Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea
Abstract
:1. Introduction
2. Results
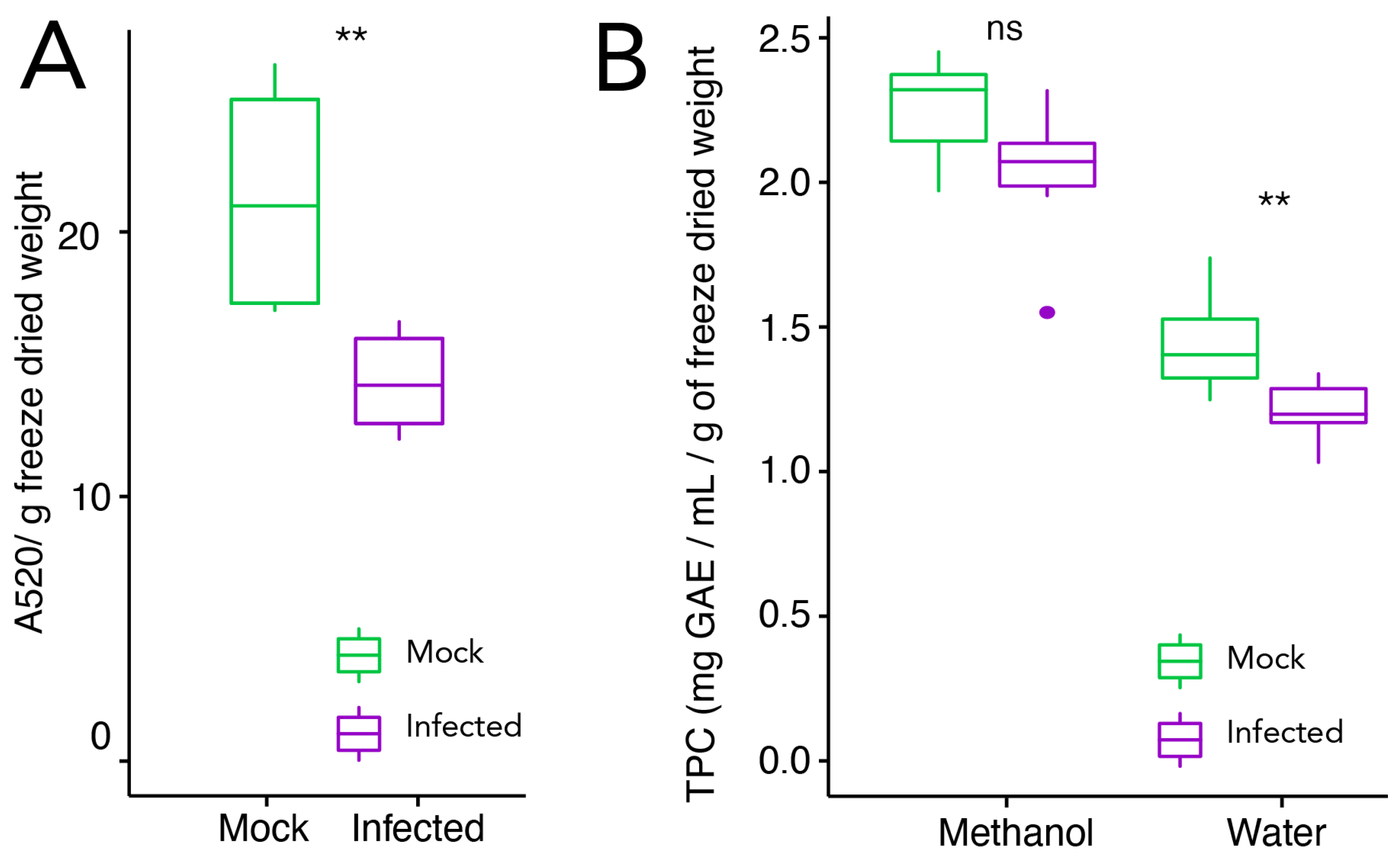
2.1. Phenotypic Characterization, Total Phenolic Content, and Anthocyanin Quantification in Infected and Mock-Treated Grape Berries
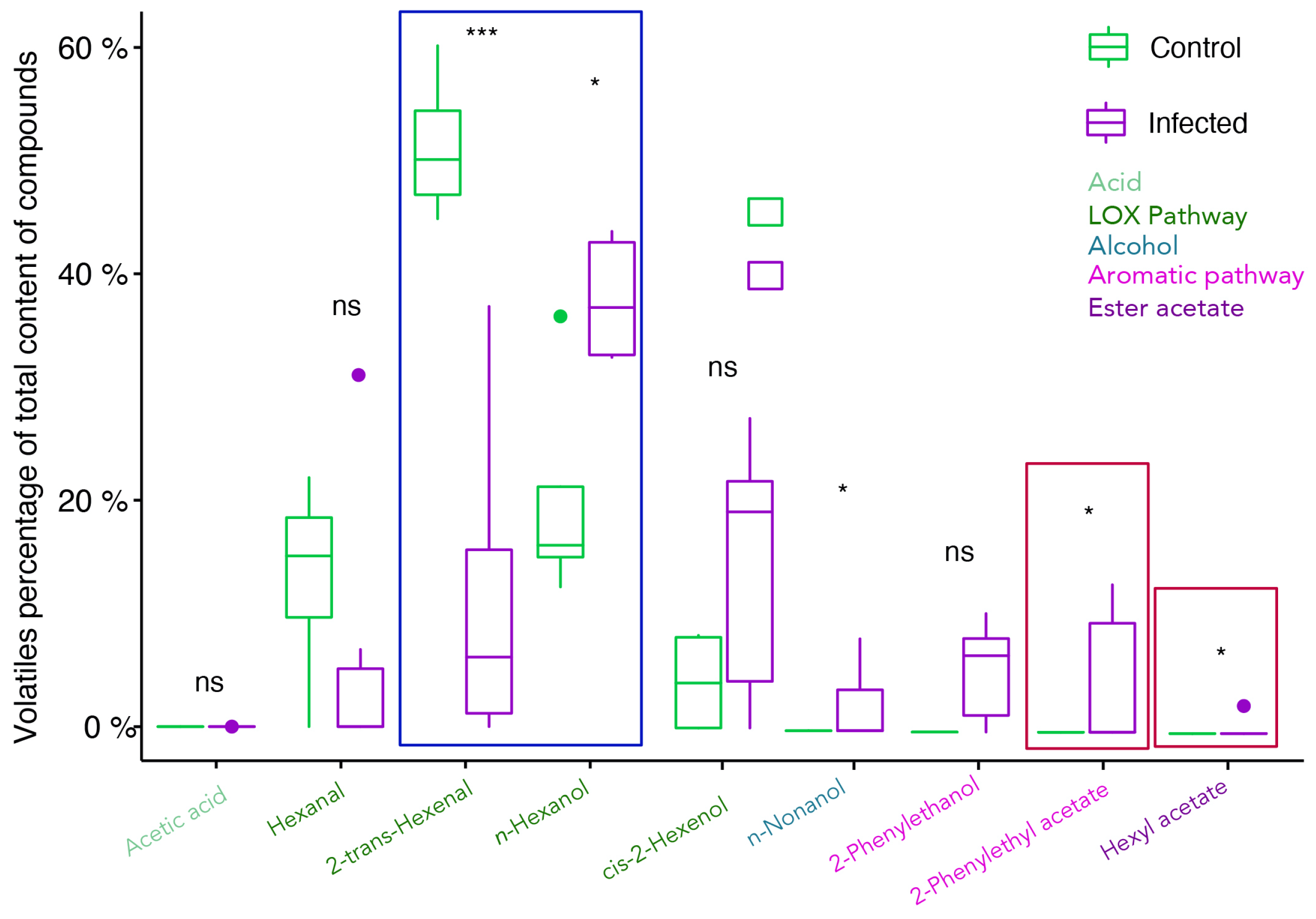
2.2. Profiling of Volatile Organic Compounds in Healthy and Infected Berries
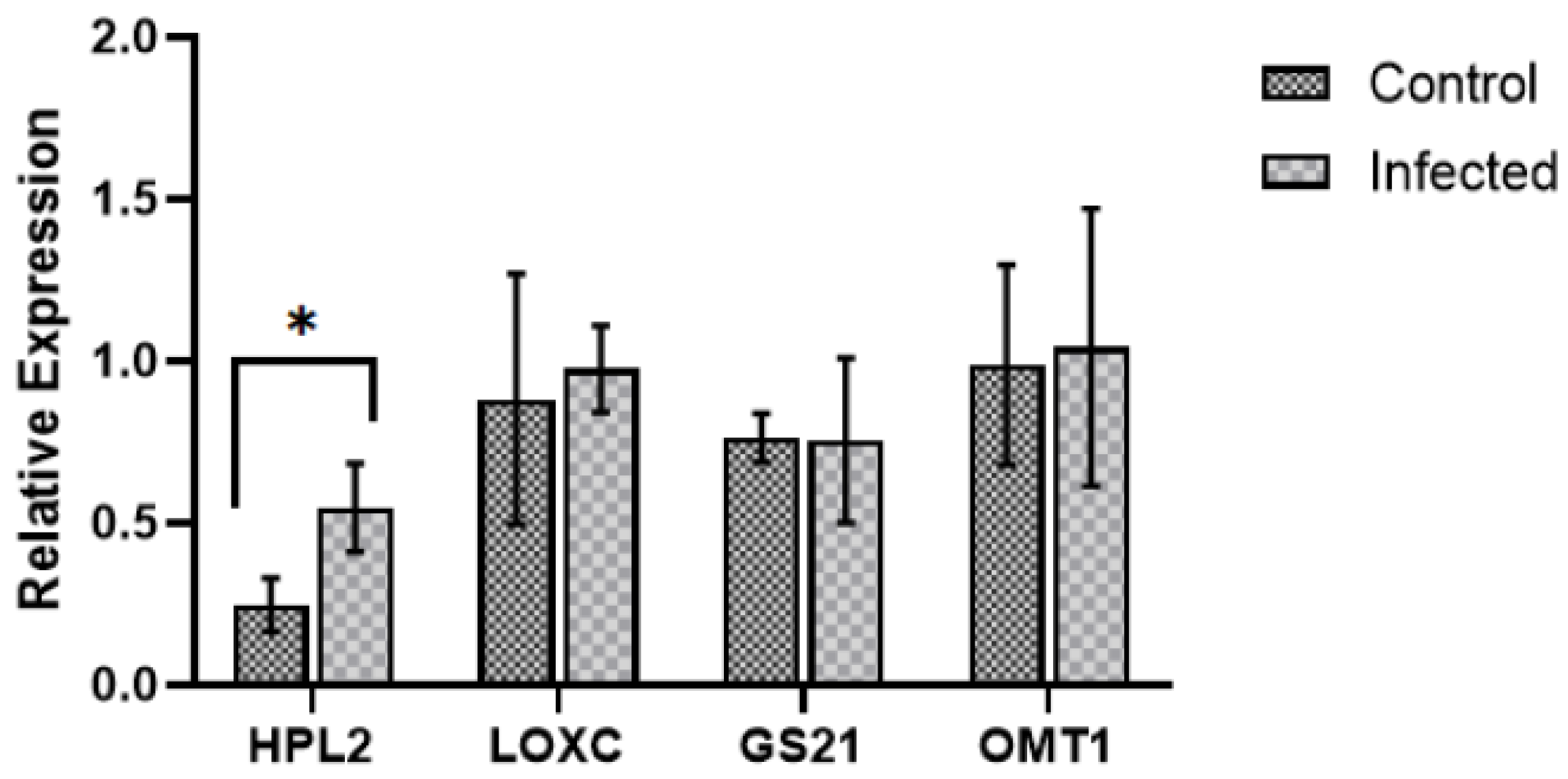
2.3. Expression of Genes Involved in Volatile Compound Metabolism
3. Discussion
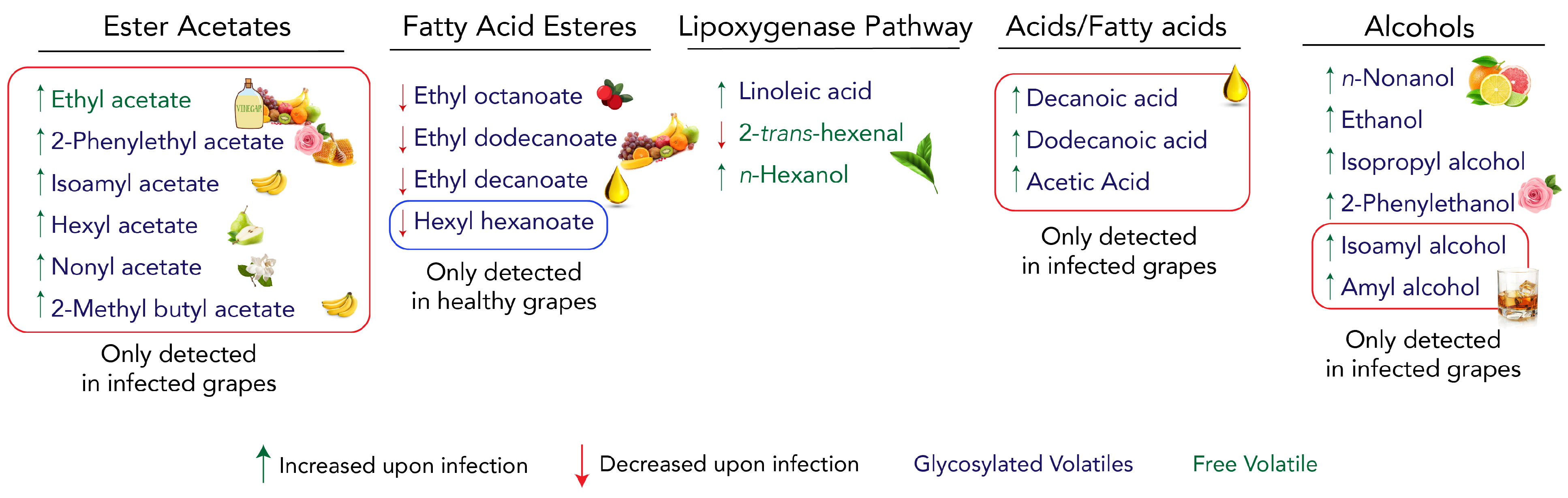
3.1. The C6-Volatile Compounds Are Altered in Both Free and Glycosidic Fractions
3.2. Alcohols Increase upon Infection, Contributing to Specific Grape Aroma
3.3. Ester Acetates Are Only Present in Infected Samples and Fatty Acid Esters Decrease under Infection
3.4. Volatile Organic Compounds Blend for a Global Aroma
4. Materials and Methods
4.1. Fungal Infection of Berries, Sample Collection, and Processing
4.2. Isolation of Free and Glycosidic Bound Volatile Organic Compounds
4.3. Headspace HP–SPME Chemical Analysis
4.4. Determination of Total Phenolic Content
4.5. Determination of Total Anthocyanin Content
4.6. RNA Extraction and Purification for Transcriptional Profiling
4.7. Quantitative RT-PCR for Genes Involved in Volatile Metabolism
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B. Handbook of Enology, 2nd ed.; John Wiley: Chichester, UK; Hoboken, NJ, USA, 2006; pp. 205–206. [Google Scholar]
- Agudelo-Romero, P.; Erban, A.; Rego, C.; Carbonell-Bejerano, P.; Nascimento, T.; Sousa, L.; Martínez-Zapater, J.M.; Kopka, J.; Fortes, A.M. Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. J. Exp. Bot. 2015, 66, 1769–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortes, A.M.; Pais, M.S. Chapter 12—Grape (Vitis species). In Nutritional Composition of Fruit Cultivars; Simmonds, M.S., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 257–286. [Google Scholar]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlevy, J.; Kalua, C.; Keyzers, R.; Boss, P. The production of Flavour & aroma compounds in grape berries. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 293–340. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Skouroumounis, G.K.; Sefton, M.A. The Formation of β-Damascenone in Wine. In Carotenoid-Derived Aroma Compounds; Winterhalter, P., Rouseff, R.L., Eds.; American Chemical Society: Washington, DC, USA, 2001; Volume 802, pp. 241–254. [Google Scholar]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-analysis of the core aroma components of grape and wine aroma. Front. Plant Sci. 2016, 7, 1472. [Google Scholar] [CrossRef] [Green Version]
