Fresh Compost Tea Application Does Not Change Rhizosphere Soil Bacterial Community Structure, and Has No Effects on Soybean Growth or Yield
Abstract
:1. Introduction
2. Results
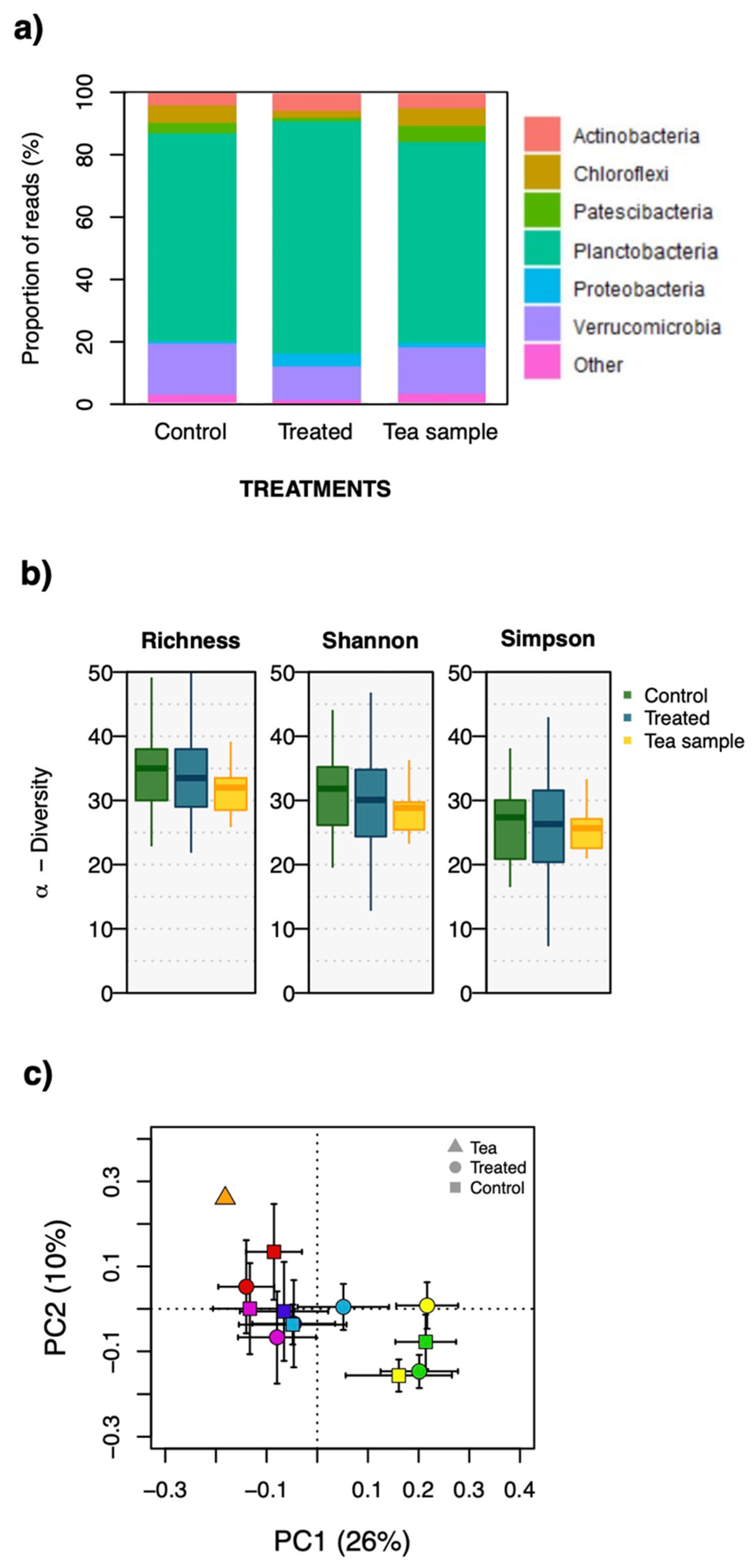
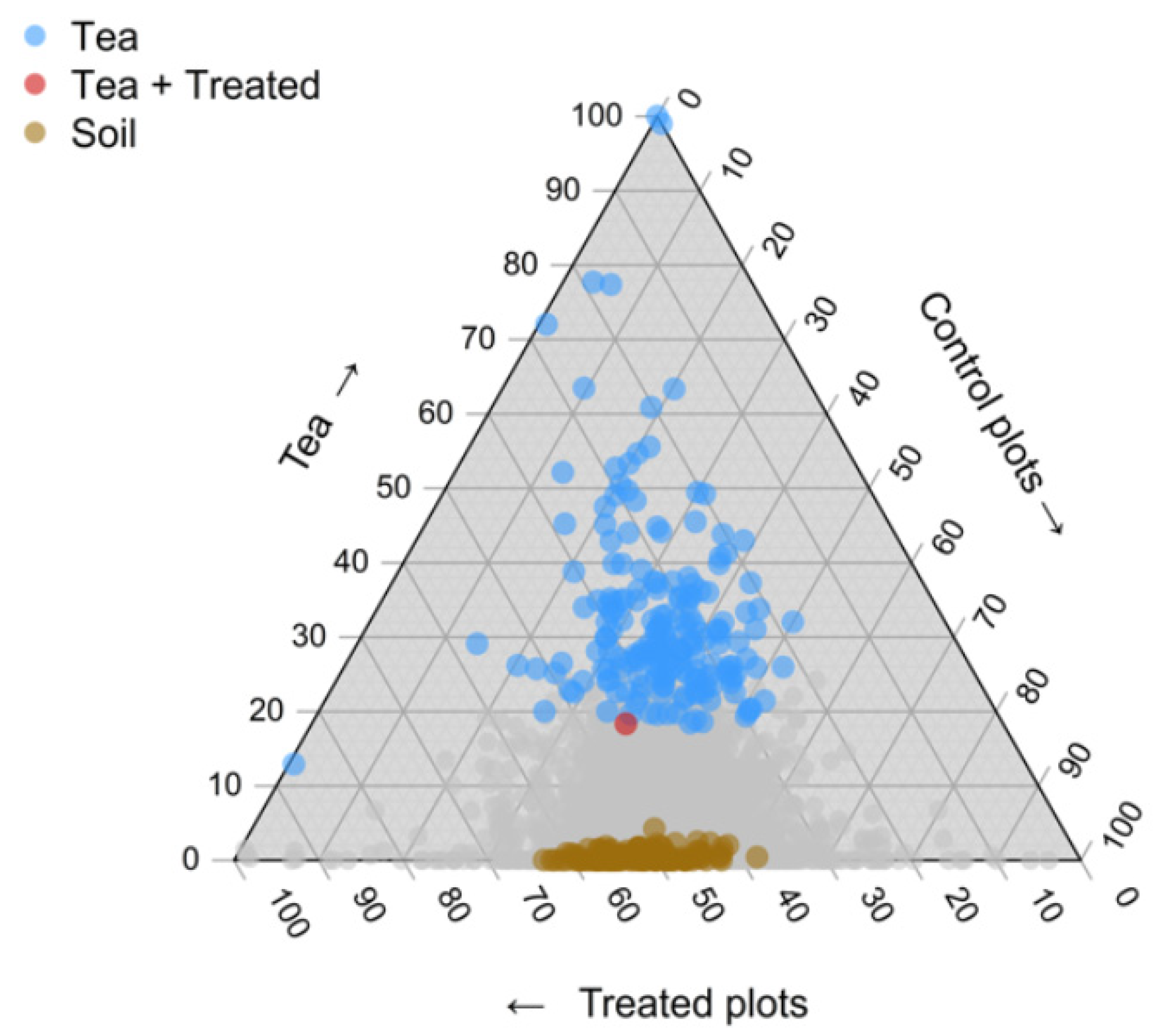
2.1. Bacterial Community Composition
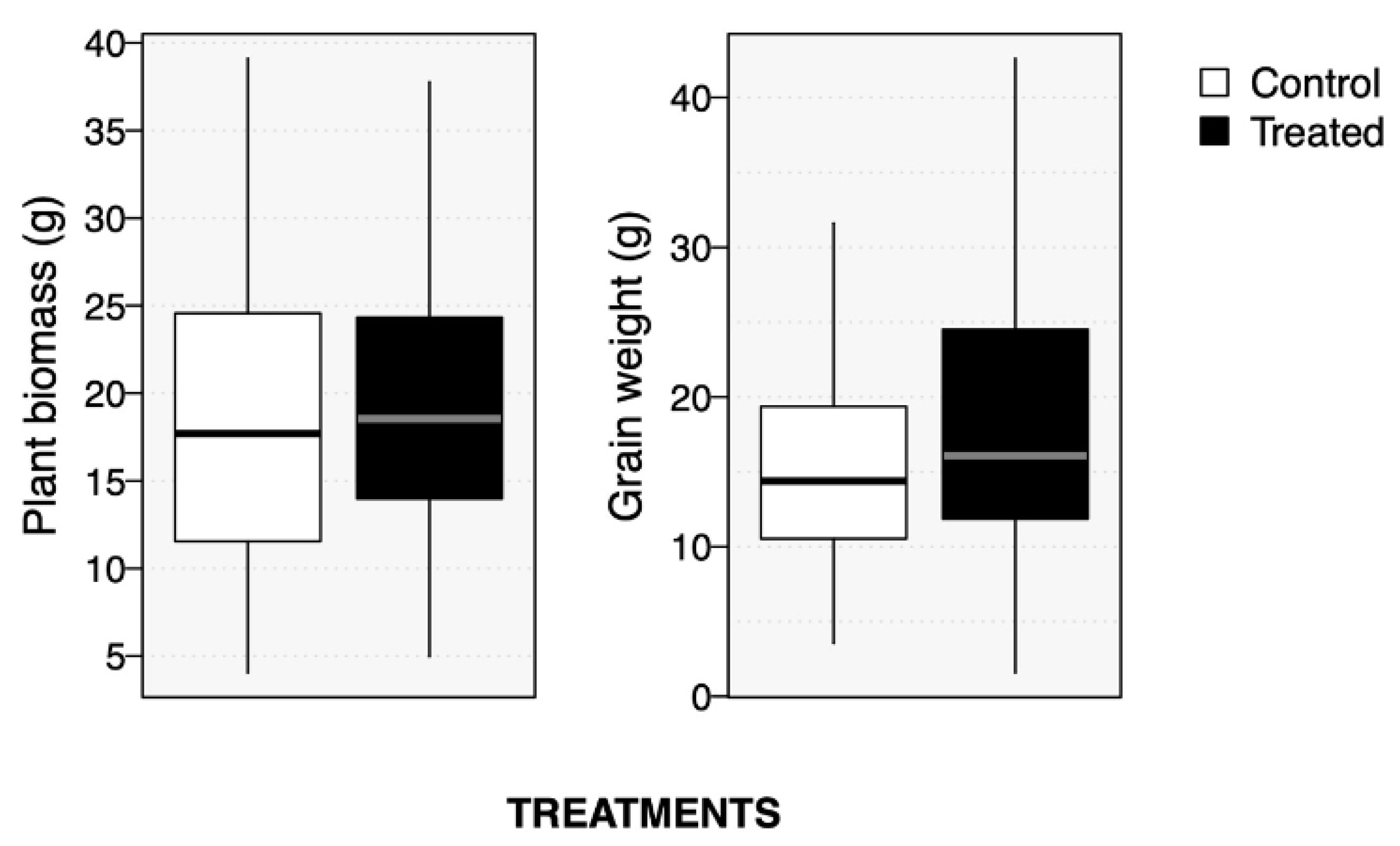
2.2. Soybean Growth and Productivity
3. Discussion
4. Materials and Methods
4.1. Site Description
4.2. Experimental Design
4.3. Compost Tea Preparation and Application
4.4. Field Samplings
4.5. Molecular Analyses
4.6. Bioinformatics
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Code Availability
References
- Davison, J. Plant Beneficial Bacteria. Nat. Biotechnol. 1988, 6, 282–286. [Google Scholar] [CrossRef]
- Kennedy, A.C. Bacterial Diversity in Agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Paoletti, M.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 65–76. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M.; Roy, J. Soil microbial diversity and soil functioning affect competition among grasses in experimental microcosms. Oecologia 2005, 143, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Hayat, R.; Ahmed, I.; Sheirdil, R.A. An Overview of Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture. In Crop Production for Agricultural Improvement; Springer: Dordrecht, The Netherlands, 2012; pp. 557–579. [Google Scholar] [CrossRef]
- Janušauskaite, D.; Kadžienė, G.; Auškalnienė, O. The effect of tillage system on soil microbiota in relation to soil structure. Pol. J. Environ. Stud. 2013, 22, 1387–1391. [Google Scholar]
- Silva, A.; Babujia, L.; Matsumoto, M.; Guimarães, M.; Hungria, M. Bacterial diversity under different tillage and crop rotation systems in an oxisol of Southern Brazil. Embrapa Soja-Artig. Periódico Indexado (ALICE) 2013, 7, 40–47. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage changes vertical distribution of soil bacterial and fungal communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, S.; Guo, L.; Cao, C.; Li, C.; Zhai, Z.; Zhou, J.; Mei, Y.; Ke, H. Advantages of nitrogen fertilizer deep placement in greenhouse gas emissions and net ecosystem economic benefits from no-tillage paddy fields. J. Clean. Prod. 2020, 263, 121322. