Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Preparation and Setup
2.2. Compost Preparation
2.3. Soil Sampling
2.4. Assessments of Soil CEC, Organic Matter, and Nutrient Contents
2.5. Soil Organic Carbon and Soil Enzyme Activity Determinations
2.6. Assessment of Growth and Yield Components
2.7. Leafy Nutrient Contents
2.8. Total Chlorophyll, Osmoprotectants, and Antioxidants Contents
2.9. Assaying the Activities of Antioxidative Enzymes
2.10. Data Analysis
3. Results
3.1. Soil Physical and Chemical Properties Response to SCF75 + FCF25 + CLB Supplementation for Two Seasons (2019 and 2020)
3.2. Response of Soil Chemical Properties and Plant Performances to the Growing Season
3.3. Response of Soil Chemical Properties and Plant Performances to Different Fertilization Strategies
3.4. Response of Soil Chemical Properties and Plant Performances to the Interaction between Growing Season and Different Fertilization Strategies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshankiti, A.; Gill, S. Integrated Plant Nutrient Management for Sandy Soil Using Chemical Fertilizers, Compost, Biochar and Biofertilizers—Case Study in UAE. J. Arid Land Stud. 2016, 26, 101–106. [Google Scholar]
- Pain, C.F.; Abdelfattah, M.A. Landform evolution in the arid northern United Arab Emirates: Impacts of tectonics, sea level changes and climate. Catena 2015, 134, 14–29. [Google Scholar] [CrossRef]
- Al-Muaini, A.H.; Green, S.R.; Abou Dahr, W.A.; Al-Yamani, W.; Abdelfattah, M.A.; Pangilinan, R.; McCann, I.; Dakheel, A.; Abdullah, A.; Kennedy, L.; et al. Sustainable Irrigation of Date Palms in the Hyper-Arid United Arab Emirates: A Review. Chron. Hortic. 2019, 4, 30–36. [Google Scholar]
- Ksiksi, T.S.; Trueman, R.; Abdelfattah, M.A.; Mousa, M.T.; Almarzouqi, A.Y.; Barahim, S.A. Above and belowground carbon pools are affected by dominant floral species in hyper-arid environments. F1000Research 2019, 8, 1043. [Google Scholar] [CrossRef]
- Abdelfattah, M.A. Pedogenesis, land management and soil classification in hyper-arid environments: Results and implications from a case study in the United Arab Emirates. Soil Use Manag. J. 2013, 29, 279–294. [Google Scholar] [CrossRef]
- Idowu, O.A.; Lorentz, S.A.; Annandale, J.G.; McCartney, M.P.; Jovanovic, N.Z. Assessment of the Impact of Irrigation with Low-quality Mine Water on Virgin and Rehabilitated Soils in the Upper Olifants Basin. Mine Water Environ. 2008, 27, 2–11. [Google Scholar] [CrossRef][Green Version]
- Wilson, I.G. Ergs. Sediment. Geol. 1973, 10, 77–106. [Google Scholar] [CrossRef]
- Fryberger, S.G. Dune forms and wind regime. In A Study of Global Sand Seas, U.S. Geological Survey Professional Paper 1052; McKee, E.D., Ed.; US Government Printing Office: Washington, DC, USA, 1979; pp. 137–170. [Google Scholar]
- Thomas, D.S.G. Arid Zone Geomorphology: Process, Forms and Change in Drylands, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 1997; p. 713. [Google Scholar]
- Pye, K.; Tsoar, H. Aeolian Sand and Sand Dunes; Unwin Hyman: London, UK, 1990; p. 396. [Google Scholar]
- Bubenzer, O.; Embabi, N.S.; Ashour, M.M. Sand Seas and Dune Fields of Egypt. Geosci 2020, 10, 101. [Google Scholar] [CrossRef]
- Brataševec, K.; Sivilotti, P.; Vodopivec, B.M. Soil and foliar fertilization affects mineral contents in Vitis vinifera L. cv. ‘rebula’ leaves. J. Soil Sci. Plant Nutr. 2013, 13, 650–663. [Google Scholar]
- Fregoni, M. Viticoltura di Qualità; Stampa Grafiche Lama: Piacenza, Italy, 1998; pp. 597–680. [Google Scholar]
- Kannan, S. Foliar Fertilization for Sustainable Crop Production. In Genetic Engineering, Biofertilization, Soil Quality and Organic Farming; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews 4; Springer: Berlin/Heidelberg, Germany, 2010; pp. 371–402. [Google Scholar]
- Tejada, M.; Gonzalez, J.L. Effects off foliar application of a byproduct of the two-step olive oil mill process on rice yield. Europ. J. Agron. 2004, 21, 31–40. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D. Response of tomato (Lycopersicon esculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Sci. Hortic. 2002, 93, 53–64. [Google Scholar] [CrossRef]
- Jamal, Z.; Hamayun, M.; Ahmed, N.; Chaudhary, M.F. Effect of soil and foliar application of different concentrations of NPK and foliar application of (NH4)2SO4 on different yield parameters in wheat. Asian J. Agron. 2006, 5, 251–256. [Google Scholar]
- Dewdar, M.D.H.; Rady, M.M. Influence of soil and foliar applications of potassium fertilization on growth, yield and fiber quality traits in two Gossypium barbadense L. varieties. Afr. J. Agric. Res. 2013, 8, 2211–2215. [Google Scholar]
- Basavaraj, P.; Chetan, H.T. Folair fertilization of nutrients. Marumegh 2018, 3, 49–53. [Google Scholar]
- Rady, M.M. Effects on growth, yield, and fruit quality in tomato (Lycopersicon esculentum Mill.) using a mixture of potassium humate and farmyard manure as an alternative to mineral-N fertiliser. J. Hortic. Sci. Biotechnol. 2011, 86, 249–254. [Google Scholar]
- Rady, M.M.; Semida, W.M.; Hemida, K.A.; Abdelhamid, M.T. The effect of compost on growth and yield of Phaseolus vulgaris plants grown under saline soil. Int. J. Recycl. Org. Waste Agric. 2016, 5, 311–321. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-Zahrani, H.S.; Hakeem, K.R.; Alsamadany, H.; Desoky, E.-S.M.; Rady, M.M. Silymarin-Enriched Biostimulant Foliar Application Minimizes the Toxicity of Cadmium in Maize by Suppressing Oxidative Stress and Elevating Antioxidant Gene Expression. Biomolecules 2021, 11, 465. [Google Scholar] [CrossRef]
- Kusparwanti, T.R.; Eliyatiningsih; Wardana, R. Application Legume Compost with Bio-Activator Trichoderma sp as Inorganic Fertilizer Substitution in Sweet Corn (Zea mays L. Saccharata) Cultivation. IOP Conf. Series. Earth Environ. Sci. 2020, 411, 012063. [Google Scholar] [CrossRef]
- Bamagoos, A.A.; Alharby, H.F.; Belal, E.E.; Khalaf, A.E.A.; Abdelfattah, M.A.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M. Phosphate-Solubilizing Bacteria as a Panacea to Alleviate Stress Effects of High Soil CaCO3 Content in Phaseolus vulgaris with Special Reference to P-Releasing Enzymes. Sustainability 2021, 13, 7063. [Google Scholar] [CrossRef]
- Giller, K.E.; McDonagh, J.F.; Cadisch, G. Can biological nitrogen fixation sustain agriculture in the tropics? In Soil Science and Sustainable Land Management in the Tropics; Syers, P.K., Rimmer, D.L., Eds.; C.A.B International: Wallingford, UK, 1994; pp. 173–191. [Google Scholar]
- Kumwenda, J.D.T.; Waddington, S.R.; Snapp, S.S.; Jones, R.B.; Blackie, M.J. Soil Fertility Managementfor Smallholder Maize-Based Cropping Systems of Southern Afnca: A Review. Network Working Paper No. 1. Soil Fertility Network for Maize-Based Cropping Systems in Countries of Southern Africa; CIMMYT: Harare, Zimbabwe, 1995; p. 34. [Google Scholar]
- Cayuela, M.L.; Mondini, C.; Insam, H.; Sinicco, T.; Whittle, F.I. Plant and animal wastes composting: Effects of the N source on process performance. Bioresour. Technol. 2009, 100, 3097–3106. [Google Scholar] [CrossRef]
- Buttery, B.R.; Park, S.l.; Hume, D.F.J. Potential for increasing nitrogen fixation in grain legumes. J. Plant Sci. 1992, 72, 323–349. [Google Scholar] [CrossRef]
- Senjobi, B.A. Comparative Assessment of the Effect of Land Use and Land Type on Soil Degradation and Agricultural Productivity in Ogun State, Nigeria. Ph.D. Thesis, Department of Agronomy, University of Ibadan, Ibadan, Nigeria, 2007, unpublished. [Google Scholar]
- Page, A.I.; Miller, R.H.; Keeny, D.R. Methods of soil analysis. In Part II. Chemical and Microbiological Methods, 2nd ed.; Amer Soc Agron: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity. Laboratory methods. Methods of Soil Analysis-Part 1. Phys. Mineral. Methods 1986, 9, 687–734. [Google Scholar]
- OECD. “Common Bean (Phaseolus Vulgaris)”, in Safety Assessment of Transgenic Organisms in the Environment, Volume 6: OECD Consensus Documents; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2006. [Google Scholar]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulases in a silt loam soil. Biol. Fertil. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for the determination of glucose. Anal. Chem. 1972, 31, 426–428. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Johnson, J.L.; Temple, K.L. Some variables affecting the measurement of “catalase activity” in soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Stepniewska, Z.; Wolinska, A.; Ziomek, J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009, 21, 1142–1147. [Google Scholar] [CrossRef]
- King, E.J. Micro-Analysis in Medical Biochemistry; J. & A. Churchill: London, UK, 1951. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Colorado, CO, USA, 1954. [Google Scholar]
- Chapman, H.D. Cation-Exchange capacity. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Norman, A.G., Ed.; Madison, WI, USA, 1965; Volume 9, pp. 891–901. Available online: https://doi.org/10.2134/agronmonogr9.2.c6 (accessed on 16 June 2021).
