Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview
Abstract
:1. Introduction
1.1. Geographic Diffusion and Nutritional Relevance of Durum Wheat
1.2. Drought and Salinity: Impact on Plant Physiology in Relation to Antioxidant Defence System
1.2.1. Drought and Salinity in the Global Climate Change Scenario
1.2.2. Physiological and Biochemical Responses to Hyperosmotic Stress: Focus on the Role of Antioxidant Defence System in Stress Tolerance
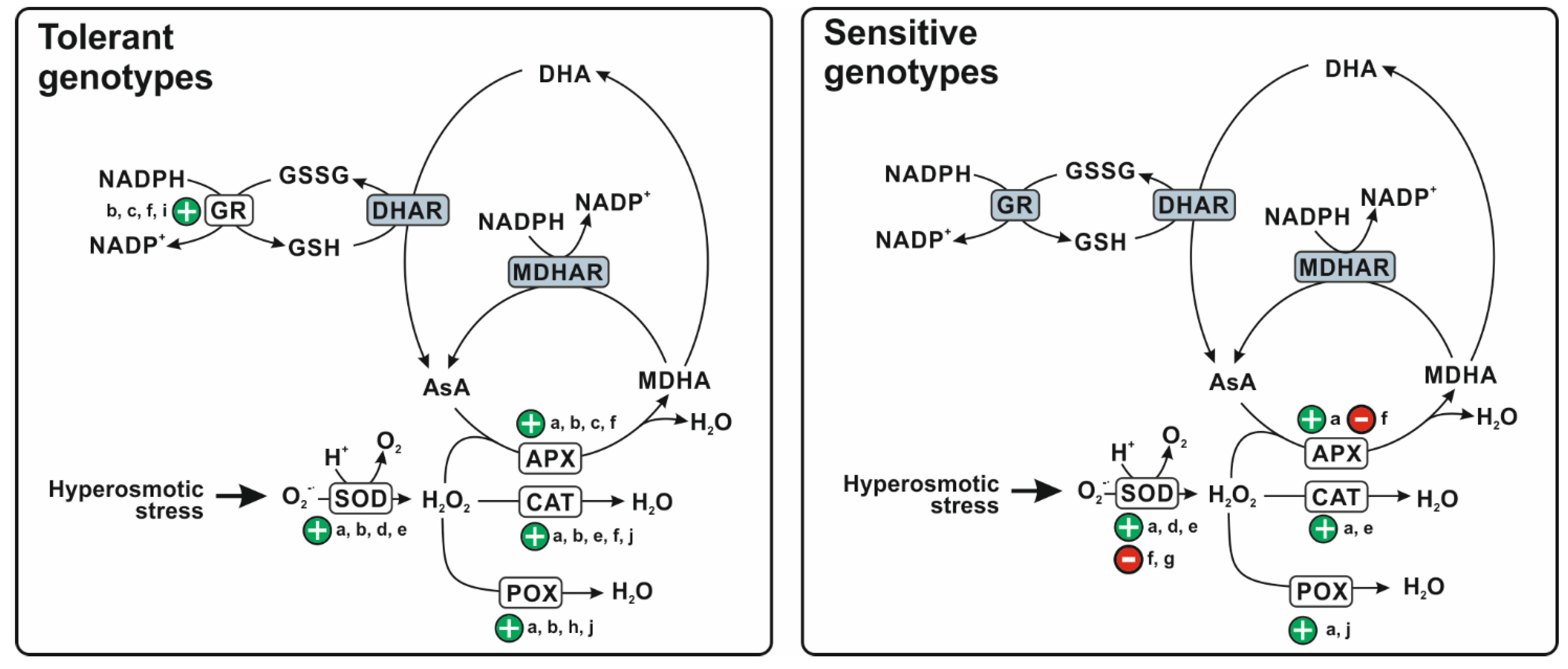
2. Effect of Hyperosmotic Stress on Antioxidant Defence System in Durum Wheat
2.1. Drought Stress
| Plant Species | Growth Conditions | Stress Treatment/s | Tissue | Changes in Antioxidant Components | Ref. |
|---|---|---|---|---|---|
| 50 durum wheat genotypes 18 Iranian landraces, 3 local Kermanshah, Iran and 29 breeding lines | Growth chamber | PEG-6000 (imposed at the three-leaf stage) for 14 days | Leaf obtained 14 days after PEG treatment | SOD activity: on average +92% CAT activity: on average +11% APX activity: on average +15% POX activity: on average +177% | [43] |
| 1 durum wheat genotype Maali 3 T3 transgenic durum wheat lines overexpressing TdPIP2 | Greenhouse | 40% of field capacity imposed after 2 weeks from sowing until the end of plant cycle | Leaf | CAT activity: about 1.6- and up to 2.2-fold increase in wild type transgenic lines, respectively SOD activity: about 1.7- and up to 3.2-fold increase in wild type and transgenic lines, respectively | [44] |
| 8 durum wheat near-isogenic lines derived from 4 different recombinant inbred lines of a cross between Kofa and Svevo | Field | Rain-fed conditions and two different sowing densities (480 seeds/m2 and 320 seeds/m2) | Leaf at flowering | APX activity: on average 70% decrease POX activity: on average 50% increase | [45] |
| 2 durum wheat genotypes Barakatli-95 (drought-tolerant) Garagylchyg-2 (drought-sensitive) 2 bread wheat genotypes Giymatli-2/17 (drought-sensitive) Azamatli-95 (less drought-sensitive) | Field | Rain-fed conditions imposed by withholding irrigation | Leaf at seven different stages of ontogenesis | APX activity: on average about 1.4- fold increase and 30% decrease in Barakatli-95 and Garagylchyg-2, respectively, with the maximal activity under stress at the end of flowering and of earing, respectively SOD activity: on average no significant change and about 60% decrease in Barakatli-95 and Garagylchyg-2, respectively CAT activity: on average 1.75- and 1.2-fold increase in Barakatli-95 and Garagylchyg-2, respectively, with the maximal activity at the milk ripeness stage GR activity: increase in Barakatli-95 at all stages of ontogenesis, with the maximal activity under normal water supply at flowering For bread wheat genotypes see literature | [46] |
| 2 durum wheat genotypes Barakatli-95 (drought-tolerant) Garagylchyg- 2 (drought-sensitive) | Field | Rain-fed conditions imposed by ceasing watering | Leaf at seven different stages of ontogenesis | SOD activity: on average no significant change and about 60% decrease in Barakatli-95 and Garagylchyg-2, respectively Changes in SOD isoenzyme composition under stress | [47] |