- Agudelo-Romero, P.; Erban, A.; Sousa, L.; Pais, M.S.; Kopka, J.; Fortes, A.M. Search for transcriptional and metabolic markers of grape pre-ripening and ripening and insights into specific aroma development in three Portuguese cultivars. PLoS ONE 2013, 8, e60422. [Google Scholar] [CrossRef] [Green Version]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Bate, N.J.; Rothstein, S.J. C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J. 1998, 16, 561–569. [Google Scholar] [CrossRef]
- Angerosa, F.; Basti, C. Olive oil volatile compounds from the lipoxygenase pathway in relation to fruit ripeness. Ital. J. Food Sci. 2001, 13, 421–428. [Google Scholar]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Kambiranda, D.; Basha, S.M.; Singh, R.K.; He, H.; Calvin, K.; Mercer, R. In depth proteome analysis of ripening muscadine grape berry cv. Carlos reveals proteins associated with flavor and aroma compounds. J. Proteome Res. 2016, 15, 2910–2923. [Google Scholar] [CrossRef] [PubMed]
- Lopez Pinar, A.; Rauhut, D.; Ruehl, E.; Buettner, A. Effects of Botrytis cinerea and Erysiphe necator fungi on the aroma character of grape must: A comparative approach. Food Chem. 2016, 207, 251–260. [Google Scholar] [CrossRef] [PubMed]
- van Kan, J.A.L. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.M.C.; Miebach, M.; Kleist, E.; van Henten, E.J.; Wildt, J. Release of lipoxygenase products and monoterpenes by tomato plants as an indicator of Botrytis cinerea—Induced stress. Plant Biol. 2009, 11, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Brás, E.J.S.; Fortes, A.M.; Esteves, T.; Chu, V.; Fernandes, P.; Conde, J.P. Microfluidic device for multiplexed detection of fungal infection biomarkers in grape cultivars. Analyst 2020, 145, 7973–7984. [Google Scholar] [CrossRef] [PubMed]
- Laothawornkitkul, J.; Jansen, R.M.C.; Smid, H.M.; Bouwmeester, H.J.; Muller, J.; van Bruggen, A.H.C. Volatile organic compounds as a diagnostic marker of late blight infected potato plants: A pilot study. Crop Prot. 2010, 29, 872–878. [Google Scholar] [CrossRef]