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Li, P.M.; Cai, R.G.; Gao, H.Y.; Peng, T.; Wang, Z.L. Partitioning of excitation energy in two wheat cultivars with different grain protein contents grown under three nitrogen applications in the field. Physiol. Plant. 2007, 129, 822–829. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Landolt, W.; Bucher, J.B.; Strasser, R.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Osman, A.S.; Rady, M.M. Ameliorative effects of sulphur and humic acid on the growth, antioxidant levels, and yields of pea (Pisum sativum L.) plants grown in reclaimed saline soil. J. Hortic. Sci. Biotechnol. 2014, 87, 626–632. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Emerich, D.W.; Sanchez-Diaz, M. Water stress induced changes in the concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Plant Physiol. 1992, 8, 455–460. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Griffth, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2 vinyl pyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Choudhari, M.A. Implications of water stress induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983, 58, 116–170. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Putter, J. Peroxidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinhan, MA, USA, 1974; pp. 685–690. [Google Scholar]
- Bradford, M.M. A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Razali, N.; Wahi, Y.B. Power comparisons of Shapiro–Wilk, Kolmogorov–Smirnov, Lilliefors and Anderson–Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Abdelfattah, M.A. Climate Change Impacts on Water Resources and Food Security in Egypt and Possible Adaptive Measures—A review. In Climate Change, Food Security, and Sustainable Smart Agriculture; Behnassi, M., Mirza, B.B., El Haiba, M., Reed, M., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2021; in press. [Google Scholar]
- Shahid, S.A.; Abdelfattah, M.A.; Taha, F.K. Developments in Soil Salinity Assessment and Reclamation, Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; p. 808. ISBN 978-94-007-5684-7. [Google Scholar]
- Shahid, S.A.; Taha, F.K.; Abdelfattah, M.A. Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; p. 858. ISBN 978-94-007-5331-0. [Google Scholar]
- Shahid, S.A.; Abdelfattah, M.A.; Wilson, M.A.; Kelley, J.A.; Chiaretti, J.V. United Arab Emirates Keys to Soil Taxonomy; Springer Science + Business Media, B.V.: Berlin, Germany, 2014; ISBN 978-94-007-7419-3. [Google Scholar]
- Pain, C.F.; Abdelfattah, M.A.; Shahid, S.A.; Ditzler, C. Soil-landform relationships in the arid Northern United Arab Emirates. In Geopedology, An Integration of Geomorphology and Pedology for Soil and Landscape Studies; Zinck, J.A., Metternicht, G., Bocco, G., Del Valle, H.F., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2016; pp. 211–225. [Google Scholar] [CrossRef]
- Shahid, S.A.; Abdelfattah, M.A.; Mahmoudi, H. Innovations in soil chemical analyses—New ECe and total ions relationship in Abu Dhabi Emirate soils. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 799–812. ISBN 978-94-007-5331-0. [Google Scholar]