| 2 durum wheat genotypes Barakatli 95 (drought-tolerant) Garagylchyg-2 (drought-sensitive) | Field | Rain-fed conditions (imposed by ceasing watering from April to June) | Leaf and root at three different stages of ontogenesis | APX activity: on average no significant change in leaves Barakatli-95 and about 1.2-fold increase in leaves and roots of Garagylchyg-2, with the maximal activity at flowering Guaiacol-type POX activity: on average about 7-fold increase in leaves and roots of Barakatli-95 and 1.5-fold increase in leaves and about 50% decrease in roots of Garagylchyg-2, with the maximal activity in leaves and roots at wax ripeness and flowering, respectively Benzidine-type POX activity: on average 2.3- and 1.8-fold increase in leaves and roots of Barakatli-95, respectively, and 1.6- and 1.4-fold increase in leaves and roots of Garagylchyg-2, respectively, with the maximal activity at wax ripeness CAT activity: on average 1.4- and 1.2-fold increase in leaves of Barakatli-95 and Garagylchyg-2, respectively, and no significant change and about 20% decrease in roots of Barakatli-95 and Garagylchyg-2, respectively | [48] |
| 2 durum wheat genotypes Barakatli-95 (drought-tolerant) Garagylchyg-2 (drought-sensitive) | Field | Rain-fed conditions (imposed by ceasing watering from April to June) | Leaf and roots at three different stages of ontogenesis | AsA content: similar decrease in all stages of ontogenesis ranging from 20% to 25% in leaves and roots of both Barakatli-95 and Garagylchyg-2 | [49] |
| 1 durum wheat genotype A 9-30-1 1 Triticum dicoccum genotype HW 24 2 bread wheat genotypes C 306 (drought-resistant) and Hira (drought-sensitive) | Natural conditions in earthen pots | Drought stress imposed by withholding water supply for 7 days during 3 different phases, at 50% anthesis and 10 and 20 days after anthesis (DAA) | Leaf at 7, 17 and 27 DAA | SOD activity: up to about 30% increase at 27 DAA in durum wheat CAT activity: slight increase in durum wheat POX activity: slight increase in durum wheat For the other cereal species, see literature | [50] |
| 3 durum wheat genotypes Kızıltan-91, Kunduru 414-44 and Ç.1252 3 bread wheat genotypes Bezostaya-1, Seri-82 and Kıraç-66 | Growth chamber | Drought stress (imposed on 6-day-old seedlings), in combination with low (5/−5 °C, day/night) or high (40/30 °C) temperatures conditions, for 6 days | Leaf from 12-day-old seedlings | CAT activity: no significant change in Kızıltan-91, and about 30% increase and 40% decrease in Kunduru 414-44 and Ç.1252, respectively, under drought stress and normal temperature GR activity: no significant change in Ç.1252 and about 2- and 1.4-fold increase in Kızıltan-91 and Kunduru 414-44, respectively, under drought stress and normal temperature AsA+DHA content: about 30% increase in Kızıltan-91 and about 25% decrease in both Kunduru 414-44 and Ç.1252, under drought stress and normal temperature For combined effects of drought and low or high temperatures, see literature | [51] |
2.2. Salinity Stress
| Plant Species | Growth Conditions | Stress Treatment/s | Tissue | Changes in Antioxidant Components | Ref. |
|---|---|---|---|---|---|
| 2 durum wheat genotypes Om Rabia3 (salt-tolerant) Mahmoudi (salt-sensitive) | Glasshouse | 50, 100, and 200 mM NaCl (imposed on 7-day-old seedlings) for 3 days | Shoot from 10-day-old seedlings | SOD activity: up to about 5- and 3-fold increase at 200 mM NaCl in Om Rabia3 and Mahmoudi, respectively POX activity: up to about 2- and 1.6-fold increase at 200 mM NaCl, in Om Rabia3 and Mahmoudi, respectively CAT activity: up to about 8- and 6-fold (increase at 200 mM NaCl in Om Rabia3 and Mahmoudi, respectively APX activity: up to about 2-fold increase at 100 mM NaCl in both genotypes AsA content: up to about −20% and −35% at 200 mM NaCl in Om Rabia3 and Mahmoudi, respectively Expression level of CAT, MnSOD and APX genes: from 2- to 4-fold increase | [52] |