- Fang, Y.; Ramasamy, R.P. Detection of p-ethylphenol, a major plant volatile organic compound, by tyrosinase–based electrochemical biosensor. ECS J. Solid State Sci. Technol. 2016, 5, M3054–M3059. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Keulemans, J.; Geeraerd, A.; Nicolai, B.M.; Hertog, M.L.A.T.M. Evaluation of fast volatile analysis for detection of Botrytis cinerea infections in strawberry. Food Microbiol. 2012, 32, 406–414. [Google Scholar] [CrossRef]
- Aprea, E.; Carlin, S.; Giongo, L.; Grisenti, M.; Gasperi, F. Characterization of 14 raspberry cultivars by solid-phase microextraction and relationship with gray mold susceptibility. J. Agric. Food Chem. 2010, 58, 1100–1105. [Google Scholar] [CrossRef]
- Niederbacher, B.; Winkler, J.; Schnitzler, J. Volatile organic compounds as non-invasive markers for plant phenotyping. J. Exp. Bot. 2015, 66, 5403–5416. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Schwab, W.; Wüst, M. Understanding the constitutive and induced biosynthesis of mono- and sesquiterpenes in grapes (Vitis vinifera): A key to unlocking the biochemical secrets of unique grape aroma profiles. J. Agric. Food Chem. 2015, 63, 10591–10603. [Google Scholar] [CrossRef]
- Coombe, B. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Butelli, E.; De Stefano, R.; Schoonbeek, H.J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Ky, I.; Lorrain, B.; Jourdes, M.; Pasquier, G.; Fermaud, M.; Gény, L.; Rey, P.; Doneche, B.; Teissedre, P.L. Assessment of grey mould (Botrytis cinerea) impact on phenolic and sensory quality of Bordeaux grapes, musts and wines for two consecutive vintages. Aust. J. Grape Wine Res. 2012, 18, 215–226. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defence against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [Green Version]
- Lopez Pinar, A.; Rauhut, D.; Ruehl, E.; Buettner, A. Effects of bunch rot (Botrytis cinerea) and powdery mildew (Erysiphe necator) fungal diseases on wine aroma. Front. Chem. 2017, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.E.; Loeb, G.M.; Cadle-Davidson, L.; Evans, K.J.; Wilcox, W.F. Grape sour rot: A four-way interaction involving the host, yeast, acetic acid bacteria, and insects. Phytopathology 2018, 108, 1429–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortina, M.G.; Acquati, A.; Rossi, P.; Manachini, P.L.; Gennaro, C. Production of laccase by Botrytis cinerea and fermentation studies with strain F226. J. Ind. Microbiol. 1996, 17, 69–72. [Google Scholar] [CrossRef]