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; De Ruiter, P.C.; Van Der Putten, W.H.; Birkhofer, K.; Hemerik, L.; De Vries, F.T.; Bardgett, R.D.; Brady, M.V. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef]
- Ji, L.; Wu, Z.; You, Z.; Yi, X.; Ni, K.; Guo, S.; Ruan, J. Effects of organic substitution for synthetic N fertilizer on soil bacterial diversity and community composition: A 10-year field trial in a tea plantation. Agric. Ecosyst. Environ. 2018, 268, 124–132. [Google Scholar] [CrossRef]
- Ma, W.; Abdulai, A.; Goetz, R. Agricultural cooperatives and investment in organic soil amendments and chemical fertilizer in China. Am. J. Agric. Econ. 2018, 100, 502–520. [Google Scholar] [CrossRef]
- Bai, Y.-C.; Chang, Y.-Y.; Hussain, M.; Lu, B.; Zhang, J.-P.; Song, X.-B.; Lei, X.-S.; Pei, D. Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Hou, R.; Li, J.; Lyu, Y.; Hang, S.; Gong, H.; Ouyang, Z. Effects of Different Fertilizers on Rhizosphere Bacterial Communities of Winter Wheat in the North China Plain. Agronomy 2020, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar]
- Lo, C.-C. Effect of pesticides on soil microbial community. J. Environ. Sci. Health Part B 2010, 45, 348–359. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural soils, pesticides and microbial diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Swami, S. Soil Microbes for Securing the Future of Sustainable Farming. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2687–2706. [Google Scholar] [CrossRef]
- Raina, S.A.; Bhat, R.A.; Qadri, H.; Dutta, A. Values of Biofertilizers for Sustainable Management in Agricultural Industries. In Bioremediation and Biotechnology; Springer: Berlin, Germany, 2020; Volume 2, pp. 121–137. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafique, M.; Sultan, T.; Ortas, I.; Chaudhary, H.J. Enhancement of maize plant growth with inoculation of phosphate-solubilizing bacteria and biochar amendment in soil. Soil Sci. Plant Nutr. 2017, 63, 460–469. [Google Scholar] [CrossRef]