- Shahid, S.A.; Abdelfattah, M.A.; Othman, Y.; Kumar, A.; Taha, F.K.; Kelley, J.A.; Wilson, M.A. Innovative Thinking for Sustainable Use of Terrestrial Resources in Abu Dhabi Emirate Through Scientific Soil Inventory and Policy Development. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 3–49. ISBN 978-94-007-5331-0. [Google Scholar]
- Shendi, M.M.; Abdelfattah, M.A.; Harbi, A. Spatial monitoring of soil salinity and prospective conservation study for Sinnuris District soils, Fayoum, Egypt. In Developments in Soil Salinity Assessment and Reclamation, Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Shahid, S.A., Abdelfattah, M.A., Taha, F.K., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 199–217. ISBN 978-94-007-5684-7. [Google Scholar]
- Kelley, J.A.; Wilson, M.A.; Abdelfattah, M.A.; Shahid, S.A. Quality Assurance Standards: USDA Perspective of the Extensive Soil Survey of Abu Dhabi Emirate. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 813–822. ISBN 978-94-007-5331-0. [Google Scholar]
- King, P.; Grealish, G.; Shahid, S.A.; Abdelfattah, M.A. Land evaluation interpretations—Soil Survey of Abu Dhabi Emirate. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 147–164. ISBN 978-94-007-5331-0. [Google Scholar]
- Wilson, M.A.; Shahid, S.A.; Abdelfattah, M.A.; Kelley, J.A.; Thomas, J.E. Anhydrite formation on the coastal sabkha of Abu Dhabi, United Arab Emirates. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 175–202. ISBN 978-94-007-5331-0. [Google Scholar]
- Verboom, W.H.; Pate, J.S.; Abdelfattah, M.A.; Shahid, S.A. Effects of plants on soil forming processes: Case studies from arid environments. In Developments in Soil Classification, Land Use Planning and Policy Implications, Innovative Thinking of Soil Inventory for Land Use Planning and Management of Land Resources; Shahid, S.A., Taha, F.K., Abdelfattah, M.A., Eds.; Springer Science + Business Media, B.V.: Berlin, Germany, 2013; pp. 329–344. ISBN 978-94-007-5331-0. [Google Scholar]
- Clark, S. Organic farming and climate change: The need for innovation: An opinion. Sustainability 2020, 12, 7012. [Google Scholar] [CrossRef]
- Gransee, A.; Fuhrs, H. Magnesium mobility in soils as a challenge for soil and plant analysis magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Rehman, H.; Alharby, H.F.; Alzahrani, Y.; Rady, M.M. Magnesium and organic biostimulant integrative application induces physiological and biochemical changes in sunflower plants and its harvested progeny on sandy soil. Plant Physiol. Biochem. 2018, 126, 97–105. [Google Scholar] [CrossRef]
- Shahid, S.A.; Taha, F.K.; Ismail, S.; Al Dakheel, A.; Abdelfattah, M.A. Turning Adversity into an Advantage for Food Security Through Improving Soil Quality and Providing Production Systems for Marginal Saline Lands: ICBA Perspectives and Approaches. In Sustainable Agriculture Development, Recent Approaches in Resources Management and Environmentally-Balanced Production Enhancement; Behnassi, M., Shahid, S.A., D’Silva, J., Eds.; Springer Science + Business Media B.V.: Berlin, Germany, 2011. [Google Scholar]
- Buragohain, S.; Sarma, B.; Nath, D.J.; Gogoi, N.; Meena, R.S.; Lal, R. Effect of 10 years of biofertiliser use on soil quality and rice yield on an Inceptisol in Assam, India. Soil Res. 2018, 56, 49–58. [Google Scholar] [CrossRef]
