Genetic Features of Triticale–Wheat Hybrids with Vaviloid-Type Spike Branching
Abstract
1. Introduction
2. Results
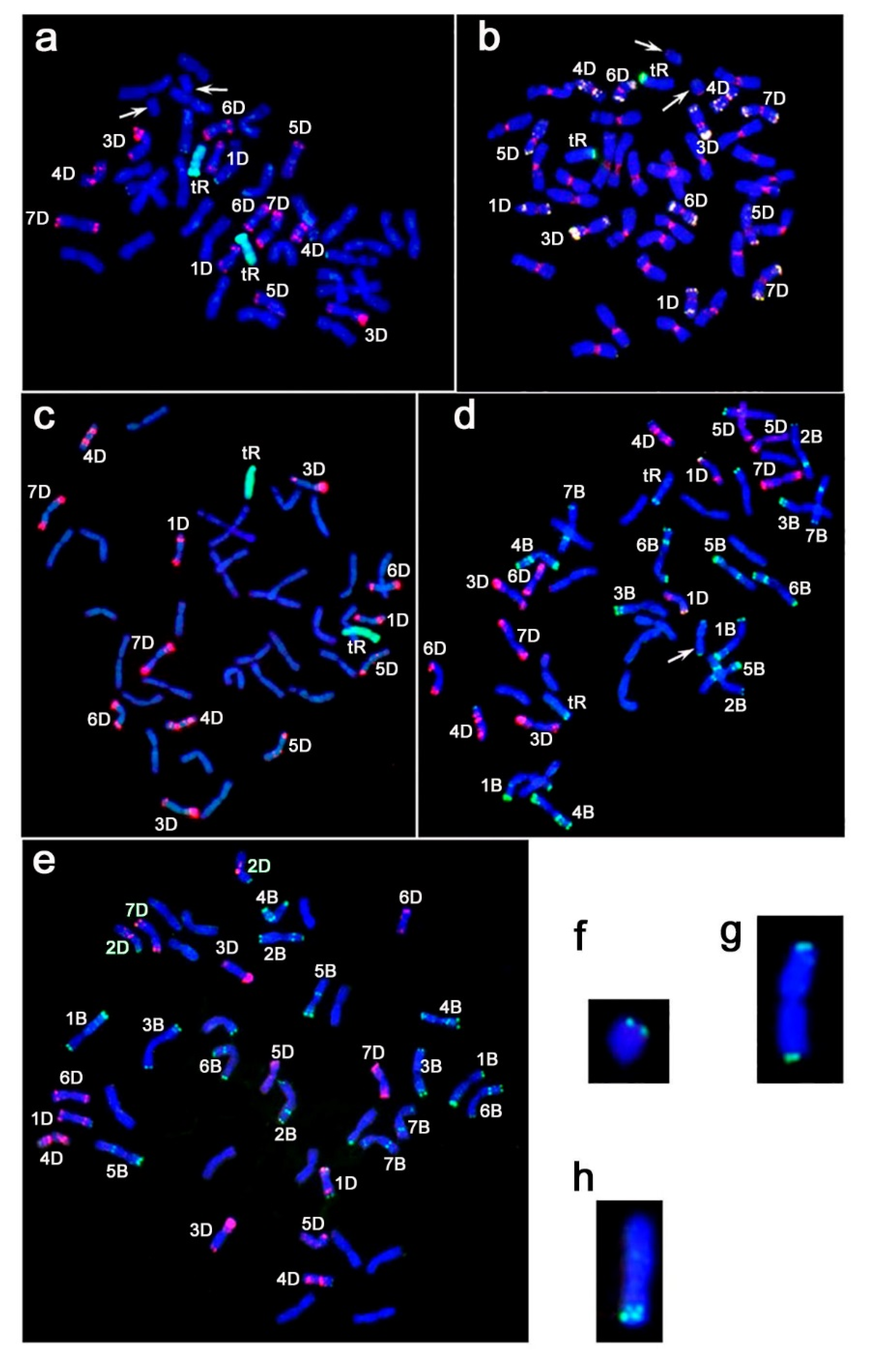
2.1. Genome Structure of Triticale–Wheat Hybrids
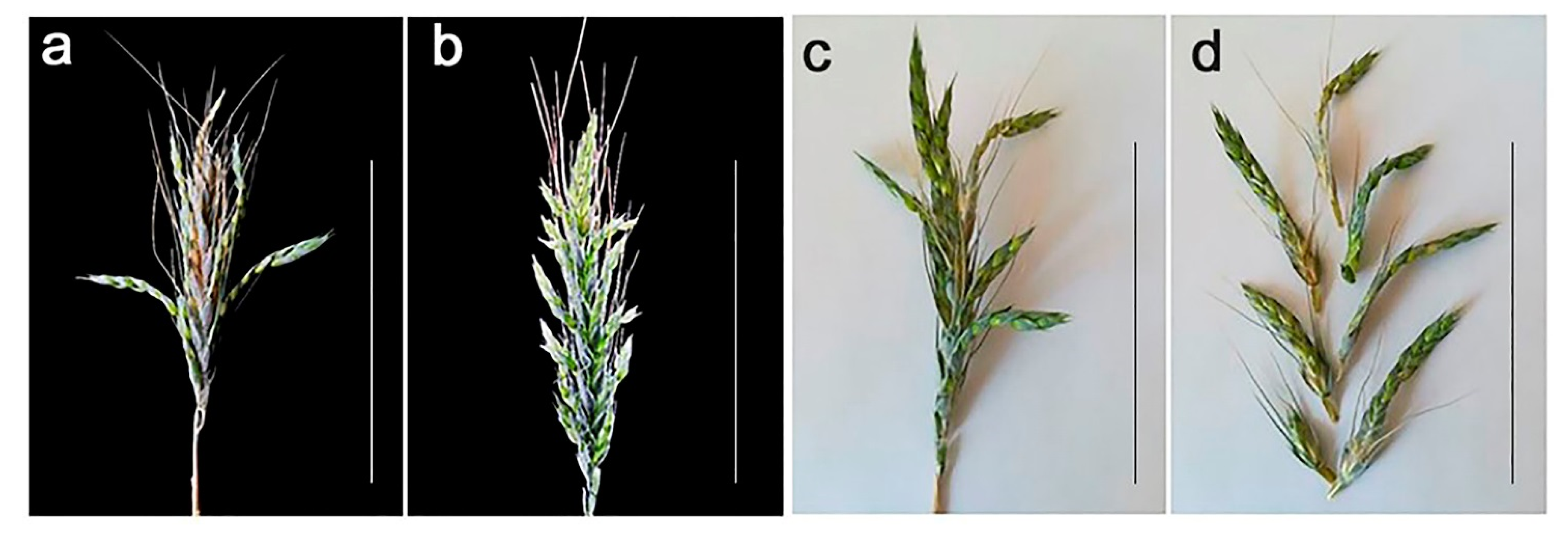
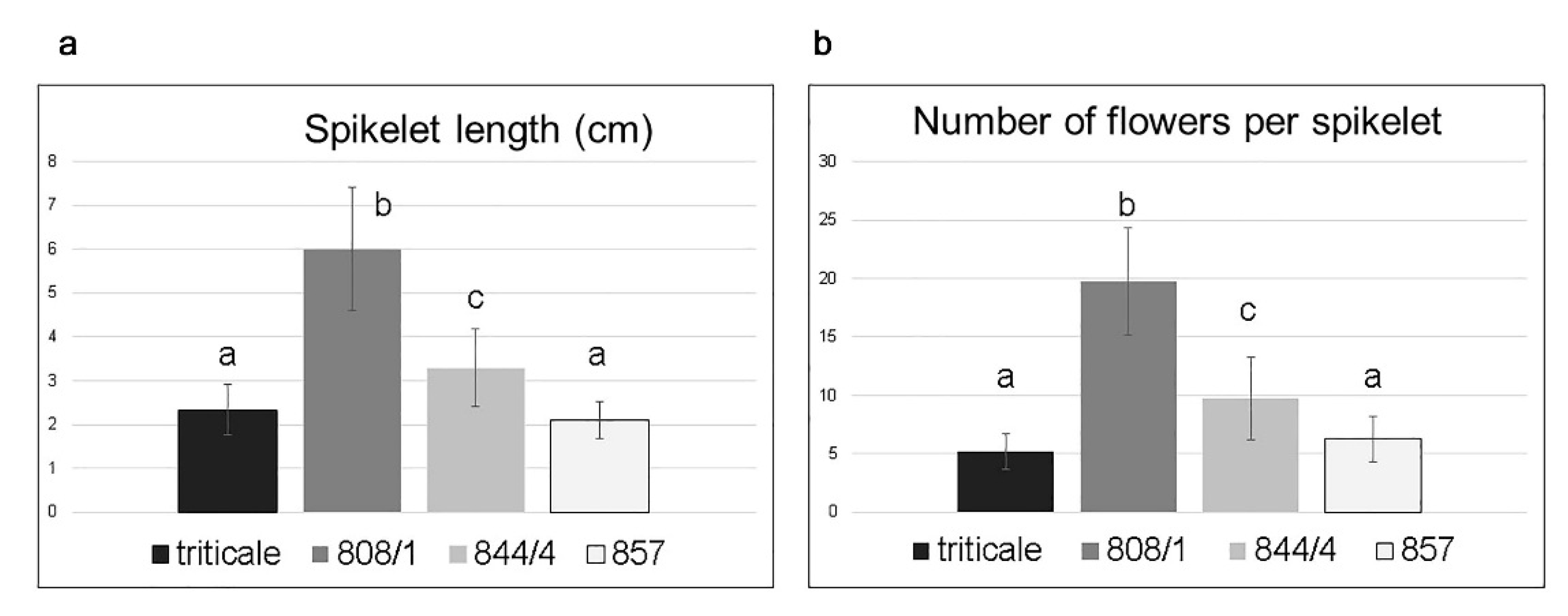
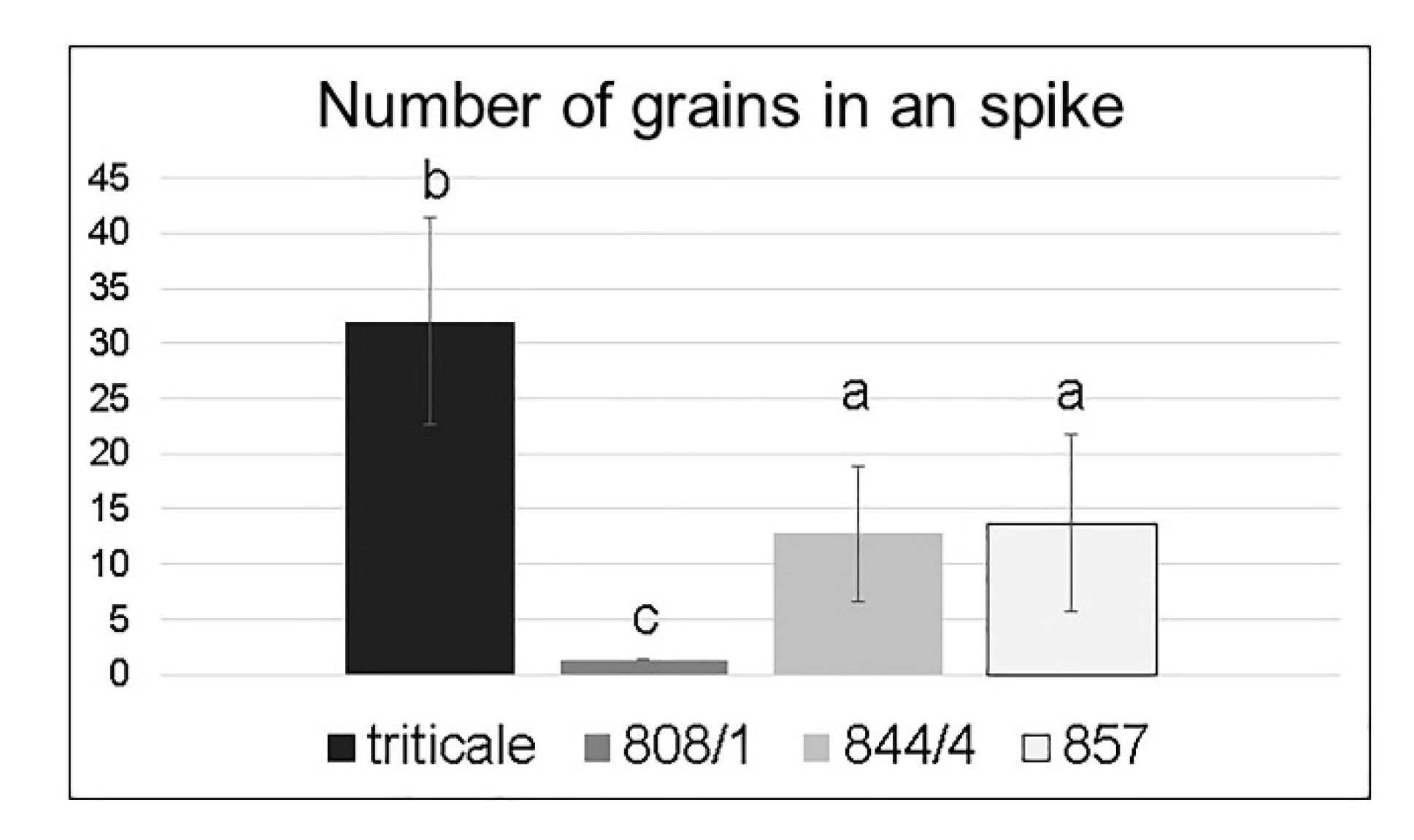
2.2. Analysis of Spike Morphology in Different Karyotypes
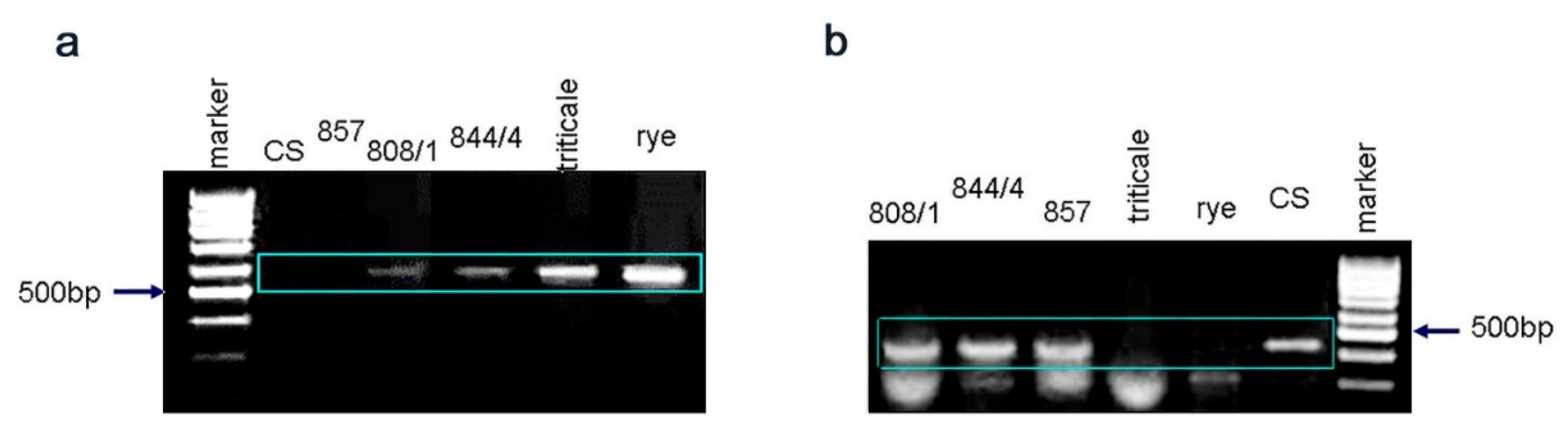
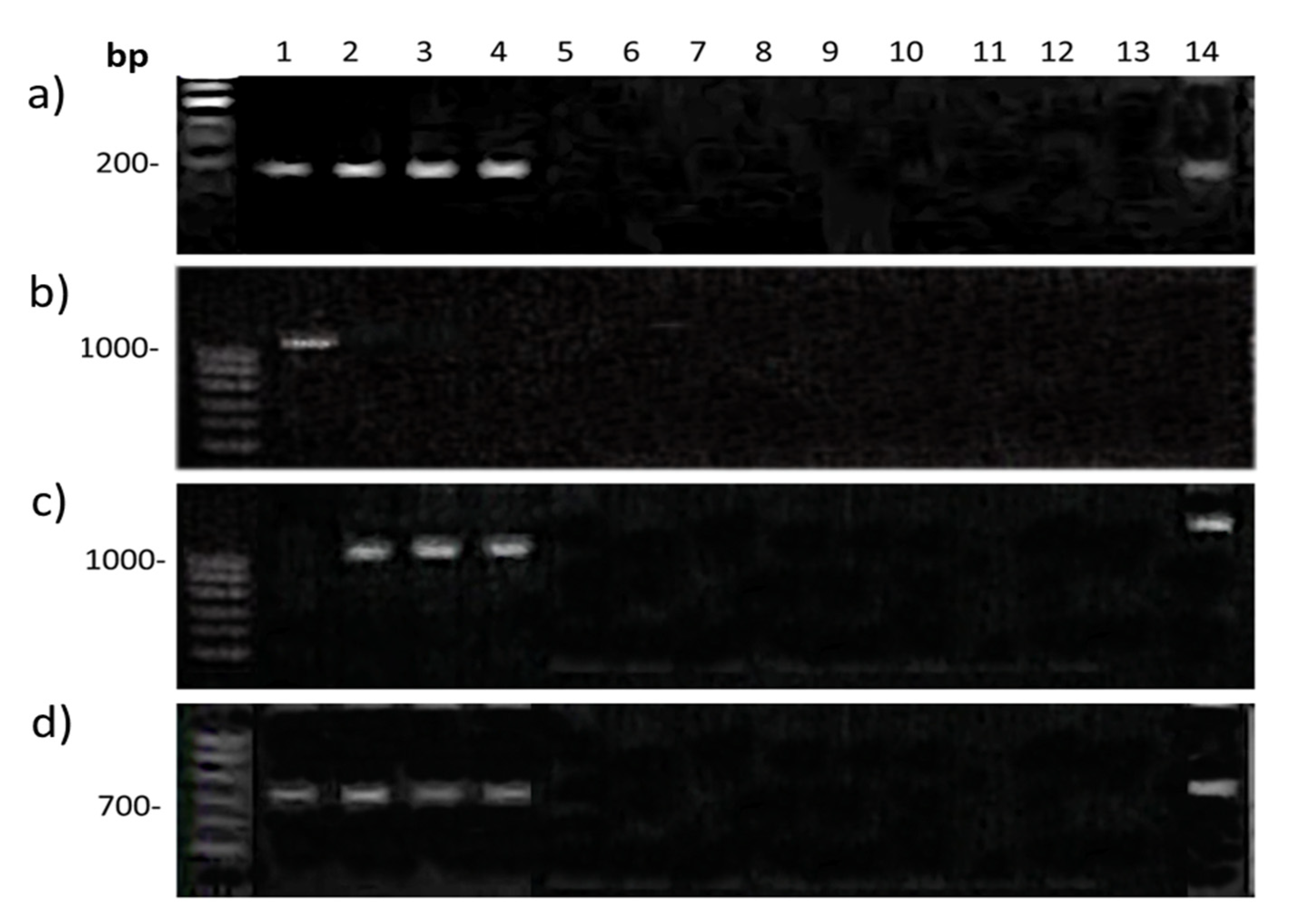
2.3. Gene Q Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Phenotypic Analysis
4.3. Cytogenetic Analysis
4.4. DNA Isolation and Molecular Genetic Analysis
4.5. Gene Q Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobrovolskaya, O.; Pont, C.; Sibout, R.; Martinek, P.; Badaeva, E.; Murat, F.; Chosson, A.; Watanabe, N.; Prat, E.; Gautier, N.; et al. FRIZZY PANICLE drives supernumerary spikelets in bread wheat (T. aestivum L.). Plant Physiol. 2015, 167, 189–199. [Google Scholar] [CrossRef]
- Poursarebani, N.; Seidensticker, T.; Koppolu, R.; Trautewig, C.; Gawronski, P.; Bini, F.; Govind, G.; Rutten, T.; Sakuma, S.; Tagiri, A.; et al. The genetic basis of composite spike form in barley and ‘Miracle-Wheat’. Genetics 2015, 201, 155–165. [Google Scholar] [CrossRef]