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. Embrapa Soja-Artig. Periódico Indexado (ALICE) 2015, 6, 811–817. [Google Scholar]
- Requena, N.; Perez-Solis, E.; Azcón-Aguilar, C.; Jeffries, P.; Barea, J.-M. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl. Environ. Microbiol. 2001, 67, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The effect of plant growth promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. World Acad. Sci. Eng. Technol. 2009, 49, 19–24. [Google Scholar]
- Chaudhary, V.B.; Akland, K.; Johnson, N.C.; Bowker, M.A. Do soil inoculants accelerate dryland restoration? A simultaneous assessment of biocrusts and mycorrhizal fungi. Restor. Ecol. 2020, 28, S115–S126. [Google Scholar] [CrossRef]
- Girvan, M.; Campbell, C.; Killham, K.; Prosser, J.I.; Glover, L.A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Naidu, Y.; Meon, S.; Kadir, J.; Siddiqui, Y. Microbial starter for the enhancement of biological activity of compost tea. Int. J. Agric. Biol. 2010, 12, 51–56. [Google Scholar]
- Ingham, E. The Compost Tea Brewing Manual; Soil Foodweb Incorporated: Corvallis, OR, USA, 2005; Volume 728. [Google Scholar]
- Kannangara, T.; Forge, T.; Dang, B. Effects of aeration, molasses, kelp, compost type, and carrot juice on the growth of Escherichia coli in compost teas. Compost. Sci. Util. 2006, 14, 40–47. [Google Scholar] [CrossRef]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. Are compost teas an effective nutrient amendment in the cultivation of strawberries? Soil and plant tissue effects. J. Sci. Food Agric. 2009, 89, 390–397. [Google Scholar] [CrossRef]
- Pant, A.P.; Radovich, T.J.; Hue, N.V.; Paull, R.E. Biochemical properties of compost tea associated with compost quality and effects on pak choi growth. Sci. Hortic. 2012, 148, 138–146. [Google Scholar] [CrossRef]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 259. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Li, W.; Lv, X.; Ruan, J.; Yu, M.; Song, Y.-B.; Yu, J.; Dong, M. Variations in soil bacterial composition and diversity in newly formed coastal wetlands. Front. Microbiol. 2019, 9, 3256. [Google Scholar] [CrossRef]