- Rambla, J.L.; Trapero-Mozos, A.; Diretto, G.; Rubio-Moraga, A.; Granell, A.; Gómez-Gómez, L.; Ahrazem, O. Gene-metabolite networks of volatile metabolism in Airen and Tempranillo grape cultivars revealed a distinct mechanism of aroma bouquet production. Front. Plant Sci. 2016, 7, 1619. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [Green Version]
- Dankó, T.; Szelényi, M.; Janda, T.; Molnár, B.P.; Pogány, M. Distinct volatile signatures of bunch rot and noble rot. Physiol. Mol. Plant Pathol. 2021, 114, 101626. [Google Scholar] [CrossRef]
- Vincenti, S.; Mariani, M.; Alberti, J.C.; Jacopini, S.; Brunini-Bronzini de Caraffa, V.; Berti, L.; Maury, J. Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 2019, 9, 873. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Ulate, B.; Vincenti, E.; Cantu, D.; Powell, A.L.T. Ripening of tomato fruit and susceptibility to Botrytis cinerea. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 387–412. [Google Scholar]
- Shiojiri, K.; Kishimoto, K.; Ozawa, R.; Kugimiya, S.; Urashimo, S.; Arimura, G.; Horiuchi, J.; Nishioka, T.; Matsui, K.; Takabayashi, J. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. USA 2006, 103, 16672–16676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry 2008, 69, 2127–2132. [Google Scholar] [CrossRef]
- Schueuermann, C.; Steel, C.C.; Blackman, J.W.; Clark, A.C.; Schwarz, L.J.; Moraga, J.; Collado, I.G.; Schmidtke, L.M. A GC-MS untargeted metabolomics approach for the classification of chemical differences in grape juices based on fungal pathogen. Food Chem. 2019, 270, 375–384. [Google Scholar] [CrossRef]
- Tosi, E.; Azzolini, M.; Lorenzini, M.; Torriani, S.; Fedrizzi, B.; Finato, F.; Cipriani, M.; Zapparoli, G. Induction of grape botrytization during withering affects volatile composition of Recioto di Soave, a “passito”-style wine. Eur. Food Res. Technol. 2013, 236, 853–862. [Google Scholar] [CrossRef]
- Bassolino, L.; Zhang, Y.; Schoonbeek, H.J.; Kiferle, C.; Perata, P.; Martin, C. Accumulation of anthocyanins in tomato skin extends shelf life. New Phytol. 2013, 200, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Steel, C.C.; Blackman, J.W.; Schmidtke, L.M. Grapevine bunch rots: Impacts on wine composition, quality, and potential procedures for the removal of wine faults. J. Agric. Food Chem. 2013, 61, 5189–5206. [Google Scholar] [CrossRef]