- Tanaka, W.; Pautler, M.; Jackson, D.; Hirano, H.Y. Grass meristems II: Inflorescence architecture, flower development and meristem fate. Plant Cell Physiol. 2013, 54, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Zhang, D.; Yuan, J.; Feng, M.; Li, Z.; Wang, Z.; Zhang, Z.; Li, X.; Ke, W.; Li, R.; et al. FRIZZY PANICLE defines a regulatory hub for simultaneously controlling spikelet formation and awn elongation in bread wheat. New Phytologist. 2021, 231, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Wolde, G.M.; Schreiber, M.; Trautewig, C.; Himmelbach, A.; Sakuma, S.; Mascher, M.; Schnurbusch, T. Genome-wide identification of loci modifying spike-branching in tetraploid wheat. Theor. Appl. Genet. 2021, 134, 1925–1943. [Google Scholar] [CrossRef] [PubMed]
- Martinek, P. Gene resources with non-standard spike morphology in wheat. In Proceedings of the 9th International Wheat Genetics Symposium, Saskatoon, SK, Canada, 2–7 August 1998; Volume 2, pp. 286–288. [Google Scholar]
- Dorofeev, V.F.; Filatenko, A.A.; Migushova, E.F.; Udachin, R.A.; Jakubtsiner, M.M. Pshenitsa (Wheat). In Kul’turnaya Flora SSSR (Flora of Cultivated Plants of the USSR); Kolos: Leningrad, Russia, 1979; Volume 1, pp. 7–31. [Google Scholar]
- Amagai, Y.; Aliyeva, A.J.; Aminov, N.K.; Martinek, P.; Watanabe, N.; Kuboyama, T. Microsatellite mapping of the genes for sham ramification and extra glume in spikelets of tetraploid wheat. Genet. Resour. Crop Evol. 2014, 61, 491–498. [Google Scholar] [CrossRef]