- Belal, E.E.; El Sowfy, D.M.; Rady, M.M. Integrative soil application of humic acid and sulfur improves saline calcareous soil properties and barley plant performance. Commun. Soil Sci. Plant Anal. 2019, 50, 1919–1930. [Google Scholar] [CrossRef]
- Manirakiza, N.; Şeker, C. Effects of compost and biochar amendments on soil fertility and crop growth in a calcareous soil. J. Plant Nutr. 2020, 43, 3002–3019. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Shewy, A.A.; Seif El-Yazal, M.A.; Abd El-Gawwad, I.F.M. Integrative application of soil P-solubilizing bacteria and foliar nano p improves Phaseolus vulgaris plant performance and antioxidative defense system components under calcareous soil conditions. J. Soil Sci. Plant Nutr. 2020, 20, 820–839. [Google Scholar] [CrossRef]
- Sinha, S.K.; Kumar, V.; Jha, C.K. Effect of Integrated use of Bio-Compost and Nitrogen on Productivity and Soil Properties of Sugarcane Plant–Ratoon System in Calcareous Soil. SugarTech 2017, 19, 485–491. [Google Scholar] [CrossRef]
- Wu, S.C.; Cao, Z.H.; Li, Z.G.; Cheung, K.C.; Wong, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: A greenhouse trial. Geoderma 2005, 125, 155–166. [Google Scholar] [CrossRef]
- Valarini, P.J.; Díaz, M.C.; Gascó, J.M.; Guerrero, F.; Tokeshi, H. Assessment of soil properties by organic matter and EM-Microorganisms incorporation. Rev. Bras. De Ciência Do Solo 2003, 27, 519–525. [Google Scholar] [CrossRef]
- Fischer, D.; Glaser, B. Synergisms between compost and biochar for sustainable soil amelioration. In Management of Organic Waste; Kumar, S., Ed.; In Tech.: London, UK, 2012; pp. 168–198. ISBN 978-953-307-925-7. [Google Scholar]
- Aboukila, E.F.; Nassar, I.N.; Rashad, M.; Hafez, M.; Norton, J.B. Reclamation of calcareous soil and improvement of squash growth using brewers’ spent grain and compost. J. Saudi Soc. Agric. Sci. 2018, 17, 390–397. [Google Scholar] [CrossRef]
- Nath, D.J.; Ozha, B.; Barooah, R.C.; Borah, D.K. Effect of integrated nutrient management on soil enzymes, microbial biomass carbon and bacterial populations under rice (Oryza sativa)–wheat (Triticum aestivum) sequence. Indian J. Agric. Sci. 2012, 81, 1143–1148. [Google Scholar]
- Macci, C.; Doni, S.; Peruzzi, E.; Masciandaro, G.; Mennone, C.; Ceccanti, B. Almond tree and organic fertilization for soil quality improvement in southern Italy. J. Environ. Manag. 2010, 95, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Swarnalakshmi, K.; Prasanna, R.; Kumar, A.; Pattnaik, S.; Chakravarty, K.; Shivay, Y.S.; Singh, R.; Saxena, A.K. Evaluating the influence of novel cyanobacterial bio filmed biofertilizers on soil fertility and plant nutrition in wheat. Euro. J. Soil Biol. 2013, 55, 107–116. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shinwari, Z.K.; Ahmad, N.; Kim, Y.H.; Lee, I.J. Effect of foliar and soil application of nitrogen, phosphorus and potassium on yield components of lentil. Pak. J. Bot. 2011, 43, 391–396. [Google Scholar]
- Rahman, M.A.; Rahman, M.M.; Begum, M.F.; Alam, M.F. Effect of bio compost, cow dung compost and NPK fertilizers on growth, yield and yield components of chili. Int. J. Biosci. 2012, 2, 51–55. [Google Scholar]
