Detecting Introgressed Populations in the Iberian Endemic Centaurea podospermifolia through Genome Size
Abstract
:1. Introduction
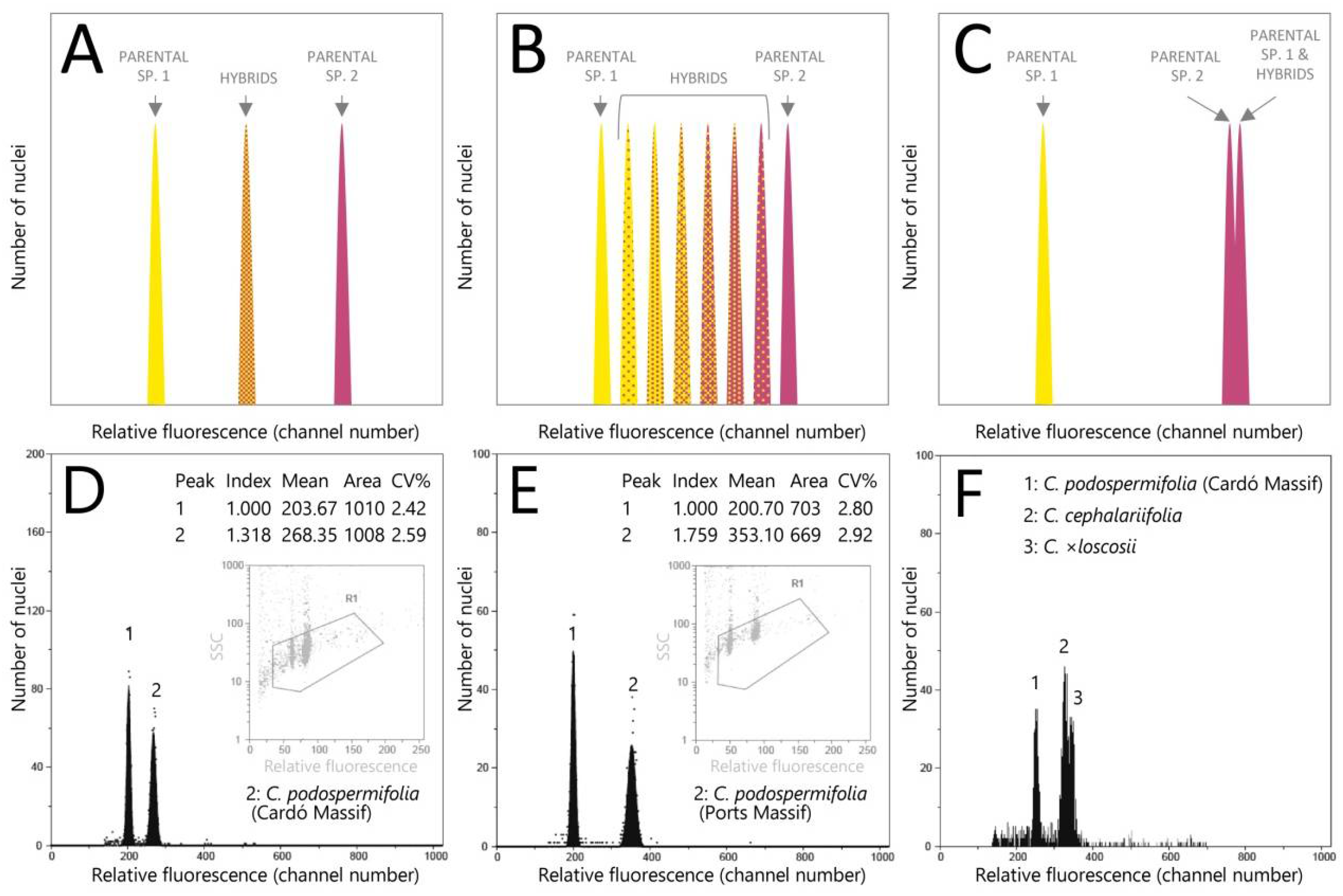
2. Results
3. Discussion
3.1. Genome Size Enables Discrimination between Populations of C. podospermifolia
3.2. Interspecific Hybridisation and Introgression: Impact on Genome Size
4. Materials and Methods
4.1. Plant Sampling
4.2. Flow Cytometry Measurements
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López-Pujol, J.; Garcia-Jacas, N.; Susanna, A.; Vilatersana, R. Should we conserve pure species or hybrid species? Delimiting hybridization and introgression in the Iberian endemic Centaurea podospermifolia. Biol. Conserv. 2012, 152, 271–279. [Google Scholar] [CrossRef]
- Fernández Casas, F.J.; Susanna, A. Monografía de la Sección Chamaecyanus Willk. del género Centaurea L.; Consejo Superior de Investigaciones Científicas, Instituto Botánico de Barcelona: Barcelona, Spain, 1985; Volume 10, ISBN 0210-8062. [Google Scholar]
- DARPAMN RESOLUCIÓ AAM/732/2015, de 9 D’abril, per la qual S’aprova la Catalogació, Descatalogació i Canvi de Categoria D’espècies i Subespècies del Catàleg de Flora Amenaçada de Catalunya. D. Of. General. Catalunya 2015, 6854: CVE-DOGC-A-15106031. 2015. Available online: https://www.conservacionvegetal.org/wp-content/uploads/legislaciones/121/Cataluña2015.pdf (accessed on 17 March 2021).