| 2 durum wheat genotypes Karim and Azizi | Hydroponic culture in growth chamber | 100 mM NaCl for 11 days | Root and leaf from 21-day-old seedlings | GSH content: −82% and −64% in leaf and root of Karim, respectively, and −34% and −47% in leaf and root of Azizi, respectively GSSG content: no significant change in Karim and 51% decrease in root of Azizi | [53] |
| 2 durum wheat genotypes Ofanto (drought tolerant) Adamello | Growth chamber | 50 and 100 mM NaCl for 9 days | Root and shoot from 9-day-old seedlings | APX, MDHAR, DHAR and GR activities: general increasing and decreasing trends in shoots and roots, respectively AsA, DHA, GSH, and GSSG contents: variable, depending on genotype, tissue, and stress intensity | [54] |
| 1 durum wheat genotype Ofanto (drought tolerant) | Hydroponic culture in growth chamber | 20% (v/v) sea water for 14 and 21 days | Root and shoot from 14- and 21-day-old seedlings | APX and GR activities: variable depending on tissue and stress duration AsA, DHA, GSH, GSSG contents: variable depending on tissue and stress duration | [55] |
| 1 durum wheat genotype Duilio | Hydroponic culture in growth chamber | 100 and 200 mM NaCl (imposed on 5-day-old seedlings) for 10 days | Leaf from 15-day-old seedlings | POXs proteomic analysis: 1 isoform upregulated at both salinity levels, 1 isoform upregulated at 100 mM NaCl and 1 isoform downregulated at 100 mM NaCl | [56] |
| 1 durum wheat genotype Ofanto (drought tolerant) | Hydroponic culture in phytotron | 100 mM NaCl (imposed on the 10th day of hydroponic culture) for 10 days, the last 5 of which in combination with high light (900 μmol m−2 s−1 PAR) | Shoot from 20-day-old seedlings | APX, GR and SOD activities: about 2.2-fold increase under salt stress and low light CAT activity: about 1.7-fold increase under salt stress and low light POX activity: about 2.1-fold increase under salt stress and low light AsA content: 2.75-fold increase under salt stress and low light GSH and GSSG contents: 3.47-fold and 2.1-fold increase under salt stress and low light, respectively For combined effects of salinity and high light, see literature | [57] |
| 1 durum wheat genotype AS 780 (salt-tolerant) 1 emmer wheat genotype AS 847 (salt-sensitive) | Growth chamber | 10 and 100 mM NaCl (imposed on 1 month-old seedlings for 2 weeks), supplemented with 3 mM MnSO4, for two weeks | Leaf | APX activity: 5-fold and +85% increase at 100 mM NaCl in durum wheat and emmer, respectively GR activity: +68% at 100 mM NaCl in both durum wheat and emmer SOD and DHAR activities: no significant change under salt stress For combined salt-manganese effects, see literature | [58] |
| 3 durum wheat genotypes Kızıltan-91, Kunduru 414-44 and Ç.1252 3 bread wheat genotypes Bezostaya-1, Seri-82 and Kıraç-66 | Growth chamber | 0.7% NaCl (imposed on 6-day-old seedlings), in combination with low (5/−5 °C day/night) or high (40/30 °C) temperatures, for 6 days | Leaf from 12-day-old seedlings | CAT activity: about 2-fold increase in Kızıltan-91 and Kunduru 414-44 and about +30% in Ç.1252 under salt stress and normal temperature GR activity: slight effect under salt stress and normal temperature AsA+DHA content: about 2.4-fold increase in Kızıltan-91 and about +30% in Kunduru 414-44 and Ç.1252 under salt stress and normal temperature For combined effects of salinity and low or high temperatures, see literature | [51] |