- Aliyeva, A.J.; Aminov, N.K. Inheritance of the branching in hybrid populations among tetraploid wheat species and the new branched spike line 166-Schakheli. Genet. Resour. Crop Evol. 2011, 58, 621–628. [Google Scholar] [CrossRef]
- Aliyeva, A.J.; Aminov, N.K. Influence of D genome of wheat on expression of novel type spike branching in hybrid populations of 171ACS line. Russ. J. Genet. 2013, 49, 1119–1126. [Google Scholar] [CrossRef]
- Faris, J.D.; Simons, K.J.; Zhang, Z.; Gill, B.S. The wheat super domestication gene Q. Wheat Inf. Serv. 2005, 100, 129–148. [Google Scholar]
- Simons, K.J.; Fellers, J.P.; Trick, H.N.; Zhang, Z.; Tai, Y.S.; Gill, B.S.; Faris, J.D. Molecular characterization of the major wheat domestication gene. Q. Genet. 2006, 172, 547–555. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Lin, H.; Chuck, G.; Faris, J.D.; Dubcovsky, J. microRNA172 plays a crucial role in wheat spike morphogenesis and grain threshability. Development 2017, 144, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, J.R.; Finnegan, E.J.; Watanabe, N.; Trevaskis, B.; Swain, S.M. New alleles of the wheat domestication gene Q reveal multiple roles in growth and reproductive development. Development 2017, 144, 1959–1965. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Greenwood, J.R.; Finnegan, E.J.; Jernstedt, J.; Dubcovsky, J. APETALA 2-like genes AP2L2 and Q specify lemma identity and axillary floral meristem development in wheat. Plant J. 2020, 101, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Adonina, I.G.; Mehdiyeva, S.P.; Prokopjeva, M.V.; Aminov, N.K.; Salina, E.A. Karyotyping of triticale-wheat hybrid lines with the sham ramification of spike. Curr. Chall. Plant Genet. Genom. Bioinform. Biotechnol. 2019, 244–246. [Google Scholar] [CrossRef]
- Bedbrook, J.R.; Jones, J.; O’Dell, M.; Thompson, R.D.; Flavell, R.B. A molecular description of telomeric heterochromatin in Secale species. Cell 1980, 19, 545–560. [Google Scholar] [CrossRef]
- Rayburn, A.L.; Gill, B.S. Isolation of a D-genome specific repeated DNA sequence from Aegilops squarrosa. Plant Mol. Biol. Rep. 1986, 4, 102–109. [Google Scholar] [CrossRef]
- Francki, M.G. Identification of Bilby, a diverged centromeric Ty1-copia retrotransposon family from cereal rye (Secale cereale L.). Genome 2001, 44, 266–274. [Google Scholar] [CrossRef]
- Zhang, P.; Wanlong, L.; Fellers, J.; Friebe, B.; Gill, B.S. BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 2004, 112, 288–299. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Tereshchenko, O.Y.; Salina, E.A. Anthocyanin biosynthesis genes location and expression in wheat–rye hybrids. Mol. Genet. Genom. 2009, 282, 475–485. [Google Scholar] [CrossRef]