- Sandoval, A.P.; Yáñez-Chávez, L.G.; Sánchez-Cohen, I.; Samaniego-Gaxiola, J.A.; Trejo-Calzada, R. Hydrogel, biocompost and its effect on photosynthetic activity and production of forage maize (Zea mays L.) plants. Acta Agron. 2017, 66, 63–68. [Google Scholar]
- Sinclair, T.R.; Ludlow, M.M. Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust. J. Plant Physiol. 1986, 13, 329–341. [Google Scholar] [CrossRef]
- Nusrat, N.; Shahbaz, M.; Perveen, S. Modulation in growth, photosynthetic efficiency, activity of antioxidants and mineral ions by foliar application of glycinebetaine on pea (Pisum sativum L.) under salt stress. Acta Physiol. Plant. 2014, 36, 2985–2998. [Google Scholar] [CrossRef]
- Rady, M.M.; Rehman, H.U. Supplementing organic biostimulants into growing media enhances growth and nutrient uptake of tomato transplants. Sci. Hortic. 2016, 203, 192–198. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, E.S.; Abo El-Maati, M.E.; Desoky, E.M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Hemida, K.A.; Rady, M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018, 154, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Alharby, H.F.; Alzahrani, H.S.; Alzahrani, Y.; Alsamadany, H.; Hakeem, K.R.; Rady, M.M. Maize grain extract enriched with polyamines alleviates drought stress in Triticum aestivum through up-regulation of the ascorbate-glutathione cycle, glyoxalase system, and polyamine gene expression. Agronomy 2021, 11, 949. [Google Scholar] [CrossRef]
- Rady, M.M.; Desoky, E.-S.M.; Ahmed, S.M.; Majrashi, A.; Ali, E.F.; Arnaout, S.M.A.; Selem, E. Foliar Nourishment with Nano-Selenium Dioxide Promotes Physiology, Biochemistry, Antioxidant Defenses, and Salt Tolerance in Phaseolus vulgaris. Plants 2021, 10, 1189. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Boriek, S.H.K.; Abd El-Mageed, T.A.; Seif El-Yazal, M.A.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Desoky, E.S.; Mansour, E.; Ali, M.M.A.; Yasin, M.A.T.; Abdul-Hamid, M.I.E.; Rady, M.M.; Ali, E.F. Exogenously used 24-epibrassinolide promotes drought tolerance in maize hybrids by improving plant and water productivity in an arid environment. Plants 2021, 10, 354. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Geros, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef] [PubMed]
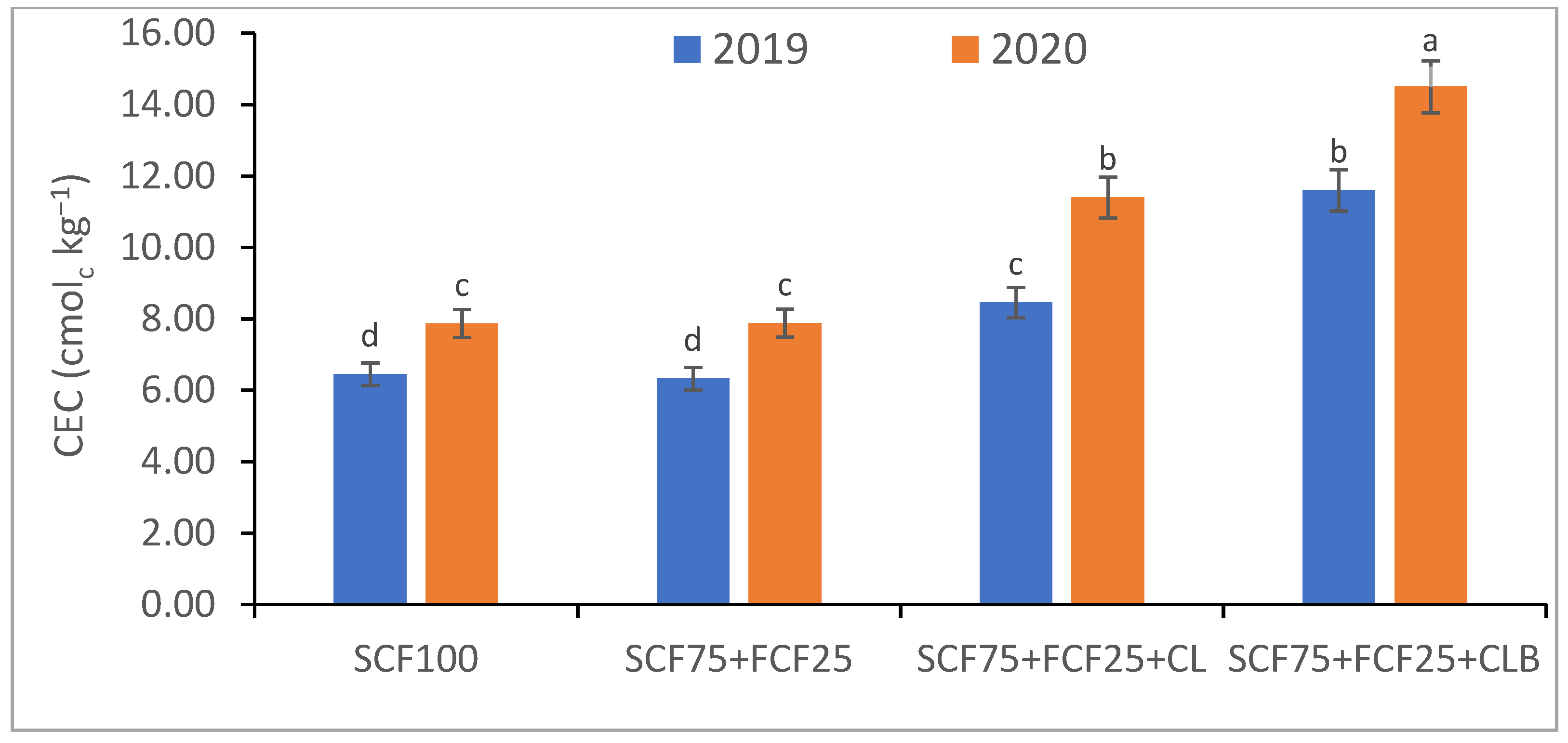
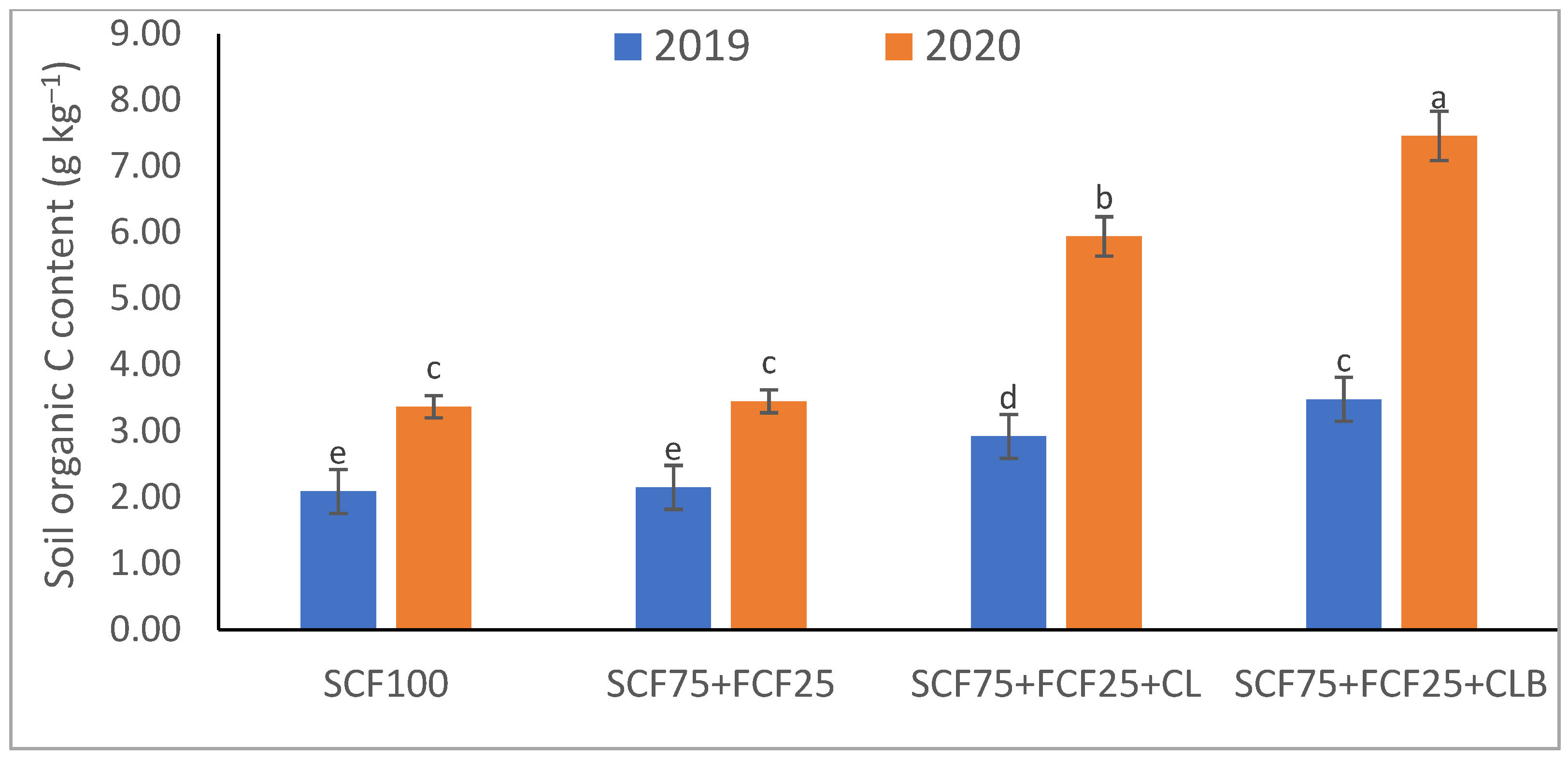
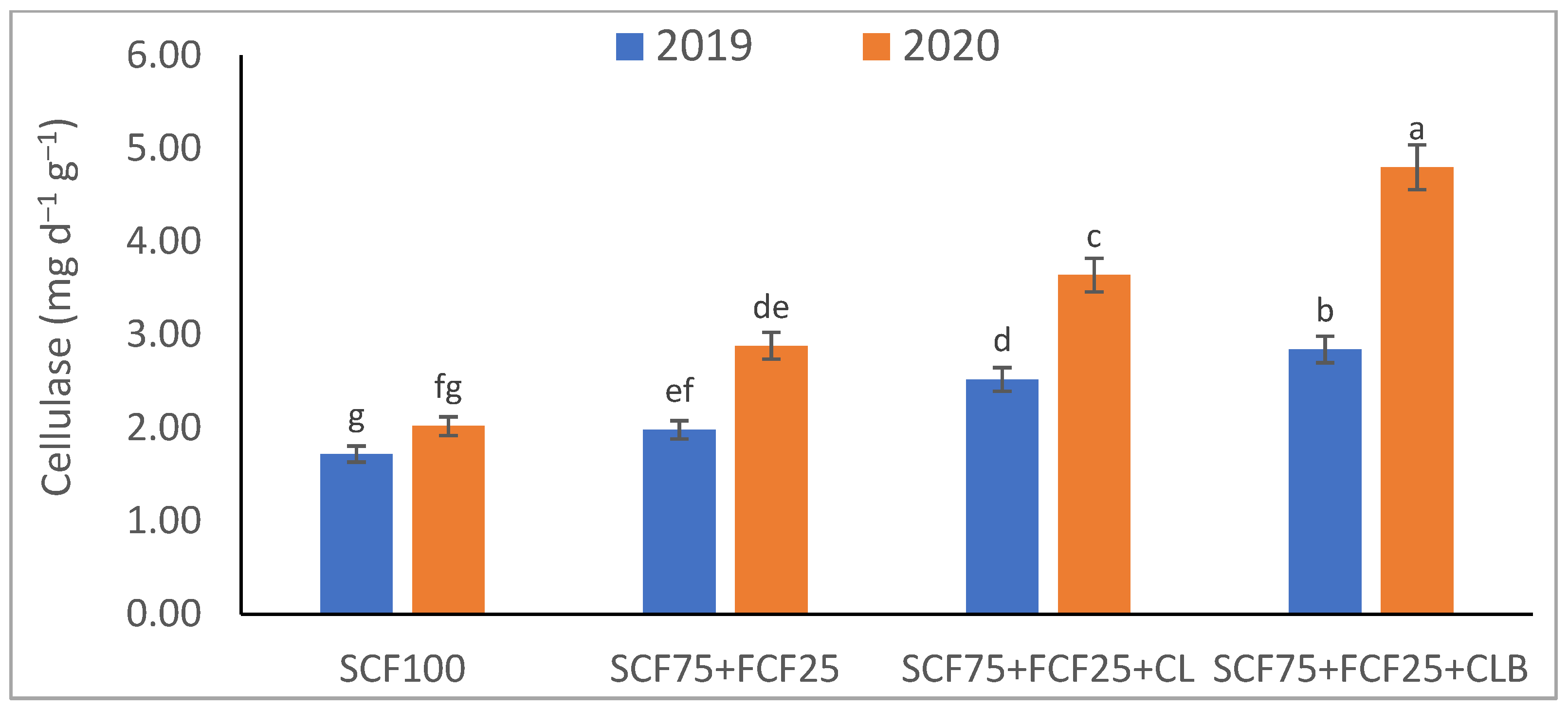
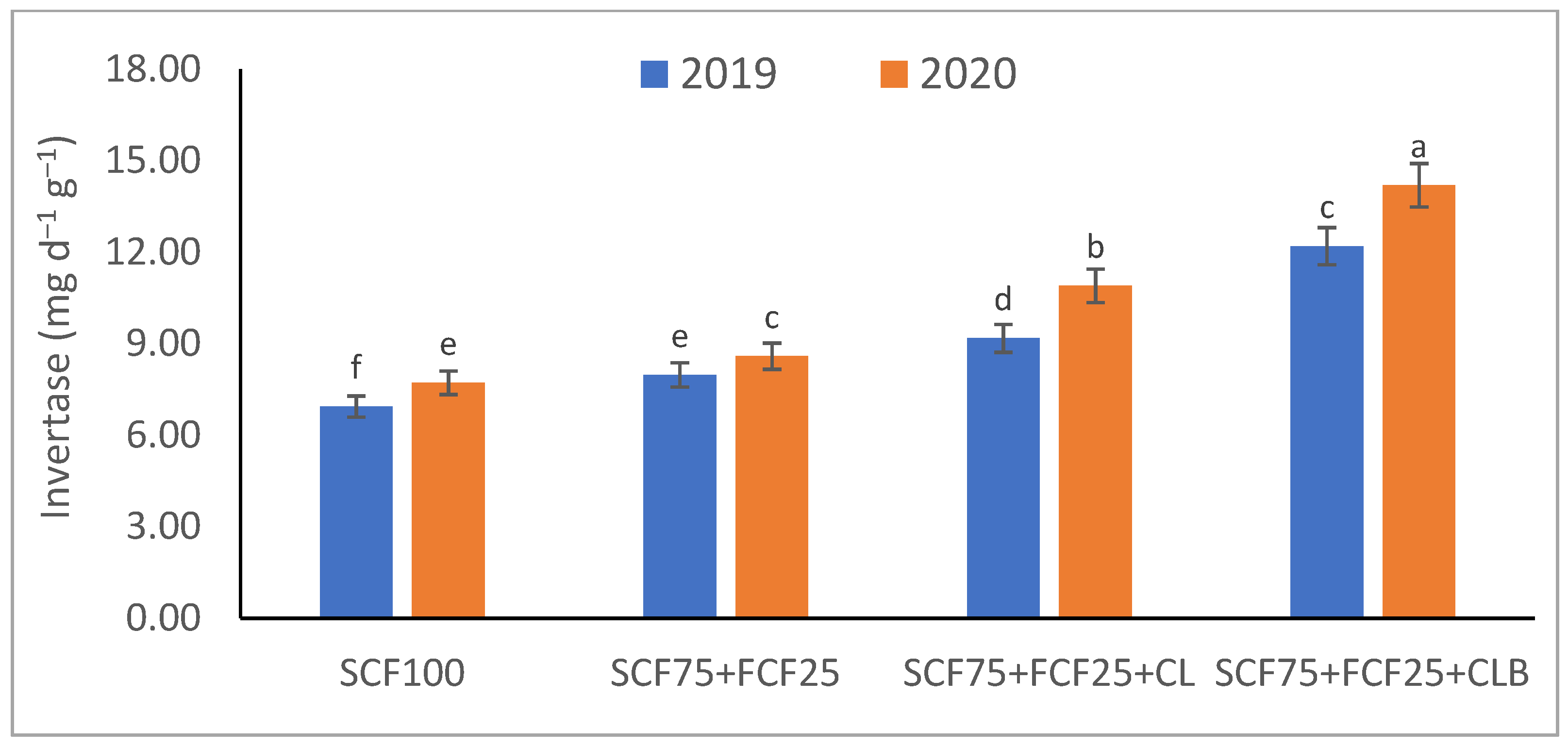
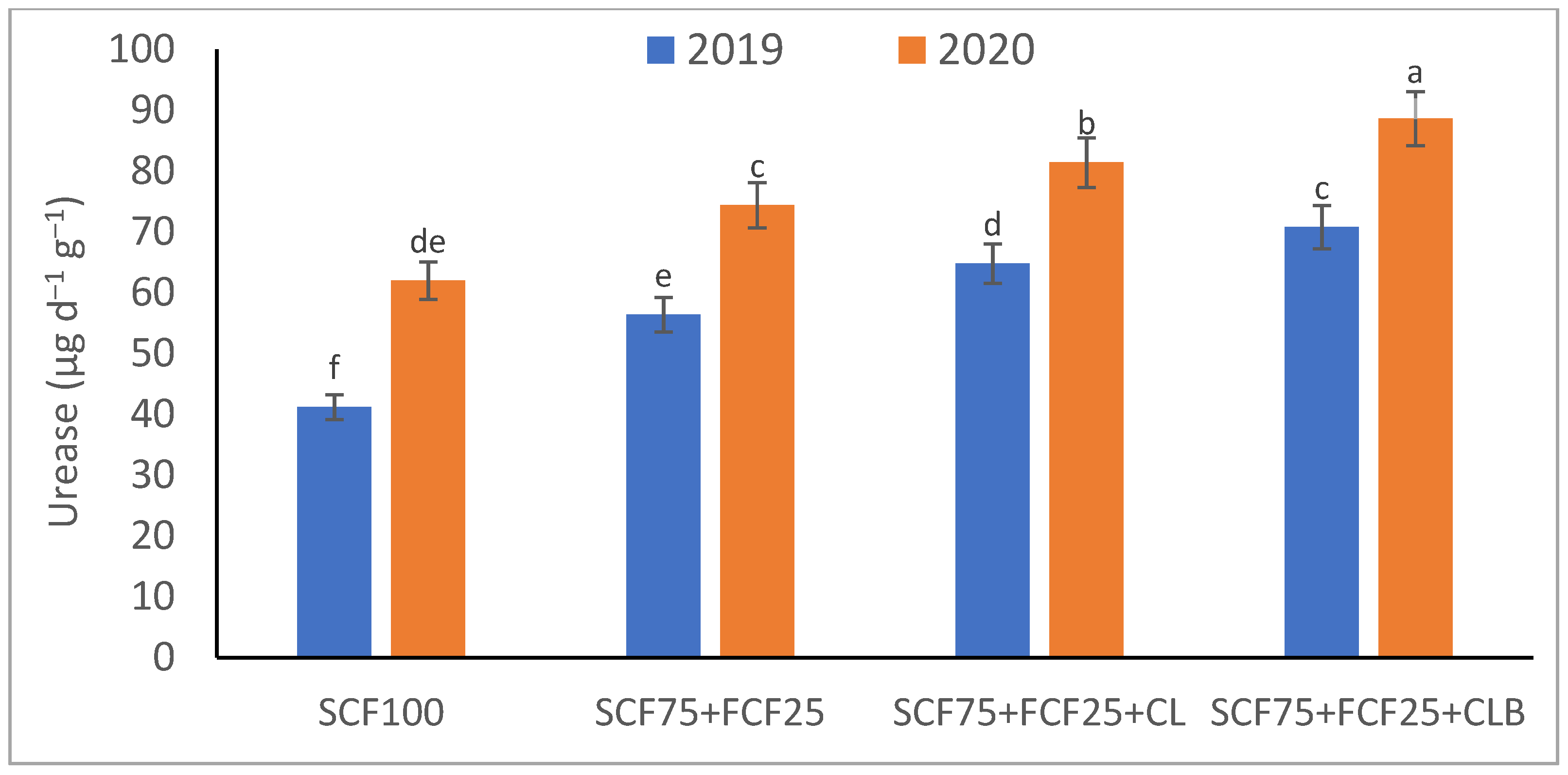
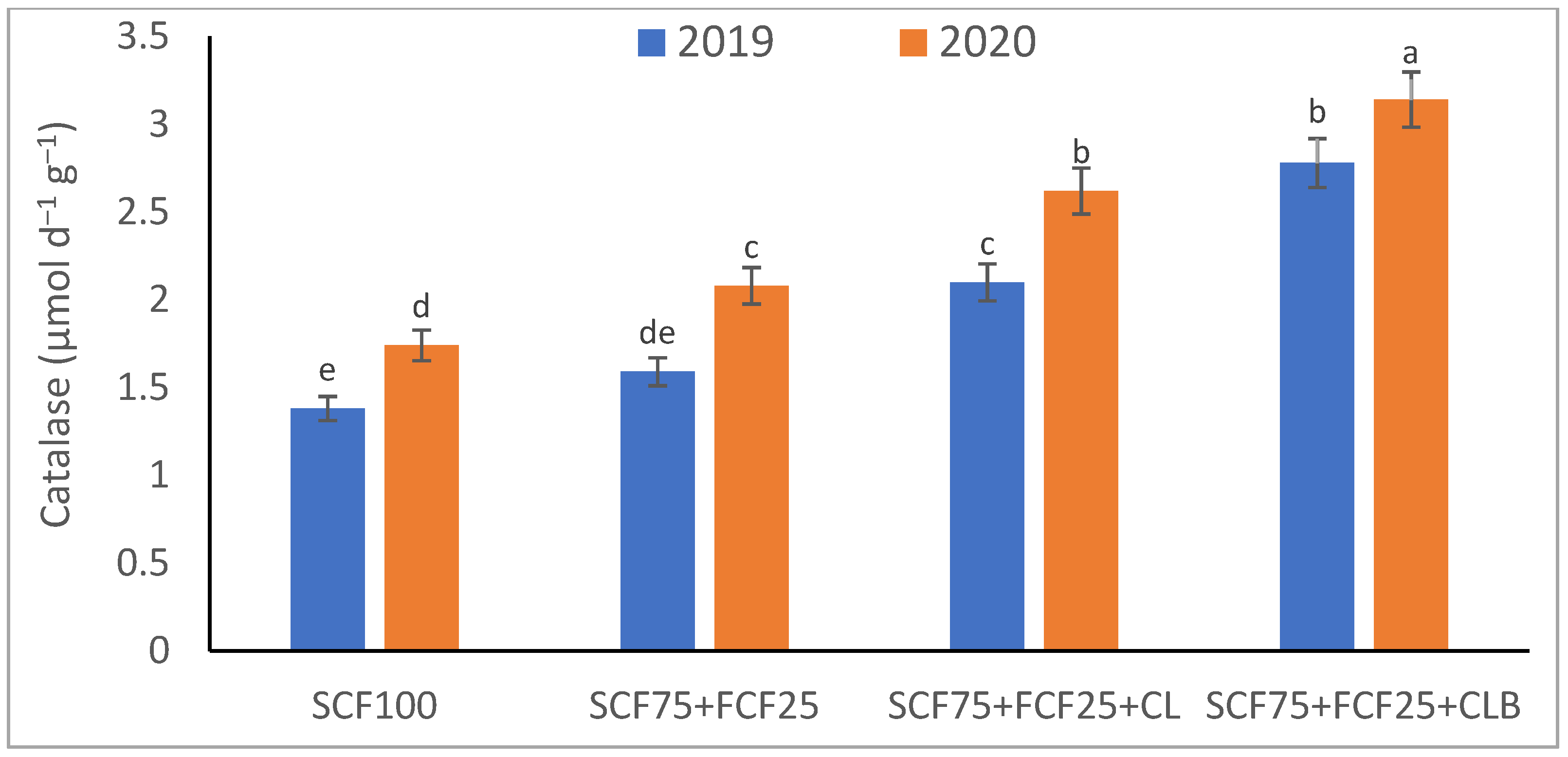
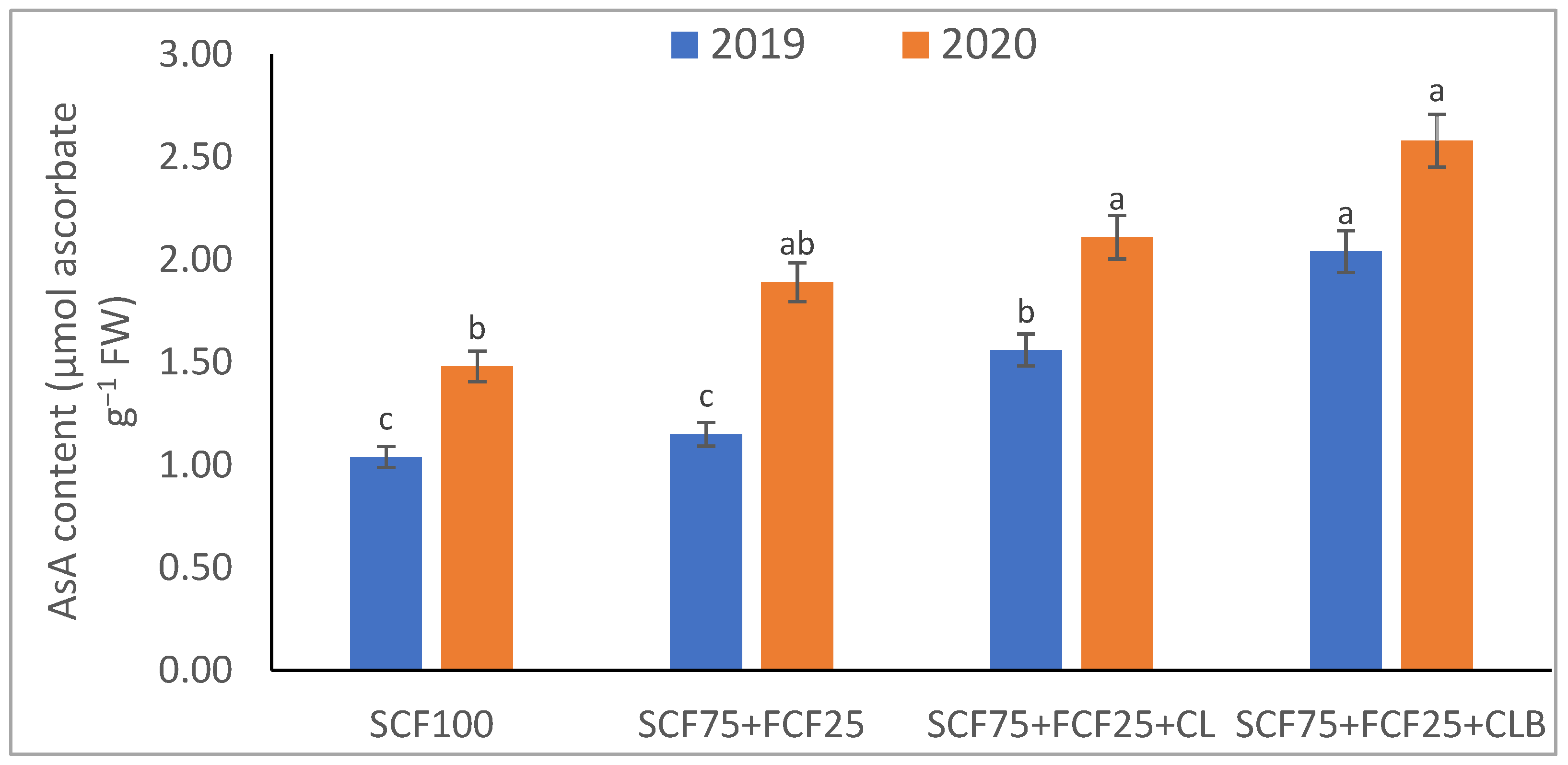
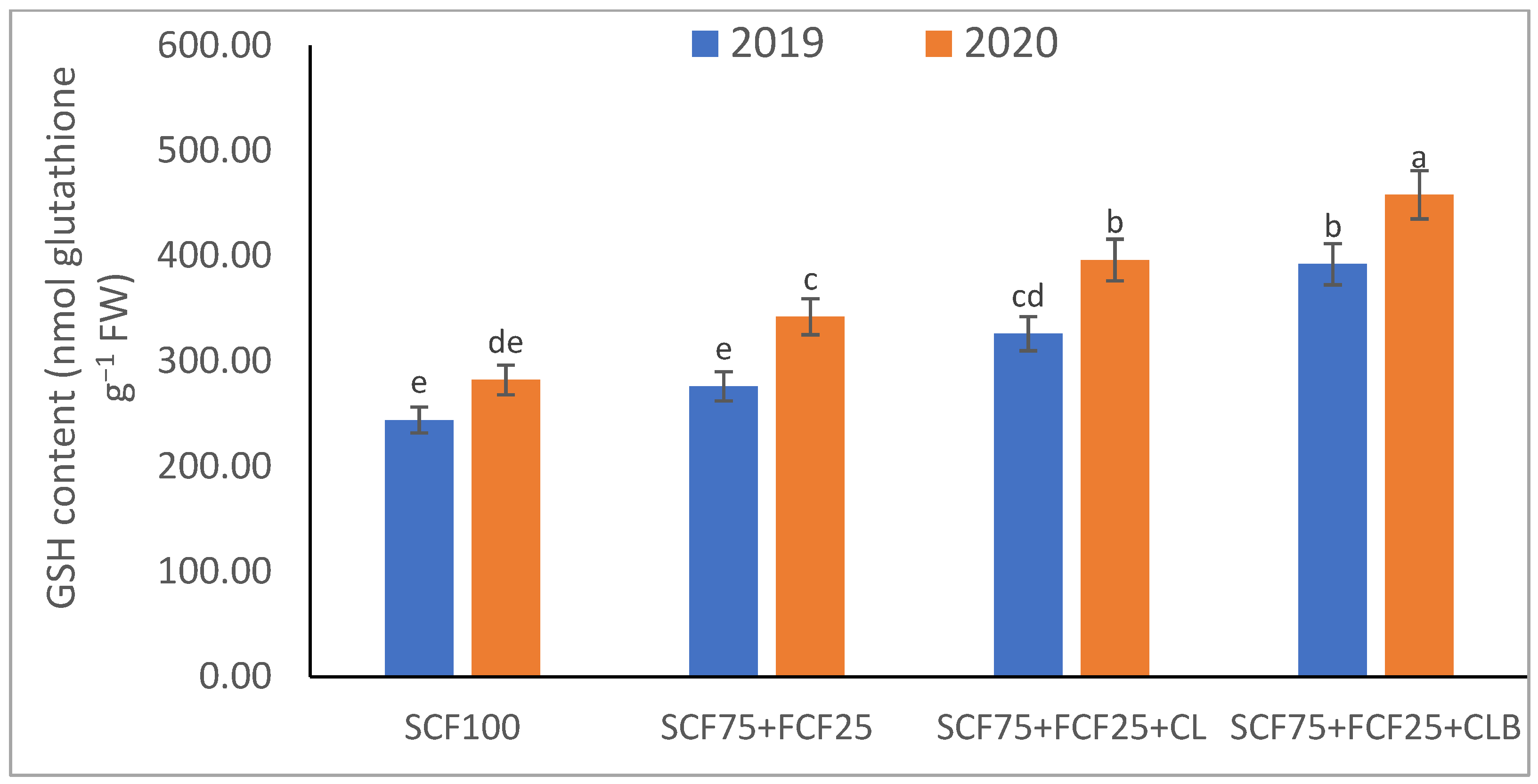
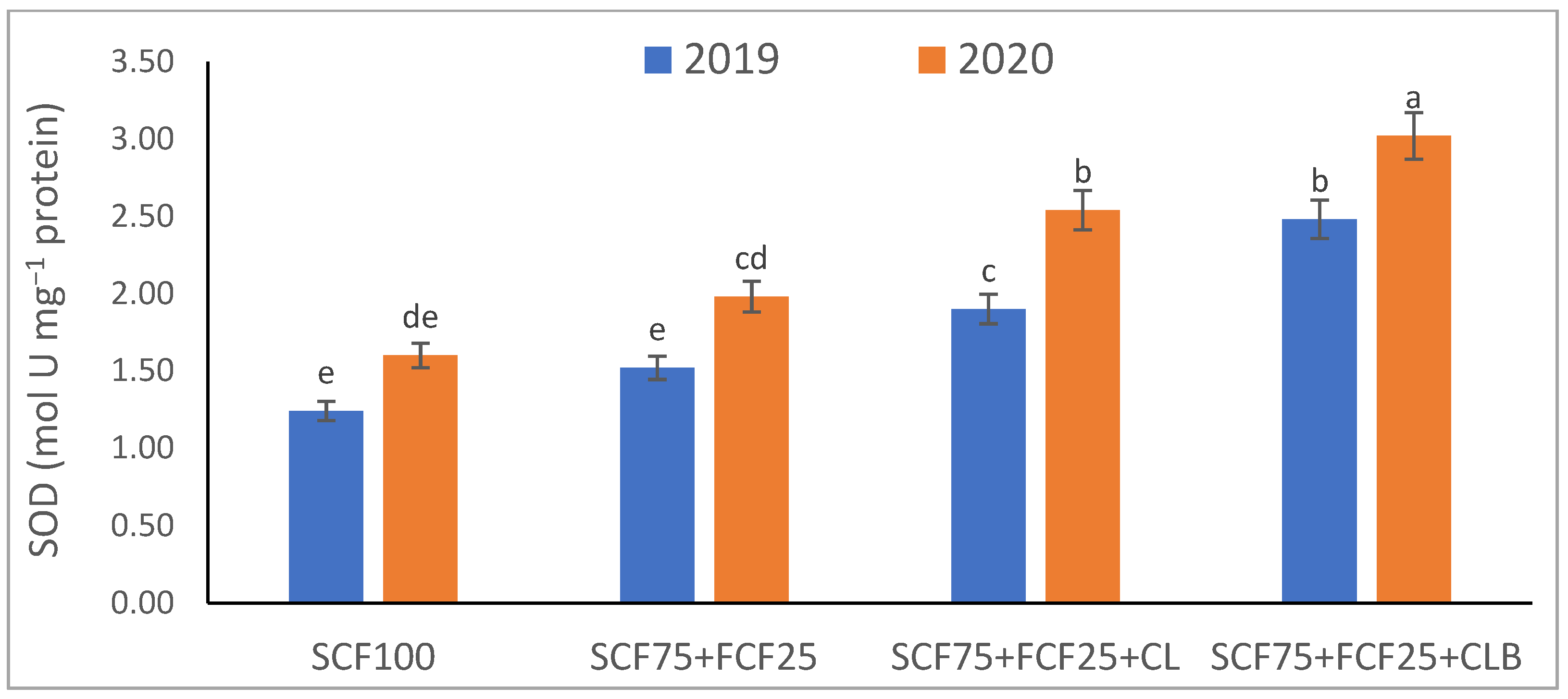
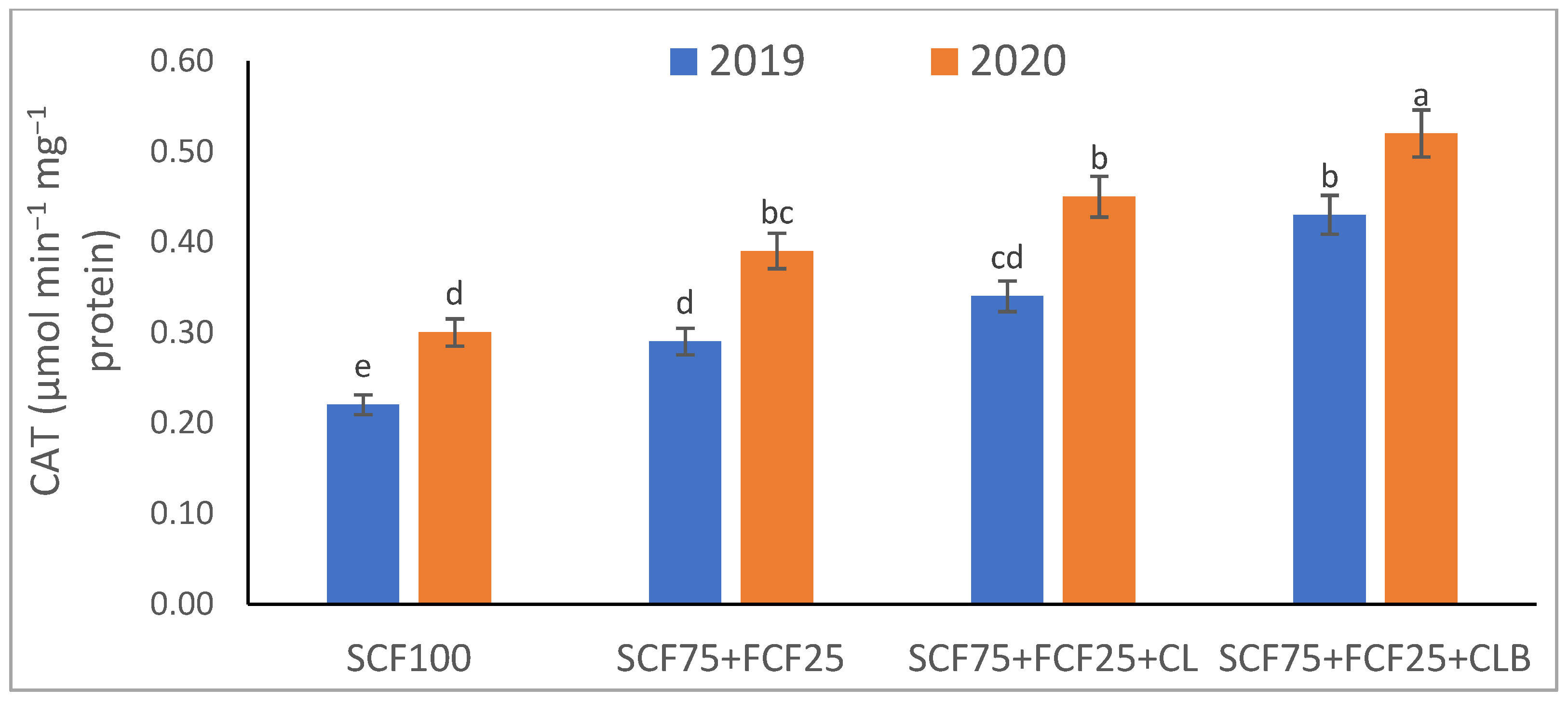
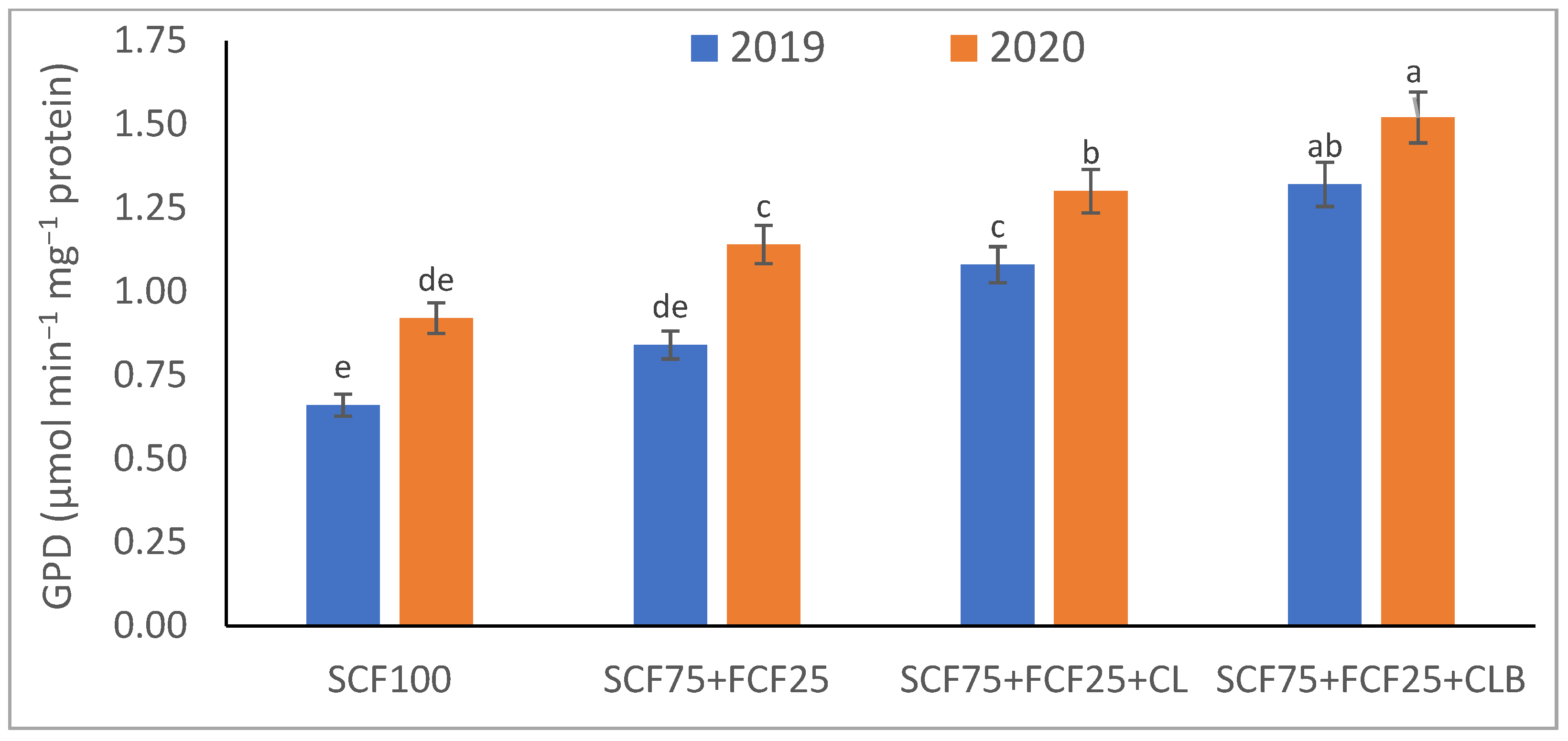

| Treatment | Description |
|---|---|
| 1. SCF100 | 100% of the recommended NPK doses were applied to the soil as a control. |
| 2. SCF75 + FCF25 | 75% of the recommended NPK doses were applied to the soil, +25% of the recommended NPK doses were applied as three foliar sprays (each spraying with the third of the 25%). |
| 3. SCF75 + FCF25 + CL | 75% of the recommended NPK doses were applied to the soil, +25% of the recommended NPK doses were applied as three foliar sprays (each spraying with the third of the 25%) + 1.2 kg leguminous compost m−2. |