| 2 durum wheat genotypes Mohamed Ben Bachir (relatively drought-tolerant) and Hedba 3 (relatively drought-sensitive) | Growth chamber | 10 g/L NaCl (imposed on 3-day-old seedlings), supplemented with 20 mM proline, for 10 days | Shoot from 13-day-old seedlings | SOD activity: 3- and 2-fold increase under salt stress in Hedba 3 and Mohamed Ben Bachir, respectively For combined salt-proline effects, see literature | [59] |
| 5 durum wheat genotypes F7 recombinant inbred lines derived from a cross between durum wheat cv. Langdon and wild emmer wheat accession G18-16 | Growth chamber | 50 mM NaCl, supplemented with Na2SeO4 or Na2SeO3 at different concentrations (0.1, 1, 2, 4, 8, 10 μM), for 10 days | Root and shoot from 10-day-old seedlings | SOD activity: on average −12% under salt stress in shoots CAT activity: on average −9.5% and −9.7% under salt stress in roots and shoots, respectively POX activity: on average −5.5% and −4.8% under salt stress in roots and shoots, respectively For combined salt-selenium effects, see literature | [60] |
| 1 durum wheat genotype Svevo | Hydroponic culture in growth chamber | 200 mM NaCl (imposed on 10-day-old seedlings), combined with foliar treatment with chitosan (100 mg/L), for 7 days | Shoot from 17-day-old seedlings | SOD activity: +89% under salt stress CAT activity: +86% under salt stress POX activity: slight increase under salt stress For combined salt-chitosan effects, see literature | [61] |
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Colasuonno, P.; Marcotuli, I.; Gadaleta, A.; Soriano, J.M. From Genetic Maps to QTL Cloning: An Overview for Durum Wheat. Plants 2021, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Marcotuli, I.; Colasuonno, P.; Hsieh, Y.S.Y.; Fincher, G.B.; Gadaleta, A. Non-Starch Polysaccharides in Durum Wheat: A Review. Int. J. Mol. Sci. 2020, 21, 2933. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; De Vita, P.; Mastrangelo, A.M. Effects of Heat Stress on Metabolite Accumulation and Composition, and Nutritional Properties of Durum Wheat Grain. Int. J. Mol. Sci. 2015, 16, 30382–30404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the Healthgrain Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Rana, G.; Katerji, N. Measurement and estimation of actual evapotranspiration in the field under Mediterranean climate: A review. Eur. J. Agron. 2000, 13, 125–153. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, A.; De Pascale, S.; Fagnano, M.; Barbieri, G. Saline agriculture in Mediterranean environments. Ital. J. Agron. 2011, 6, e7. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Lewis, J.M.; Ammar, K.; Basnet, B.R.; Crespo-Herrera, L.; Crossa, J.; Dhugga, K.S.; Dreisigacker, S.; Juliana, P.; Karwat, H.; et al. Harnessing translational research in wheat for climate resilience. J. Exp. Bot. 2021, 72, 5134–5157. [Google Scholar] [CrossRef] [PubMed]
- Diffenbaugh, N.S.; Giorgi, F. Climate change hotspots in the CMIP5 global climate model ensemble. Clim. Chang. 2012, 114, 813–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef] [PubMed]
- Qamer, Z.; Chaudhary, M.T.; DU, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigorova, B.; Vaseva, I.; Demirevska, K.; Feller, U. Combined drought and heat stress in wheat: Changes in some heat shock proteins. Biol. Plant. 2011, 55, 105–111. [Google Scholar] [CrossRef]