- Beales, J.; Turner, A.; GriYths, S.; ·Snape, J.W.; Laurie, D.A. A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef]
- Zhang, Z.; Belcram, H.; Gornicki, P.; Charles, M.; Just, J.; Huneau, C.; Magdelenat, G.; Couloux, A.; Samain, S.; Gill, B.S.; et al. Duplication and partitioning in evolution and function of homoeologous Q loci governing domestication characters in polyploid wheat. Proc. Natl. Acad. Sci. USA 2011, 108, 18737–18742. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, K.; Jin, D.; Sun, L.; Chu, J.; Wu, W.; Xin, P.; Li, X.; Sun, J.; Yang, W.; et al. ALI-1, candidate gene of B1 locus, is associated with awn length and grain weight. bioRxiv 2019. [Google Scholar] [CrossRef]
- Shingh, H.B.; Anderson, E.; Pal, B.P. Studies in the genetics of Triticum vavilovii Jakub. Agron. J. 1957, 49, 4–11. [Google Scholar] [CrossRef]
- Prabhakara-Rao, M.V.; Swaminathan, M.S. Vavilovoid mutant in Triticum aestivum and the origin of T. vavilovii. Curr. Sci. 1963, 32, 132–133. [Google Scholar]
- Kosuge, K.; Watanabe, N.; Kuboyama, T.; Melnik, V.M.; Yanchenko, V.I.; Rosova, M.A.; Goncharov, N.P. Cytological and microsatellite mapping of mutant genes for spherical grain and compact spikes in durum wheat. Euphytica 2008, 159, 289–296. [Google Scholar] [CrossRef]