| 4. SCF75 + FCF25 + CLB | 75% of the recommended NPK doses were applied to the soil, +25% of the recommended NPK doses were applied as three foliar sprays (each spraying with the third of the 25%) + 1.2 kg biocompost (leguminous compost containing bacteria; Bacillus subtilis) m−2. |
| Parameters | Properties | |||
|---|---|---|---|---|
| 2019 | 2020 | |||
| Before Sowing | After Harvest | Before Sowing | After Harvest | |
| Clay | 8.2 ± 0.6 * | 8.2 ± 0.5 | 8.2 ± 0.7 | 8.3 ± 0.8 |
| Silt | 10.7 ± 0.8 | 10.8 ± 0.8 | 10.8 ± 0.7 | 10.9 ± 1.0 |
| Sand | 81.1 ± 7.2 | 81.0 ± 6.9 | 81.0 ± 6.7 | 80.8 ± 6.2 |
| Soil texture | Loamy Sand | Loamy Sand | Loamy Sand | Loamy Sand |
| pH | 8.35 ± 0.51 | 8.12 ± 0.48 | 8.02 ± 0.42 | 7.82 ± 0.38 |
| EC (dS m−1) | 3.20 ± 0.24 | 3.24 ± 0.24 | 3.31 ± 0.26 | 3.44 ± 0.25 |
| CaCO3 (%) | 8.62 ± 0.54 | 8.52 ± 0.55 | 8.44 ± 0.50 | 8.10 ± 0.48 |
| Available nutrients (mg kg−1 soil) before sowing | ||||
| N | 12.7 ± 0.2 | 27.8 ± 0.5 | ||
| P | 7.6 ± 0.1 | 15.3 ± 0.3 | ||
| K | 16.4 ± 0.3 | 40.1 ± 0.8 | ||
| Source of Variation | Available Macronutrient Contents (mg kg−1 Soil) | ||
| Available N | Available P | Available K | |
| Season (S) | * | ns | * |
| 2019 | 30.3 ± 0.2 b | 17.5 ± 0.2 a | 37.5 ± 0.3 b |
| 2020 | 35.9 ± 0.3 a | 17.5 ± 0.2 a | 45.5 ± 0.4 a |
| Fertilization (F) | * | ** | ** |
| SCF100 | 27.5 ± 0.3 c | 11.8 ± 0.1 c | 34.3 ± 0.4 c |
| SCF75 + FCF25 | 27.1 ± 0.2 c | 11.5 ± 0.1 c | 33.4 ± 0.3 c |
| SCF75 + FCF25 + CL | 34.9 ± 0.3 b | 17.5 ± 0.2 b | 43.9 ± 0.4 b |
| SCF75 + FCF25 + CLB | 43.1 ± 0.4 a | 21.8 ± 0.2 a | 54.5 ± 0.4 a |
| S × F | * | ** | ** |
| 2019 × SCF100 | 25.6 ± 0.2 d | 10.4 ± 0.1 e | 31.2 ± 0.3 d |
| 2019 × SCF75 + FCF25 | 24.8 ± 0.2 d | 10.0 ± 0.1 e | 29.9 ± 0.3 d |
| 2019 × SCF75 + FCF25 + CL | 31.4 ± 0.2 c | 15.3 ± 0.1 c | 39.4 ± 0.3 c |
| 2019 × SCF75 + FCF25 + CLB | 39.5 ± 0.3 b | 19.4 ± 0.2 b | 49.3 ± 0.4 b |
| 2020 × SCF100 | 29.4 ± 0.3 c | 13.2 ± 0.1 d | 37.4 ± 0.4 c |
| 2020 × SCF75 + FCF25 | 29.3 ± 0.2 c | 12.9 ± 0.1 d | 36.8 ± 0.3 c |
| 2020 × SCF75 + FCF25 + CL | 38.4 ± 0.3 b | 19.6 ± 0.2 b | 48.3 ± 0.4 b |
| 2020 × SCF75 + FCF25 + CLB | 46.6 ± 0.4 a | 24.1 ± 0.2 a | 59.6 ± 0.4 a |
| Source of Variation | Available Micronutrient Contents (mg kg−1 Soil) | ||
| Available Fe | Available Mn | Available Zn | |
| Season (S) | * | * | * |
| 2019 | 4.61 ± 0.26 b | 3.13 ± 0.16 b | 2.22 ± 0.14 b |
| 2020 | 6.08 ± 0.37 a | 4.56 ± 0.24 a | 3.16 ± 0.16 a |
| Fertilization (F) | ** | * | * |
| SCF100 | 4.09 ± 0.25 c | 3.15 ± 0.16 c | 2.28 ± 0.13 c |
| SCF75 + FCF25 | 4.11 ± 0.27 c | 3.10 ± 0.16 c | 2.24 ± 0.11 c |
| SCF75 + FCF25 + CL | 5.70 ± 0.33 b | 4.06 ± 0.21 b | 2.80 ± 0.17 b |
| SCF75 + FCF25 + CLB | 7.47 ± 0.41 a | 5.08 ± 0.26 a | 3.44 ± 0.18 a |
| S × F | ** | * | * |
| 2019 × SCF100 | 3.52 ± 0.22 d | 2.28 ± 0.14 d | 1.74 ± 0.10 d |
| 2019 × SCF75 + FCF25 | 3.64 ± 0.23 d | 2.22 ± 0.12 d | 1.70 ± 0.08 d |
| 2019 × SCF75 + FCF25 + CL | 4.48 ± 0.25 c | 3.55 ± 0.16 c | 2.38 ± 0.18 c |
| 2019 × SCF75 + FCF25 + CLB | 6.79 ± 0.34 b | 4.48 ± 0.22 b | 3.04 ± 0.18 b |
| 2020 × SCF100 | 4.66 ± 0.27 c | 4.02 ± 0.18 bc | 2.82 ± 0.16 c |