- Edelstein, M.; Plaut, Z.; Ben-Hur, M. Water salinity and sodicity effects on soil structure and hydraulic properties. Adv. Hortic. Sci. 2010, 24, 154–160. [Google Scholar]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Land and Plant Nutrition Management Service. 2008. Available online: http://www.fao.org/ag/agl/agll/spush (accessed on 5 February 2021).
- Bar, S.; Kumari, B.; Gupta, S.K. Salinization of Coastal Groundwater Resource in the Perspective of Climate Change. In Fate and Transport of Subsurface Pollutants; Gupta, P.K., Bharagava, R.N., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2021; Volume 24. [Google Scholar] [CrossRef]
- Wang, W.-X.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The Combined Effect of Drought Stress and Heat Shock on Gene Expression in Tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. CRC. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Hyskova, V.; Ryslava, H. Hyperosmotic versus Hypoosmotic Stress in Plants. Biochem. Anal. Biochem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Raza, M.A.; Singh, K.; Hossain, M.A.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 43. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Foreman, J.; Demidchik, V.; Bothwell, J.H.F.; Mylona, P.; Miedema, H.; Torres, M.A.; Linstead, P.; Costa, S.; Brownlee, C.; Jones, J.D.G.; et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Heiber, I.; Ströher, E.; Raatz, B.; Busse, I.; Kahmann, U.; Bevan, M.W.; Dietz, K.-J.; Baier, M. The redox imbalanced Mutants of Arabidopsis Differentiate Signaling Pathways for Redox Regulation of Chloroplast Antioxidant Enzymes. Plant Physiol. 2007, 143, 1774–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A Large Family of Class III Plant Peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Pour-Aboughadareh, A.; Etminan, A.; Abdelrahman, M.; Siddique, K.H.M.; Tran, L.-S.P. Assessment of biochemical and physiological parameters of durum wheat genotypes at the seedling stage during polyethylene glycol-induced water stress. Plant Growth Regul. 2020, 92, 81–93. [Google Scholar] [CrossRef]
- Ayadi, M.; Brini, F.; Masmoudi, K. Overexpression of a Wheat Aquaporin Gene, Td PIP2;1, Enhances Salt and Drought Tolerance in Transgenic Durum Wheat cv. Maali. Int. J. Mol. Sci. 2019, 20, 2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bányai, J.; Maccaferri, M.; Láng, L.; Mayer, M.; Tóth, V.; Cséplő, M.; Pál, M.; Mészáros, K.; Vida, G. Abiotic Stress Response of Near-Isogenic Spring Durum Wheat Lines under Different Sowing Densities. Int. J. Mol. Sci. 2021, 22, 2053. [Google Scholar] [CrossRef] [PubMed]
- Huseynova, I.M. Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Huseynova, I.M.; Aliyeva, D.R.; Aliyev, J.A. Subcellular localization and responses of superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 2014, 81, 54–60. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Aliyeva, D.R.; Mammadov, A.C.; Aliyev, J.A. Hydrogen peroxide generation and antioxidant enzyme activities in the leaves and roots of wheat cultivars subjected to long-term soil drought stress. Photosynth. Res. 2015, 125, 279–289. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Rustamova, S.M.; Suleymanov, S.Y.; Aliyeva, D.R.; Mammadov, A.C.; Aliyev, J.A. Drought-induced changes in photosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth. Res. 2016, 130, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.K.; Chandrasekhar, V.; Srivastava, G.C.C. Comparison of Hexaploid and Tetraploid Wheat Cultivars in their Responses to Water Stress. Biol. Plant. 2001, 44, 89–94. [Google Scholar] [CrossRef]