- Whitney, K.D.; Ahern, J.R.; Campbell, L.G.; Albert, L.P.; King, M.S. Patterns of hybridization in plants. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 175–182. [Google Scholar] [CrossRef]
- Soltis, P.S.; Soltis, D.E. The Role of Hybridization in Plant Speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerbs, B.; Ressler, J.; Kelly, J.K.; Mort, M.E.; Santos-Guerra, A.; Gibson, M.J.S.; Caujapé-Castells, J.; Crawford, D.J. The potential role of hybridization in diversification and speciation in an insular plant lineage: Insights from synthetic interspecific hybrids. AoB Plants 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, S.J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, P.-C.; Twyford, A.D.; Sun, S.-S.; Wang, H.-Y.; Xia, M.-Z.; Tan, C.-X.; Zhou, X.-J.; Chen, S.-L. Recurrent hybridization underlies the evolution of novelty in Gentiana (Gentianaceae) in the Qinghai-Tibetan Plateau. AoB Plants 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Clermont, S.; Houston, L.; Rich, T.C.G.; Fay, M.F. Cytotype diversity in the Sorbus complex (Rosaceae) in Britain: Sorting out the puzzle. Ann. Bot. 2012, 110, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Rosato, M.; Castro, M.; Rosselló, J.A. Relationships of the woody Medicago species (Section Dendrotelis) assessed by molecular cytogenetic analyses. Ann. Bot. 2008, 102, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakimowski, S.B.; Rieseberg, L.H. The role of homoploid hybridization in evolution: A century of studies synthesizing genetics and ecology. Am. J. Bot. 2014, 101, 1247–1258. [Google Scholar] [CrossRef]
- Nieto Feliner, G.; Álvarez, I.; Fuertes-Aguilar, J.; Heuertz, M.; Marques, I.; Moharrek, F.; Piñeiro, R.; Riina, R.; Rosselló, J.A.; Soltis, P.S.; et al. Is homoploid hybrid speciation that rare? An empiricist’s view. Heredity 2017, 118, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Żabicka, J.; Migdałek, G.; Słomka, A.; Sliwinska, E.; Mackiewicz, L.; Keczyński, A.; Kuta, E. Interspecific hybridization and introgression influence biodiversity-based on genetic diversity of Central European Viola epipsila-V. palustris complex. Diversity 2020, 12, 321. [Google Scholar] [CrossRef]
- Levin, J.; Fay, M.F.; Pellicer, J.; Hedrén, M. Multiple independent origins of intermediate species between Sorbus aucuparia and S. hybrida (Rosaceae) in the Baltic region. Nord. J. Bot. 2018, 36. [Google Scholar] [CrossRef]
- Rice, A.; Glick, L.; Abadi, S.; Einhorn, M.; Kopelman, N.M.; Salman-Minkov, A.; Mayzel, J.; Chay, O.; Mayrose, I. The Chromosome Counts Database (CCDB)—a community resource of plant chromosome numbers. New Phytol. 2015, 206, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Font Quer, P. Flórula de Cardó; Talleres Tipográficos Mariano Clavé: Barcelona, Spain, 1950. [Google Scholar]
- Folch, R. La flora de les comarques litorals compreses entre la Riera d’Alforja i el riu Ebre; Institut d’Estudis Catalans. Arxius de la Secció de Ciènces LX: Barcelona, Spain, 1980; ISBN 84-7283-028-4. [Google Scholar]
- Aparicio Rojo, J.M. Centaurea scabiosa L. subsp. scabiosa (num. 3850). In Atlas Corològic de la Flora Vascular dels Països Catalans; Font i Castell, X., Vigo i Bonada, J., Eds.; Institut d’Estudis Catalans: Barcelona, Spain, 2008; Volume 15, ISBN 978-84-92583-25-6. [Google Scholar]