- Bommert, P.; Whipple, C. Grass inflorescence architecture and meristem determinacy. Semin. Cell Dev. Biol. 2018, 79, 37–47. [Google Scholar] [CrossRef]
- Vavilova, V.; Konopatskaia, I.; Blinov, A.; Kondratenko, E.Y.; Kruchinina, Y.V.; Goncharov, N.P. Genetic variability of spelt factor gene in Triticum and Aegilops species. BMC Plant Biol. 2020, 20 (Suppl. S1), 310. [Google Scholar] [CrossRef]
- Ning, S.Z.; Wang, N.; Sakuma, S.; Pourkheirandish, M.; Koba, T.; Komatsuda, T. Variation in the wheat AP2 homoeologs, the genes underlying lodicule development. Breed. Sci. 2013, 63, 255–266. [Google Scholar] [CrossRef][Green Version]
- Salina, E.A.; Lim, Y.K.; Badaeva, E.D.; Shcherban, A.B.; Adonina, I.G.; Amosova, A.V.; Samatadze, T.E.; Vatolina, T.Y.; Zoshchuk, S.A.; Leitch, A.R. Phylogenetic reconstruction of Aegilops section Sitopsis and the evolution of tandem repeats in the diploids and derived wheat polyploids. Genome 2006, 49, 1023–1035. [Google Scholar] [CrossRef]
- Schubert, I.; Shi, F.; Fuchs, J.; Endo, T.R. An efficient screening for terminal deletions and translocations of barley chromosomes added to common wheat. Plant J. 1998, 14, 489–495. [Google Scholar] [CrossRef]
