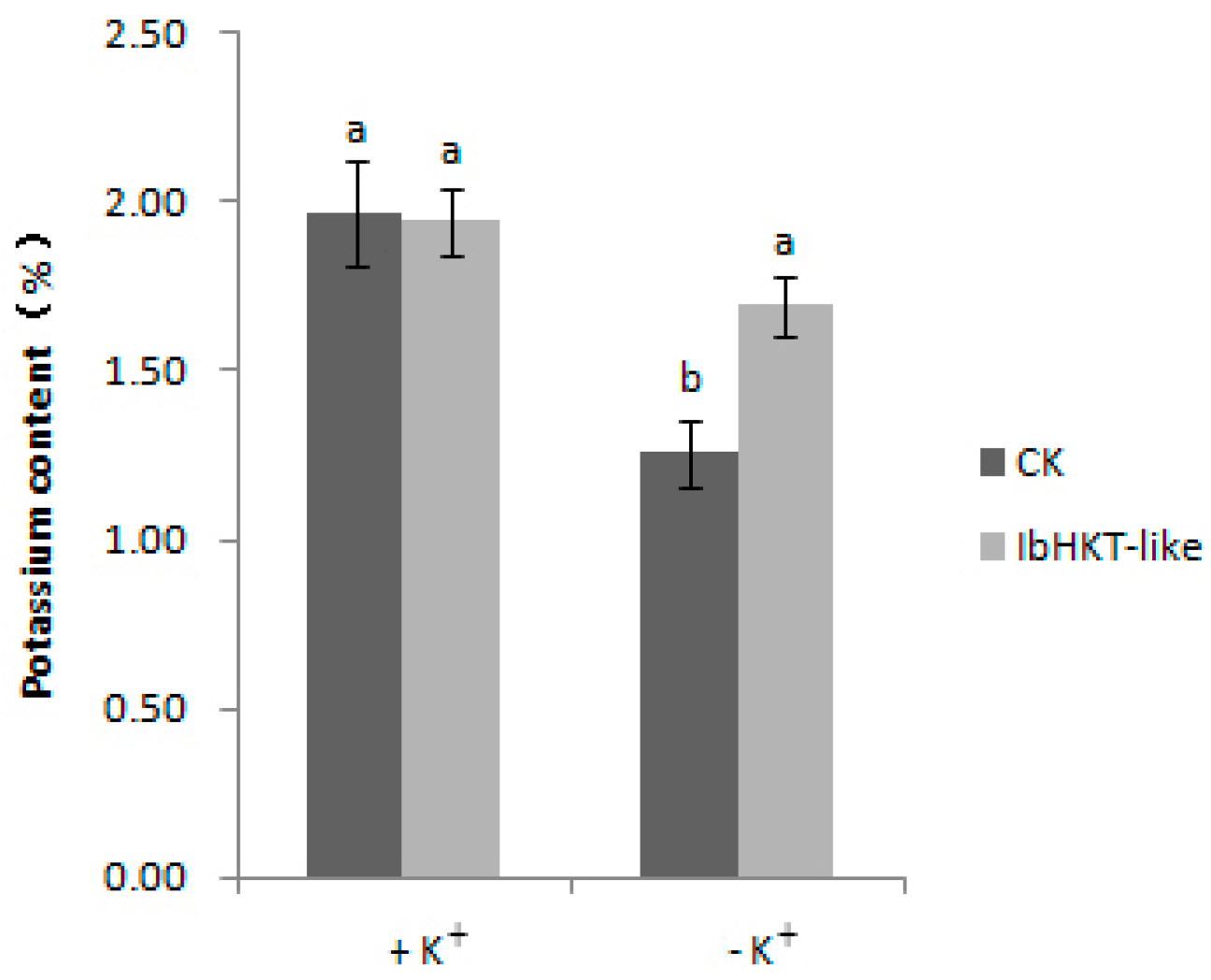
2.5. Increased Potassium Content in Plants with IbHKT-like Gene Overexpressing Roots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Expression Analysis and Phylogenetic Analysis
4.3. Vector Construction and Agrobacterium Rhizogenes-Mediated Transformation
4.4. Observation of Ultrastructure of Root Tip
4.5. Measurement of K+ Fluxes
4.6. Determination of Total Potassium Content
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, I.; Maathuis, F.J.M. Cellular and tissue distribution of potassium: Physiological relevance, mechanisms and regulation. J. Plant Physiol. 2014, 171, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Y.; Bai, B.W.; Almutairi, B.O.; Kudla, J. Emerging roles of the CBL-CIPK calcium signaling network as key regulatory hub in plant nutrition. J. Plant Physiol. 2021, 257, 153335. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, X.H.; Xia, L.; Jiang, C.J.; Wang, X.G.; Han, Y.; Wang, J.; Yu, H.Q. Effects of potassium deficiency on photosynthesis, chloroplast ultrastructure, ROS, and antioxidant activities in maize (Zea mays L.). J. Integr. Agric. 2019, 18, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.G.; Zhao, X.H.; Jiang, C.J.; Li, C.H.; Cong, S.; Wu, D.; Chen, Y.Q.; Yu, H.Q.; Wang, C.Y. Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J. Integr. Agric. 2015, 14, 856–863. [Google Scholar] [CrossRef]
- Wang, J.D.; Wang, H.Y.; Zhang, Y.C.; Zhou, J.M.; Chen, X.Q. Intraspecific variation in potassium uptake and utilization among sweet potato (Ipomoea batatas L.) genotypes. Field Crops Res. 2015, 170, 76–82. [Google Scholar] [CrossRef]
- Zorb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture-Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Cai, J.; Chen, L.; Qu, H.Y.; Lian, J.; Liu, W.; Hu, Y.B.; Xu, G.H. Alteration of nutrient allocation and transporter genes expression in rice under N, P, K, and Mg deficiencies. Acta Physiol. Plant. 2012, 34, 939–946. [Google Scholar] [CrossRef]
- Kim, M.J.; Ciani, S.; Schachtman, D.P. A Peroxidase Contributes to ROS Production during Arabidopsis Root Response to Potassium Deficiency. Mol. Plant 2010, 3, 420–427. [Google Scholar] [CrossRef]
- Borjigin, C.; Schilling, R.K.; Bose, J.; Hrmova, M.; Qiu, J.E.; Wege, S.; Situmorang, A.; Byrt, C.; Brien, C.; Berger, B.; et al. A single nucleotide substitution inTaHKT1; 5-D controls shoot Na+ accumulation in bread wheat. Plant Cell Environ. 2020, 43, 2158–2171. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, A.J.; Sun, J.; Chen, X.G.; Liu, M.; Zhao, P.; Jiang, W.; Tang, Z.H. Identification of Shaker K+ channel family members in sweetpotato and functional exploration of IbAKT1. Gene 2021, 768, 145311. [Google Scholar] [CrossRef]
- Durell, S.R.; Hao, Y.L.; Nakamura, T.; Bakker, E.P.; Guy, H.R. Evolutionary relationship between K+ channels and symporters. Biophys. J. 1999, 77, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Sassi, A.; Mieulet, D.; Khan, I.; Moreau, B.; Gaillard, I.; Sentenac, H.; Very, A.A. The Rice Monovalent Cation Transporter OsHKT2;4: Revisited Ionic Selectivity. Plant Physiol. 2012, 160, 498–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riedelsberger, J.; Miller, J.K.; Valdebenito, M.B.; Piñeros, M.A.; González, W.; Dreyer, I. Plant HKT Channels: An Updated View on Structure, Function and Gene Regulation. Int. J. Mol. Sci. 2021, 22, 1892. [Google Scholar] [CrossRef] [PubMed]