- Li, H.; Xu, Z.; Yang, S.; Li, X.; Top, E.M.; Wang, R.; Zhang, Y.; Cai, J.; Yao, F.; Han, X. Responses of soil bacterial communities to nitrogen deposition and precipitation increment are closely linked with aboveground community variation. Microb. Ecol. 2016, 71, 974–989. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.d.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarrete, A.A.; Cannavan, F.S.; Taketani, R.G.; Tsai, S.M. A molecular survey of the diversity of microbial communities in different Amazonian agricultural model systems. Diversity 2010, 2, 787–809. [Google Scholar] [CrossRef] [Green Version]
- da C Jesus, E.; Marsh, T.L.; Tiedje, J.M.; de S Moreira, F.M. Changes in land use alter the structure of bacterial communities in Western Amazon soils. ISME J. 2009, 3, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Griepenburg, U.; Ward-Rainey, N.; Mohamed, S.; Schlesner, H.; Marxsen, H.; Rainey, F.A.; Stackebrandt, E.; Auling, G. Phylogenetic diversity, polyamine pattern and DNA base composition of members of the order Planctomycetales. Int. J. Syst. Evol. Microbiol. 1999, 49, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Schlesner, H. The development of media suitable for the microorganisms morphologically resembling Planctomyces spp., Pirellula spp., and other Planctomycetales from various aquatic habitats using dilute media. Syst. Appl. Microbiol. 1994, 17, 135–145. [Google Scholar] [CrossRef]
- Fuerst, J.A. The planctomycetes: Emerging models for microbial ecology, evolution and cell biology. Microbiology 1995, 141, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neef, A.; Amann, R.; Schlesner, H.; Schleifer, K.-H. Monitoring a widespread bacterial group: In situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 1998, 144, 3257–3266. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jenkins, C.; Webb, R.I.; Fuerst, J.A. Isolation of Gemmata-like and Isosphaera-like planctomycete bacteria from soil and freshwater. Appl. Environ. Microbiol. 2002, 68, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Dedysh, S.N.; Ivanova, A.A. Planctomycetes in boreal and subarctic wetlands: Diversity patterns and potential ecological functions. FEMS Microbiol. Ecol. 2019, 95, fiy227. [Google Scholar] [CrossRef] [Green Version]