- Plaschke, J.; Ganal, M.W.; Roeder, M.S. Detection of genetic diversity in closely related bread wheat using microsatellite markers. Theor. Appl. Genet. 1995, 91, 1001–1007. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequence 5′–3′ | Gene | Chromosomal Localization | Expected PCR Product Size (bp) |
|---|---|---|---|---|
| F3h–F | CTGGGCGTGCTATCGGAGGT | Rye flavanone 3-hydroxylase (F3h-R1) | 2RL | 580 |
| F3h–R | TTGGCGAGGTCGAGGTCGCGCTT | |||
| Ppd_F | ACGCCTCCCACTACACTG | Photoperiod response (Ppd-D1) | 2DS | 414, 288 |
| Ppd_R1 | GTTGGTTCAAACAGAGAGC | |||
| Ppd_R2 | CACTG-GTGGT-AGCTG-AGATT | |||
| Qex10F | AGGCAAGGCCCCCTGAGCA | Q * exon 10 | 5AL | 175 |
| Qex10R | CGGTGGTGGTCCGGGTACGG | |||
| QTaF | CCCTGAATCGTCAACCACAATGA | exon 8–10 (Q allele) | 5AL | 1059 |
| QTaR | CGCCGGCGGCGGCGGTAGAA | |||
| QTsF | CCCTGAATCGTCAACCACAATGG | exon 8–10 (q allele) | 5AL | 1059 |
| QTsR | CGCCGGCGGCGGCGGTAGAG | |||
| QpromF | TGATGTACGCTCCGTGTGA | promoter region | 5AL | 709 |
| QpromR | TGGAGGACGACGAGGAGAG | |||
| B1for | ATAAACTCCCACATAATTACTTCG | B1 * promoter region | 5AL | 1177 |
| b1for | AAACTCCCACATAATTACTCCC | |||
| Znfrev | CTCTTCCATCTCCATGCCCA |
| Lines | Karyotype | Phenotype | |
|---|---|---|---|
| Spikelet Length, cm | Number of Florets per Spikelet | ||
| Mean (Range) of | Mean (Range) of | ||
| 808/1 | 14A + 14B + 12D+2(2DS) + 2(2RL) = 44 | 6.0 (2.6–8.3) b | 19.7 (7–27) b |