| 2020 × SCF75 + FCF25 | 4.58 ± 0.31 c | 3.98 ± 0.20 bc | 2.78 ± 0.14 c |
| 2020 × SCF75 + FCF25 + CL | 6.92 ± 0.40 b | 4.57 ± 0.26 b | 3.21 ± 0.16 b |
| 2020 × SCF75 + FCF25 + CLB | 8.15 ± 0.48 a | 5.68 ± 0.30 a | 3.84 ± 0.18 a |
| Source of Variation | Growth Parameters | Yield Parameters | ||
|---|---|---|---|---|
| Shoot FW (g Plant−1) | Shoot DW (g Plant−1) | Green Pod Weight (ton ha−1) | Dry Seed Weight (ton ha−1) | |
| Season (S) | * | * | * | * |
| 2019 | 18.3 ± 1.7 b | 2.25 ± 2.1 b | 2.62 ± 0.28 b | 0.67 ± 0.07 b |
| 2020 | 22.1 ± 2.2 a | 2.60 ± 2.6 a | 3.00 ± 0.30 a | 0.86 ± 0.09 a |
| Fertilization (F) | ** | * | * | ** |
| SCF100 | 15.3 ± 1.4 d | 1.95 ± 2.1 d | 2.16 ± 0.23 d | 0.52 ± 0.06 d |
| SCF75 + FCF25 | 17.9 ± 1.9 c | 2.22 ± 2.1 c | 2.62 ± 0.26 c | 0.63 ± 0.07 c |
| SCF75 + FCF25 + CL | 21.6 ± 2.1 b | 2.59 ± 2.6 b | 3.07 ± 0.32 b | 0.83 ± 0.08 b |
| SCF75 + FCF25 + CLB | 26.1 ± 2.5 a | 2.95 ± 2.8 a | 3.39 ± 0.34 a | 1.09 ± 0.11 a |
| S × F | ** | * | * | ** |
| 2019 × SCF100 | 14.2 ± 1.3 e | 1.79 ± 1.8 e | 1.96 ± 0.21 e | 0.46 ± 0.05 e |
| 2019 × SCF75 + FCF25 | 16.6 ± 1.7 d | 2.09 ± 1.9 d | 2.41 ± 0.25 d | 0.55 ± 0.05 d |
| 2019 × SCF75 + FCF25 + CL | 19.0 ± 1.8 c | 2.36 ± 2.2 c | 2.90 ± 0.31 c | 0.72 ± 0.07 c |
| 2019 × SCF75 + FCF25 + CLB | 23.4 ± 2.1 b | 2.75 ± 2.5 b | 3.19 ± 0.34 b | 0.96 ± 0.10 b |
| 2020 × SCF100 | 16.4 ± 1.5 d | 2.11 ± 2.3 d | 2.36 ± 0.25 d | 0.57 ± 0.07 d |
| 2020 × SCF75 + FCF25 | 19.1 ± 2.0 c | 2.34 ± 2.3 c | 2.83 ± 0.27 c | 0.69 ± 0.08 c |
| 2020 × SCF75 + FCF25 + CL | 24.2 ± 2.3 b | 2.81 ± 2.9 b | 3.24 ± 0.32 b | 0.94 ± 0.08 b |
| 2020 × SCF75 + FCF25 + CLB | 28.7 ± 2.8 a | 3.14 ± 3.0 a | 3.58 ± 0.34 a | 1.22 ± 0.11 a |
| Source of Variation | Macronutrient Contents (mg g−1 DW) | Micronutrient Contents (mg kg−1 DW) | ||||
|---|---|---|---|---|---|---|
| N | P | K | Fe | Mn | Zn | |
| Season (S) | * | * | * | * | * | * |
| 2019 | 21.1 ± 0.5 b | 1.93 ± 0.05 b | 24.0 ± 0.6 b | 274 ± 14 b | 180 ± 7 b | 126 ± 4 b |
| 2020 | 25.3 ± 0.8 a | 2.42 ± 0.07 a | 27.8 ± 0.8 a | 322 ± 17 a | 223 ± 9 a | 151 ± 5 a |
| Fertilization (F) | * | ** | * | * | ** | ** |
| SCF100 | 16.9 ± 0.5 d | 1.47 ± 0.04 d | 19.4 ± 0.5 d | 221 ± 12 d | 144 ± 6 d | 100 ± 4 d |
| SCF75 + FCF25 | 20.9 ± 0.6 c | 1.89 ± 0.05 c | 23.6 ± 0.6 c | 265 ± 14 c | 176 ± 7 c | 127 ± 5 c |
| SCF75 + FCF25 + CL | 25.6 ± 0.7 b | 2.43 ± 0.07 b | 28.1 ± 0.7 b | 327 ± 17 b | 222 ± 9 b | 152 ± 5 b |
| SCF75 + FCF25 + CLB | 29.3 ± 0.8 a | 2.92 ± 0.09 a | 32.6 ± 0.9 a | 378 ± 21 a | 264 ± 10 a | 176 ± 6 a |
| S × F | * | ** | * | * | ** | ** |
| 2019 × SCF100 | 15.2 ± 0.4 e | 1.22 ± 0.03 e | 17.8 ± 0.3 e | 198 ± 10 e | 128 ± 5 e | 89 ± 3 e |
| 2019 × SCF75 + FCF25 | 18.4 ± 0.5 d | 1.68 ± 0.04 d | 21.1 ± 0.5 d | 239 ± 12 d | 156 ± 6 d | 112 ± 4 d |
| 2019 × SCF75 + FCF25 + CL | 23.1 ± 0.5 c | 2.14 ± 0.06 c | 26.4 ± 0.6 c | 298 ± 15 c | 192 ± 8 c | 136 ± 4 c |
| 2019 × SCF75 + FCF25 + CLB | 27.6 ± 0.6 b | 2.67 ± 0.08 b | 30.6 ± 0.8 b | 360 ± 20 b | 244 ± 9 b | 165 ± 5 b |
| 2020 × SCF100 | 18.6 ± 0.6 d | 1.72 ± 0.05 d | 20.9 ± 0.6 d | 244 ± 14 d | 160 ± 7 d | 110 ± 4 d |
| 2020 × SCF75 + FCF25 | 23.4 ± 0.7 c | 2.10 ± 0.05 c | 26.0 ± 0.6 c | 290 ± 16 c | 196 ± 7 c | 141 ± 5 c |