- Wakai, J.; Kusama, S.; Nakajima, K.; Kawai, S.; Okumura, Y.; Shiojiri, K. Effects of trans-2-hexenal and cis-3-hexenal on post-harvest strawberry. Sci. Rep. 2019, 9, 10112. [Google Scholar] [CrossRef] [Green Version]
- Fallik, E.; Archbold, D.D.; Hamilton-Kemp, T.R.; Clements, A.M.; Collins, R.W.; Barth, M.M. (E)-2-Hexenal can stimulate Botrytis cinerea growth in vitro and on strawberries in vivo during storage. J. Am. Soc. Hortic. Sci. 1998, 123, 875–881. [Google Scholar] [CrossRef] [Green Version]
- Coelho, J.; Almeida-Trapp, M.; Pimentel, D.; Soares, F.M.; Reis, P.; Rego, C.; Mithofer, A.; Fortes, A.M. The study of hormonal metabolism of Trincadeira and Syrah cultivars indicates new roles of salicylic acid, jasmonates, ABA and IAA during grape ripening and upon infection with Botrytis cinerea. Plant Sci. 2019, 283, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Xu, X.Q.; Wu, Y.W.; Duan, C.Q.; Pan, Q.H. Isolation and characterization of two hydroperoxide lyase genes from grape berries: HPL isogenes in Vitis vinifera grapes. Mol. Biol. Rep. 2012, 39, 7443–7455. [Google Scholar] [CrossRef] [PubMed]
- Yalage Don, S.M.; Schmidtke, L.M.; Gambetta, J.M.; Steel, C.C. Aureobasidium pullulans volatilome identified by a novel, quantitative approach employing SPME-GC-MS, suppressed Botrytis cinerea and Alternaria alternata in vitro. Sci. Rep. 2020, 10, 4498. [Google Scholar] [CrossRef] [Green Version]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar]
- Maturano, Y.; Nally, M.; Assof, M.; Toro, M.; Castellanos de Figueroa, L.; Jofré, V.; Vazquez, F. Free volatile compounds of cv. Pedro Giménez (Vitis vinifera L.) white grape must grown in San Juan, Argentina. S. Afr. J. Enol. Vitic. 2018, 39, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.D.; Welke, J.E.; Nicolli, K.P.; Zanus, M.; Caramão, E.B.; Manfroi, V.; Zini, C.A. Monitoring the evolution of volatile compounds using gas chromatography during the stages of production of Moscatel sparkling wine. Food Chem. 2015, 183, 291–304. [Google Scholar] [CrossRef]
- Campo, E.; Cacho, J.; Ferreira, V. The chemical characterization of the aroma of dessert and sparkling white wines (Pedro Ximénez, Fino, Sauternes, and Cava) by Gas Chromatography–Olfactometry and chemical quantitative analysis. J. Agric. Food Chem. 2008, 56, 2477–2484. [Google Scholar] [CrossRef]