- Plett, D.; Safwat, G.; Gilliham, M.; Moller, I.S.; Roy, S.; Shirley, N.; Jacobs, A.; Johnson, A.; Tester, M. Improved Salinity Tolerance of Rice Through Cell Type-Specific Expression of AtHKT1; 1. PLoS ONE 2010, 5, e12571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, S.W.; Yao, X.; Luo, W.; Jha, D.; Tester, M.; Horie, T.; Schroeder, J.I. AtHKT1; 1 Mediates Nernstian Sodium Channel Transport Properties in Arabidopsis Root Stelar Cells. PLoS ONE 2011, 6, e24725. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Meng, S.; Tao, S.C.; Feng, J.; Fan, X.Q.; Xu, P.; Xu, Z.Z.; Shen, X.L. Overexpression of a samphire high-affinity potassium transporter gene SbHKT1 enhances salt tolerance in transgenic cotton. Acta Physiol. Plant. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Liu, W.H.; Fairbairn, D.J.; Reid, R.J.; Schachtman, D.P. Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability. Plant Physiol. 2001, 127, 283–294. [Google Scholar] [CrossRef] [Green Version]
- Rubio, F.; Gassmann, W.; Schroeder, J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 1995, 270, 1660–1663. [Google Scholar] [CrossRef]
- Wang, T.B.; Gassmann, W.; Rubio, F.; Schroeder, J.I.; Glass, A.D.M. Rapid up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998, 118, 651–659. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.B. Transcriptome Analysis Reveals a Novel High-Affinity Potassium Transporter HvHKT7 Involved in Low Potassium in Barley (Hordeum vulgare L.). In Proceedings of the Academic Annual Meeting of The Crop Science Socity of China, Nanjing, China, 29–31 October 2014. [Google Scholar]
- Gao, F.; Gong, Y.F.; Zhang, P.B. Production and deployment of virus-free sweetpotato in China. Crop Prot. 2000, 19, 105–111. [Google Scholar]
- Liu, M.; Zhang, A.J.; Chen, X.G.; Jin, R.; Li, H.M.; Tang, Z.H. The Effect of Potassium Deficiency on Growth and Physiology in Sweetpotato Ipomoea batatas (L.) Lam. during Early Growth. Hortscience 2017, 52, 1020–1028. [Google Scholar]
- Lu, G.Q.; George, M.S.; Zhou, W.J. Genotypic variation of sweetpotatoes grown under low potassium stress. J. Plant Nutr. 2003, 26, 745–756. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhang, Q.; Liu, Q.C.; Zhai, H.; Zhao, N.; He, S.Z. An AP2/ERF gene, IbRAP2-12, from sweetpotato is involved in salt and drought tolerance in transgenic Arabidopsis. Plant Sci. 2019, 281, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhai, H.; He, S.Z.; Zhang, H.; Ren, Z.T.; Zhang, D.D.; Liu, Q.C. A vacuolar Na+/H+ antiporter gene, IbNHX2, enhances salt and drought tolerance in transgenic sweetpotato. Sci. Hortic. 2016, 201, 153–166. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, X.R.; Zhi, Y.H.; Li, X.; Zhang, Q.; Niu, J.B.; Wang, J.; Zhai, H.; Zhao, N.; Li, J.G.; et al. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019, 223, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Park, S.C.; Yu, Y.C.; Kou, M.; Yan, H.; Tang, W.; Wang, X.; Liu, Y.J.; Zhang, Y.G.; Sang-soo, K.; Ma, D.F.; et al. Ipomoea batatas HKT1 transporter homolog mediates K+ and Na+ uptake in Saccharomyces cerevisiae. J. Integr. Agric. 2017, 16, 2168–2176. [Google Scholar] [CrossRef]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Jin, R.; Liu, M.; Zhao, P.; Zhang, A.J.; Wang, D.F.; Li, T.X.; Fan, W.J.; Tang, Z.H. Cloning and expression analysis of IbHKT-like gene in sweet potato. Jiangsu J. Agric. Sci. 2021, 31, 831–838. [Google Scholar]
- Cao, Y.; Jin, X.S.; Huang, H.; Derebe, M.G.; Levin, E.J.; Kabaleeswaran, V.; Pan, Y.P.; Punta, M.; Love, J.; Weng, J.; et al. Crystal structure of a potassium ion transporter, TrkH. Nature 2011, 471, 336–340. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.Y.; Shin, R.; Schachtman, D.P. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Ruzicka, D.; Shin, R.; Schachtman, D.P. The Arabidopsis AP2/ERF transcription factor RAP2.11 modulates plant response to low-potassium conditions. Mol. Plant 2012, 5, 1042–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antal, T.; Petrova, E.; Slepnyova, V.; Kukarskikh, G.; Volgusheva, A.; Dubini, A.; Baizhumanov, A.; Tyystjarvi, T.; Gorelova, O.; Baulina, O.; et al. Photosynthetic hydrogen production as acclimation mechanism in nutrient-deprived Chlamydomonas. Algal Res. Biomass Biofuels Bioprod. 2020, 49, 101951. [Google Scholar] [CrossRef]
- Ostaszewska, M.; Juszczuk, I.M.; Kolodziejek, I.; Rychter, A.M. Long-term sulphur starvation of Arabidopsis thaliana modifies mitochondrial ultrastructure and activity and changes tissue energy and redox status. J. Plant Physiol. 2014, 171, 549–558. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, A.J.; Chen, X.G.; Jin, R.; Li, H.M.; Tang, Z.H. Effects of potassium deficiency on root morphology, ultrastructure and antioxidant enzyme system in sweet potato (Ipomoea batatas L. Lam.) during early growth. Acta Physiol. Plant. 2017, 39, 211. [Google Scholar] [CrossRef]
- Rantanen, L.; Palomaki, V.; Harrison, A.F.; Lucas, P.W.; Mansfield, T.A. Interactions between combined exposure to SO2 and NO2 and nutrient status of trees-effects on nutrient content and uptake, growth, needle ultrastructure and pigments. New Phytol. 1994, 128, 689–701. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [Green Version]
- Drew, M.C.; Saker, L.R.; Barber, S.A.; Jenkins, W. Changes in the kinetics of phosphate and potassium absorption in nutrient-deficient barley roots measured by a solution-depletion technique. Planta 1984, 160, 490–499. [Google Scholar] [CrossRef]
- GLASS, A.D.M. Regulation of potassium absorption in barley roots an allosteric model. Plant Physiol. 1976, 58, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.J.; Zhang, X.Y.; Xu, Y.S.; An, L.J.; Liu, X.G.; Su, Q. The bacterial potassium transporter gene MbtrkH improves K+ uptake in yeast and tobacco. PLoS ONE 2020, 15, e0236246. [Google Scholar]