| 2020 × SCF75 + FCF25 + CL | 28.0 ± 0.9 b | 2.71 ± 0.07 b | 29.8 ± 0.8 b | 356 ± 18 b | 251 ± 9 b | 168 ± 5 b |
| 2020 × SCF75 + FCF25 + CLB | 31.0 ± 0.9 a | 3.16 ± 0.09 a | 34.6 ± 1.0 a | 396 ± 20 a | 284 ± 11 a | 186 ± 7 a |
| Source of Variation | Photosynthetic Parameters | Leaf Relative Water Content (RWC) and Osmoprotectant Contents | ||||
|---|---|---|---|---|---|---|
| TChl Content (g kg‒1 FW) | Fv/Fm | PI (%) | RWC (%) | Proline (μg g−1 DW) | TS Sugars (mg g−1 DW) | |
| Season (S) | * | * | * | * | * | * |
| 2019 | 1.76 ± 0.03 b | 0.77 ± 0.02 b | 13.7 ± 0.3 b | 65.0 ± 2.3 b | 160 ± 5 b | 18.0 ± 0.4 b |
| 2020 | 2.14 ± 0.04 a | 0.83 ± 0.03 a | 15.9 ± 0.4 a | 73.8 ± 2.5 a | 185 ± 6 a | 22.4 ± 0.5 a |
| Fertilization (F) | ** | * | * | * | ** | ** |
| SCF100 | 1.30 ± 0.03 d | 0.71 ± 0.02 d | 11.5 ± 0.3 d | 56.7 ± 2.1 d | 136 ± 4 d | 14.2 ± 0.4 d |
| SCF75 + FCF25 | 1.69 ± 0.03 c | 0.76 ± 0.02 c | 13.4 ± 0.4 c | 64.5 ± 2.3 c | 163 ± 5 c | 18.0 ± 0.5 c |
| SCF75 + FCF25 + CL | 2.17 ± 0.04 b | 0.83 ± 0.03 b | 15.9 ± 0.4 b | 74.0 ± 2.5 b | 183 ± 7 b | 22.1 ± 0.5 b |
| SCF75 + FCF25 + CLB | 2.65 ± 0.05 a | 0.89 ± 0.03 a | 18.5 ± 0.4 a | 82.3 ± 2.9 a | 205 ± 7 a | 26.4 ± 0.6 a |
| S × F | ** | * | * | * | ** | ** |
| 2019 × SCF100 | 1.12 ± 0.02 e | 0.68 ± 0.01 e | 10.4 ± 0.2 f | 52.4 ± 1.9 d | 125 ± 3 d | 12.4 ± 0.3 e |
| 2019 × SCF75 + FCF25 | 1.52 ± 0.02 d | 0.73 ± 0.02 d | 12.4 ± 0.3 e | 60.2 ± 2.1 c | 148 ± 4 d | 15.8 ± 0.4 d |
| 2019 × SCF75 + FCF25 + CL | 1.97 ± 0.03 c | 0.79 ± 0.02 c | 14.9 ± 0.3 c | 69.6 ± 2.4 b | 176 ± 6 c | 19.6 ± 0.4 c |
| 2019 × SCF75 + FCF25 + CLB | 2.44 ± 0.04 b | 0.86 ± 0.02 b | 17.2 ± 0.4 b | 77.8 ± 2.9 a | 192 ± 6 bc | 24.0 ± 0.5 b |
| 2020 × SCF100 | 1.48 ± 0.03 de | 0.74 ± 0.02 d | 12.6 ± 0.3 de | 61.0 ± 2.2 c | 147 ± 5 d | 16.0 ± 0.4 d |
| 2020 × SCF75 + FCF25 | 1.86 ± 0.03 cd | 0.79 ± 0.02 c | 14.4 ± 0.4 cd | 68.8 ± 2.5 b | 178 ± 5 c | 20.2 ± 0.5 c |
| 2020 × SCF75 + FCF25 + CL | 2.37 ± 0.04 b | 0.87 ± 0.03 b | 16.9 ± 0.4 b | 78.4 ± 2.6 a | 196 ± 7 ab | 24.6 ± 0.5 b |
| 2020 × SCF75 + FCF25 + CLB | 2.86 ± 0.05 a | 0.92 ± 0.03 a | 19.8 ± 0.4 a | 86.8 ± 2.8 a | 218 ± 7 a | 28.8 ± 0.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelfattah, M.A.; Rady, M.M.; Belal, H.E.E.; Belal, E.E.; Al-Qthanin, R.; Al-Yasi, H.M.; Ali, E.F. Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance. Plants 2021, 10, 1637. https://doi.org/10.3390/plants10081637
Abdelfattah MA, Rady MM, Belal HEE, Belal EE, Al-Qthanin R, Al-Yasi HM, Ali EF. Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance. Plants. 2021; 10(8):1637. https://doi.org/10.3390/plants10081637
Chicago/Turabian StyleAbdelfattah, Mahmoud A., Mostafa M. Rady, Hussein E. E. Belal, Eman E. Belal, Rahmah Al-Qthanin, Hatim M. Al-Yasi, and Esmat F. Ali. 2021. "Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance" Plants 10, no. 8: 1637. https://doi.org/10.3390/plants10081637
APA StyleAbdelfattah, M. A., Rady, M. M., Belal, H. E. E., Belal, E. E., Al-Qthanin, R., Al-Yasi, H. M., & Ali, E. F. (2021). Revitalizing Fertility of Nutrient-Deficient Virgin Sandy Soil Using Leguminous Biocompost Boosts Phaseolus vulgaris Performance. Plants, 10(8), 1637. https://doi.org/10.3390/plants10081637