- Magyar, I. Botrytized wines. Adv. Food Nutr. Res. 2011, 63, 147–206. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; Taylor & Francis: Boca Raton, FL, USA, 2009; p. 2159. [Google Scholar]
- Sarrazin, E.; Dubourdieu, D.; Darriet, P. Characterization of key-aroma compounds of botrytized wines, influence of grape botrytization. Food Chem. 2007, 103, 536–545. [Google Scholar] [CrossRef]
- Hong, Y.S.; Cilindre, C.; Liger-Belair, G.; Jeandet, P.; Hertkorn, N.; Schmitt-Kopplin, P. Metabolic influence of Botrytis cinereainfection in Champagne base wine. J. Agric. Food Chem. 2011, 59, 7237–7245. [Google Scholar] [CrossRef]
- Oliva, M.; Hatan, E.; Kumar, V.; Galsurker, O.; Nisim-Levi, A.; Ovadia, R.; Galili, G.; Lewinsohn, E.; Elad, Y.; Alkan, N.; et al. Increased phenylalanine levels in plant leaves reduces susceptibility to Botrytis cinerea. Plant Sci. 2020, 290, 110289. [Google Scholar] [CrossRef]
- Ohgami, S.; Ono, E.; Horikawa, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; et al. Volatile glycosylation in tea plants: Sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Cheng, Y.; Yang, M.; Liu, Y.; Chen, K.; Long, C.; Deng, X. Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 2014, 14, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape contribution to wine aroma: Production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Pearce, A.D.; Zhao, Y.; Nicholson, E.L.; Dennis, E.G.; Jeffery, D.W. Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 2015, 20, 7845–7873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Filonow, A.B. Mycoactive acetate esters from apple fruit stimulate adhesion and germination of conidia of the gray mold fungus. J. Agric. Food Chem. 2002, 50, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Maoz, I.; Rikanati, R.D.; Schlesinger, D.; Bar, E.; Gonda, I.; Levin, E.; Kaplunov, T.; Sela, N.; Lichter, A.; Lewinsohn, E. Concealed ester formation and amino acid metabolism to volatile compounds in table grape (Vitis vinifera L.) berries. Plant Sci. 2018, 274, 223–230. [Google Scholar] [CrossRef]
- Carlquist, M.; Gibson, B.; Yuceer, Y.K.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; Billerbeck, G.M.D.; et al. Process engineering for bioflavour production with metabolically active yeasts—A mini-review. Yeast 2015, 32, 123–143. [Google Scholar]