| 844/4 | 14A + 14B + 12D + 2(2DS) (centric fusion) + 2(2RL) = 43 | 3.3 (1.0–5.4) c | 9.7 (3–19) c |
| 857 | 14A + 14B + 14D = 42 | 2.1 (1.0–3.2) a | 6.2 (2–11) a |
| triticale | 14A + 14B + 14R = 42 | 2.3 (0.9–3.8) a | 5.2 (2–8) a |
| T. aestivum [7] | 14A + 14B + 14D = 42 | 0.9–1.7 | 2–5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adonina, I.G.; Shcherban, A.B.; Zorina, M.V.; Mehdiyeva, S.P.; Timonova, E.M.; Salina, E.A. Genetic Features of Triticale–Wheat Hybrids with Vaviloid-Type Spike Branching. Plants 2022, 11, 58. https://doi.org/10.3390/plants11010058
Adonina IG, Shcherban AB, Zorina MV, Mehdiyeva SP, Timonova EM, Salina EA. Genetic Features of Triticale–Wheat Hybrids with Vaviloid-Type Spike Branching. Plants. 2022; 11(1):58. https://doi.org/10.3390/plants11010058
Chicago/Turabian StyleAdonina, Irina G., Andrey B. Shcherban, Maremyana V. Zorina, Sabina P. Mehdiyeva, Ekaterina M. Timonova, and Elena A. Salina. 2022. "Genetic Features of Triticale–Wheat Hybrids with Vaviloid-Type Spike Branching" Plants 11, no. 1: 58. https://doi.org/10.3390/plants11010058
APA StyleAdonina, I. G., Shcherban, A. B., Zorina, M. V., Mehdiyeva, S. P., Timonova, E. M., & Salina, E. A. (2022). Genetic Features of Triticale–Wheat Hybrids with Vaviloid-Type Spike Branching. Plants, 11(1), 58. https://doi.org/10.3390/plants11010058