- Lu, Z.F.; Xie, K.L.; Pan, Y.H.; Ren, T.; Lu, J.W.; Wang, M.; She, Q.R.; Guo, S.W. Potassium mediates coordination of leaf photosynthesis and hydraulic conductance by modifications of leaf anatomy. Plant Cell Environ. 2019, 42, 2231–2244. [Google Scholar] [CrossRef]
- Zhang, G.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Maruo, T. Plant Growth and Photosynthesis Response to Low Potassium Conditions in Three Lettuce (Lactuca sativa) Types. Hortic. J. 2017, 86, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Jiang, W.; Yan, M.X.; Zhang, A.J.; Liu, M.; Zhao, P.; Chen, X.G.; Tang, Z.H. Genome-wide characterization and expression analysis of HAK K+ transport family in Ipomoea. 3 Biotech 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.C.; Xuan, Y.; Bian, X.F.; Zhang, L.; Pan, Z.Y.; Kou, M.; Cao, Q.H.; Tang, Z.H.; Li, Q.; Ma, D.F.; et al. Overexpression of phosphatidylserine synthase IbPSS1 affords cellular Na+ homeostasis and salt tolerance by activating plasma membrane Na+/H+ antiport activity in sweet potato roots. Hortic. Res. 2020, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; Zhang, A.J.; Wei, M.; Chen, X.G.; Liu, Z.H.; Li, H.M.; Ding, Y.F. Physiological response to potassium deficiency in three sweet potato (Ipomoea batatas L. Lam.) genotypes differing in potassium utilization efficiency. Acta Physiol. Plant. 2015, 37, 184. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.L.; Dai, S.X.; Wang, R.G.; Li, N.Y.; Shen, X.; Zhou, X.Y.; Lu, C.F.; Zheng, X.J.; Hu, Z.M.; et al. NaCl-induced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol. 2009, 149, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.C.; Kou, M.; Gao, Z.H.; Liu, Y.; Xuan, Y.; Liu, Y.J.; Tang, Z.H.; Cao, Q.H.; Li, Z.Y.; Sun, J. Involvement of phosphatidylserine and triacylglycerol in the response of sweet potato leaves to salt stress. Front. Plant Sci. 2019, 10, 1086. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Jin, R.; Wang, D.; Yang, Y.; Zhao, P.; Liu, M.; Zhang, A.; Tang, Z. A Novel High-Affinity Potassium Transporter IbHKT-like Gene Enhances Low–Potassium Tolerance in Transgenic Roots of Sweet Potato (Ipomoea batatas (L.) Lam.). Plants 2022, 11, 1389. https://doi.org/10.3390/plants11111389
Jiang W, Jin R, Wang D, Yang Y, Zhao P, Liu M, Zhang A, Tang Z. A Novel High-Affinity Potassium Transporter IbHKT-like Gene Enhances Low–Potassium Tolerance in Transgenic Roots of Sweet Potato (Ipomoea batatas (L.) Lam.). Plants. 2022; 11(11):1389. https://doi.org/10.3390/plants11111389
Chicago/Turabian StyleJiang, Wei, Rong Jin, Danfeng Wang, Yufeng Yang, Peng Zhao, Ming Liu, Aijun Zhang, and Zhonghou Tang. 2022. "A Novel High-Affinity Potassium Transporter IbHKT-like Gene Enhances Low–Potassium Tolerance in Transgenic Roots of Sweet Potato (Ipomoea batatas (L.) Lam.)" Plants 11, no. 11: 1389. https://doi.org/10.3390/plants11111389
APA StyleJiang, W., Jin, R., Wang, D., Yang, Y., Zhao, P., Liu, M., Zhang, A., & Tang, Z. (2022). A Novel High-Affinity Potassium Transporter IbHKT-like Gene Enhances Low–Potassium Tolerance in Transgenic Roots of Sweet Potato (Ipomoea batatas (L.) Lam.). Plants, 11(11), 1389. https://doi.org/10.3390/plants11111389