- Buckley, D.H.; Huangyutitham, V.; Nelson, T.A.; Rumberger, A.; Thies, J.E. Diversity of Planctomycetes in soil in relation to soil history and environmental heterogeneity. Appl. Environ. Microbiol. 2006, 72, 4522–4531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derakshani, M.; Lukow, T.; Liesack, W. Novel bacterial lineages at the (sub) division level as detected by signature nucleotide-targeted recovery of 16S rRNA genes from bulk soil and rice roots of flooded rice microcosms. Appl. Environ. Microbiol. 2001, 67, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Elshahed, M.S.; Youssef, N.H.; Luo, Q.; Najar, F.Z.; Roe, B.A.; Sisk, T.M.; Bühring, S.I.; Hinrichs, K.-U.; Krumholz, L.R. Phylogenetic and metabolic diversity of Planctomycetes from anaerobic, sulfide-and sulfur-rich Zodletone Spring, Oklahoma. Appl. Environ. Microbiol. 2007, 73, 4707–4716. [Google Scholar] [CrossRef] [Green Version]
- Fuerst, J.A. Planctomycetes—New Models for Microbial Cells and Activities. In Microbial Resources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–27. [Google Scholar]
- Kepel, B.J.; Gani, M.A.; Tallei, T.E. Comparison of bacterial community structure and diversity in traditional gold mining waste disposal site and rice field by using a metabarcoding approach. Int. J. Microbiol. 2020, 2020. [Google Scholar] [CrossRef]
- Chaichi, W.; Djazouli, Z.; Zebib, B.; Merah, O. Effect of Vermicompost Tea on Faba Bean Growth and Yield. Compost. Sci. Util. 2018, 26, 279–285. [Google Scholar] [CrossRef]
- Bidabadi, S.S.; Dehghanipoodeh, S.; Wright, G.C. Vermicompost leachate reduces some negative effects of salt stress in pomegranate. Int. J. Recycl. Org. Waste Agric. 2017, 6, 255–263. [Google Scholar] [CrossRef]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motuka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: A phytohormonal approach. Plant Soil 2020, 446, 145–162. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- Zuur, A.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; pp. 1–567. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- De Caceres, M.; Jansen, F.; De Caceres, M.M. Package ‘indicspecies’. Indicators 2016, 8, 1. [Google Scholar]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analysing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
| Block | pH | Organic Matter Content (%) | Gravimetric Moisture (%) | Melich III-PO43− (mg/kg) | KCl-NH4+ (mg/kg) | KCl-NO3− (mg/kg) |
|---|---|---|---|---|---|---|
| A | 6.20 | 12.75 | 25.26 | 75.61 | 68.91 | 5.84 |
| B | 6.25 | 9.30 | 20.79 | 26.28 | 62.74 | 8.44 |
| C | 6.16 | 14.02 | 22.53 | 26.60 | 81.77 | 9.38 |
| D | 6.36 | 10.27 | 24.46 | 32.73 | 91.31 | 20.43 |
| E | 6.64 | 6.88 | 21.42 | 32.06 | 28.38 | 14.04 |
| F | 6.56 | 12.21 | 25.16 | 24.26 | 44.48 | 19.89 |
| Mean | 6.36 | 10.91 | 23.27 | 36.26 | 62.93 | 13.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bali, R.; Pineault, J.; Chagnon, P.-L.; Hijri, M. Fresh Compost Tea Application Does Not Change Rhizosphere Soil Bacterial Community Structure, and Has No Effects on Soybean Growth or Yield. Plants 2021, 10, 1638. https://doi.org/10.3390/plants10081638
Bali R, Pineault J, Chagnon P-L, Hijri M. Fresh Compost Tea Application Does Not Change Rhizosphere Soil Bacterial Community Structure, and Has No Effects on Soybean Growth or Yield. Plants. 2021; 10(8):1638. https://doi.org/10.3390/plants10081638
Chicago/Turabian StyleBali, Rana, Jonathan Pineault, Pierre-Luc Chagnon, and Mohamed Hijri. 2021. "Fresh Compost Tea Application Does Not Change Rhizosphere Soil Bacterial Community Structure, and Has No Effects on Soybean Growth or Yield" Plants 10, no. 8: 1638. https://doi.org/10.3390/plants10081638
APA StyleBali, R., Pineault, J., Chagnon, P.-L., & Hijri, M. (2021). Fresh Compost Tea Application Does Not Change Rhizosphere Soil Bacterial Community Structure, and Has No Effects on Soybean Growth or Yield. Plants, 10(8), 1638. https://doi.org/10.3390/plants10081638