- Moreno, J. Chapter 7—Sugars in Must. In Enological Chemistry, 1st ed.; Moreno, J., Peinado, R., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 95–107. [Google Scholar]
- Barata, A.; Pais, A.; Malfeito-Ferreira, M.; Loureiro, V. Influence of sour rotten grapes on the chemical composition and quality of grape must and wine. Eur. Food Res. Technol. 2011, 233, 183–194. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Breitenlechner, S.; Bach, T. Kinetic study on the esterification of hexanoic acid with n,n-dialkylamino alcohols: Evidence for an activation by hydrogen bonding. Z. Für Nat. B 2006, 61, 583–588. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Elmer, P.A.G.; Parry, F.J.; Viñas, I.; Usall, J.; Torres, R.; Agnew, R.H.; Teixidó, N. Mode of action of a fatty acid-based natural product to control Botrytis cinerea in grapes. J. Appl. Microbiol. 2014, 116, 967–979. [Google Scholar] [CrossRef]
- Desbois, A.P. Potential applications of antimicrobial fatty acids in medicine, agriculture and other industries. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 111–122. [Google Scholar] [CrossRef]
- Wang, X.J.; Tao, Y.S.; Wu, Y.; An, R.Y.; Yue, Z.Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of some mushroom and earthy off-odors microbially induced by the development of rot on grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef]
- Eder, M.; Sanchez, I.; Brice, C.; Camarasa, C.; Legras, J.L.; Dequin, S. QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation. BMC Genom. 2018, 19, 166. [Google Scholar] [CrossRef]
- Dixon, R.A.; Liu, C.; Jun, J.H. Metabolic engineering of anthocyanins and condensed tannins in plants. Curr. Opin. Biotechnol. 2013, 24, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, A.B.; Merkx, I.J.M. A simple method for screening of fresh plant material for glycosidic bound volatile compounds. Planta Medica 1989, 55, 38–40. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Fortes, A.M.; Agudelo-Romero, P.; Silva, M.S.; Ali, K.; Sousa, L.; Maltese, F.; Choi, Y.H.; Grimplet, J.; Martinez-Zapater, J.M.; Verpoorte, R.; et al. Transcript and metabolite analysis in Trincadeira cultivar reveals novel information regarding the dynamics of grape ripening. BMC Plant Biol. 2011, 11, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| VOCs | RI | Free Form Percentage | Glycosidic Bound Percentage | ||