- Keles, Y.; Öncel, I. Response of antioxidative defence system to temperature and water stress combinations in wheat seedlings. Plant Sci. 2002, 163, 783–790. [Google Scholar] [CrossRef]
- Feki, K.; Tounsi, S.; Brini, F. Comparison of an antioxidant system in tolerant and susceptible wheat seedlings in response to salt stress. Span. J. Agric. Res. 2017, 15, e0805. [Google Scholar] [CrossRef] [Green Version]
- Bouthour, D.; Kalai, T.; Chaffei, H.C.; Gouia, H.; Corpas, F.J. Differential response of NADP-dehydrogenases and carbon metabolism in leaves and roots of two durum wheat (Triticum durum Desf.) cultivars (Karim and Azizi) with different sensitivities to salt stress. J. Plant Physiol. 2015, 179, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, S.; Navam-Izzo, F.; Izzo, R. Antioxidative Responses of Shoots and Roots of Wheat to Increasing NaCl Concentrations. J. Plant Physiol. 1999, 155, 274–280. [Google Scholar] [CrossRef]
- D’Amico, M.L.; Navari-Izzo, F.; Sgherri, C.; Izzo, R. The role of lipoic acid in the regulation of the redox status of wheat irrigated with 20% sea water. Plant Physiol. Biochem. 2004, 42, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Borrelli, G.M.; Colapicchioni, V.; Papa, R.; Piovesana, S.; Samperi, R.; Stampachiacchiere, S.; Laganà, A. Proteomic study of a tolerant genotype of durum wheat under salt-stress conditions. Anal. Bioanal. Chem. 2014, 406, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D’Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A.; et al. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Differential Responses of Two Wheat Varieties Differing in Salt Tolerance to the Combined Stress of Mn and Salinity. J. Plant Growth Regul. 2020, 39, 795–808. [Google Scholar] [CrossRef]
- Ami, K.; Planchais, S.; Cabassa, C.; Guivarc’H, A.; Very, A.-A.; Khelifi, M.; Djebbar, R.; Abrous-Belbachir, O.; Carol, P. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol. Plant. 2020, 42, 21. [Google Scholar] [CrossRef]
- Liang, Y.; Li, D.; Chen, Y.; Cheng, J.; Zhao, G.; Fahima, T.; Yan, J. Selenium mitigates salt-induced oxidative stress in durum wheat (Triticum durum Desf.) seedlings by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. 3 Biotech 2020, 10, 368. [Google Scholar] [CrossRef]
- Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-Induced Activation of the Antioxidant Defense System Counteracts the Adverse Effects of Salinity in Durum Wheat. Plants 2021, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laus, M.N.; De Santis, M.A.; Flagella, Z.; Soccio, M. Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview. Plants 2022, 11, 98. https://doi.org/10.3390/plants11010098
Laus MN, De Santis MA, Flagella Z, Soccio M. Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview. Plants. 2022; 11(1):98. https://doi.org/10.3390/plants11010098
Chicago/Turabian StyleLaus, Maura Nicoletta, Michele Andrea De Santis, Zina Flagella, and Mario Soccio. 2022. "Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview" Plants 11, no. 1: 98. https://doi.org/10.3390/plants11010098
APA StyleLaus, M. N., De Santis, M. A., Flagella, Z., & Soccio, M. (2022). Changes in Antioxidant Defence System in Durum Wheat under Hyperosmotic Stress: A Concise Overview. Plants, 11(1), 98. https://doi.org/10.3390/plants11010098