|---|---|---|---|---|---|
| Control | Infected | Control | Infected | ||
| Acetic acid | 606 | t | t | t | 6.0 |
| Amyl alcohol | 839 | 2.9 | |||
| Hexanal | 840 | 13.0 | 7.6 | t | |
| 2-trans-Hexenal | 866 | 51.3 | 11.3 | ||
| cis-2-Hexen-1-ol | 882 | 4.0 | 14.6 | ||
| n-Hexanol | 882 | 21.1 | 37.9 | 19.3 | 9.3 |
| Isoamyl acetate | 882 | 10.4 | |||
| 2-Methyl butyl acetate | 882 | t | t | 5.2 | |
| Hexanoic acid | 970 | t | 0.2 | ||
| Ethyl hexanoate | 965 | 1.6 | 0.7 | ||
| Hexyl acetate | 995 | 0.5 | t | 4.3 | |
| 2-Phenylethanol | 1064 | t | 5.4 | 1.2 | 4.7 |
| n-Nonanol | 1148 | 2.3 | 1.8 | 4.1 | |
| Octanoic acid | 1149 | t | |||
| Ethyl octanoate | 1177 | 0.6 | 4.8 | 2.3 | |
| 2-Phenylethyl acetate | 1222 | 4.3 | t | 4.7 | |
| Ethyl nonanoate | 1273 | 2.6 | 0.3 | ||
| Nonyl acetate | 1300 | t | 2.4 | ||
| Decanoic acid | 1356 | t | 4.6 | ||
| Hexyl hexanoate | 1375 | 0.8 | |||
| Ethyl decanoate | 1387 | 34.3 | 14.7 | ||
| Dodecanoic Acid | 1550 | 1.7 | |||
| Ethyl dodecanoate | 1580 | 25.5 | 9.4 | ||
| Ethyl tetradecanoate | 1774 | 2.1 | 1.0 | ||
| n-Octadecane (C18) | 1800 | 1.7 | 5.6 | ||
| n-Nonadecane (C19) | 1900 | 6.1 | 2.3 | ||
| Ethyl hexadecanoate | 1936 | 4.3 | 5.9 | ||
| n-Eicosane (C20) | 2000 | 2.7 | 4.3 | ||
| n-Heneicosane (C21) | 2100 | t | 3.3 | ||
| Linoleic acid | 2137 | 1.6 | 5.2 | ||
| n-Docosane (C22) | 2200 | t | t | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, H.; Augusto, C.; Reis, P.; Rego, C.; Figueiredo, A.C.; Fortes, A.M. Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants 2022, 11, 141. https://doi.org/10.3390/plants11010141
Santos H, Augusto C, Reis P, Rego C, Figueiredo AC, Fortes AM. Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants. 2022; 11(1):141. https://doi.org/10.3390/plants11010141
Chicago/Turabian StyleSantos, Helena, Catarina Augusto, Pedro Reis, Cecília Rego, Ana Cristina Figueiredo, and Ana Margarida Fortes. 2022. "Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea" Plants 11, no. 1: 141. https://doi.org/10.3390/plants11010141
APA StyleSantos, H., Augusto, C., Reis, P., Rego, C., Figueiredo, A. C., & Fortes, A. M. (2022). Volatile Metabolism of Wine Grape Trincadeira: Impact of Infection with Botrytis cinerea. Plants, 11(1), 141. https://doi.org/10.3390/plants11010141