- Thompson, J.D.; Gauthier, P.; Papuga, G.; Pons, V.; Debussche, M.; Farris, E. The conservation significance of natural hybridisation in Mediterranean plants: From a case study on Cyclamen (Primulaceae) to a general perspective. Plant Biol. 2018, 20, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Kelly, L.J.; McAllister, H.A.; Zohren, J.; Buggs, R.J.A. Resolving phylogeny and polyploid parentage using genus-wide genome-wide sequence data from birch trees. Mol. Phylogenet. Evol. 2021, 160, 107126. [Google Scholar] [CrossRef]
- Font, M.; Garcia-Jacas, N.; Vilatersana, R.; Roquet, C.; Susanna, A. Evolution and biogeography of Centaurea section Acrocentron inferred from nuclear and plastid DNA sequence analyses. Ann. Bot. 2009, 103, 985–997. [Google Scholar] [CrossRef]
- Gross, B.L.; Rieseberg, L.H. The ecological genetics of homoploid hybrid speciation. J. Hered. 2005, 96, 241–252. [Google Scholar] [CrossRef]
- Marques, I.; Nieto Feliner, G.; Martins-Loução, M.A.; Fuertes Aguilar, J. Fitness in Narcissus hybrids: Low fertility is overcome by early hybrid vigour, absence of exogenous selection and high bulb propagation. J. Ecol. 2011, 99, 1508–1519. [Google Scholar] [CrossRef]
- Draper, D.; Laguna, E.; Marques, I. Demystifying negative connotations of hybridization for less biased conservation policies. Front. Ecol. Evol. 2021, 9, 268. [Google Scholar] [CrossRef]
- McCann, J.; Jang, T.-S.; Macas, J.; Schneeweiss, G.M.; Matzke, N.J.; Novák, P.; Stuessy, T.F.; Villaseñor, J.L.; Weiss-Schneeweiss, H. Dating the species network: Allopolyploidy and repetitive DNA evolution in American daisies (Melampodium sect. Melampodium, Asteraceae). Syst. Biol. 2018, 67, 1010–1024. [Google Scholar] [CrossRef]
- Glombik, M.; Bačovský, V.; Hobza, R.; Kopecký, D. Competition of parental genomes in plant hybrids. Front. Plant Sci. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Quero-García, J.; Letourmy, P.; Ivancic, A.; Feldmann, P.; Courtois, B.; Noyer, J.L.; Lebot, V. Hybrid performance in taro (Colocasia esculenta) in relation to genetic dissimilarity of parents. Theor. Appl. Genet. 2009, 119, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Senerchia, N.; Felber, F.; Parisod, C. Contrasting evolutionary trajectories of multiple retrotransposons following independent allopolyploidy in wild wheats. New Phytol. 2014, 202, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Nieto Feliner, G.; Rosato, M.; Alegre, G.; San Segundo, P.; Rosselló, J.A.; Garnatje, T.; Garcia, S. Dissimilar molecular and morphological patterns in an introgressed peripheral population of a sand dune species (Armeria pungens, Plumbaginaceae). Plant Biol. 2019, 21, 1072–1082. [Google Scholar] [CrossRef]
- Balao, F.; Casimiro-Soriguer, R.; García-Castaño, J.L.; Terrab, A.; Talavera, S. Big thistle eats the little thistle: Does unidirectional introgressive hybridization endanger the conservation of Onopordum hinojense? New Phytol. 2015, 206, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridizarion and introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Ungerer, M.C.; Strakosh, S.C.; Zhen, Y. Genome expansion in three hybrid sunflower species is associated with retrotransposon proliferation. Curr. Biol. 2006, 16, R872–R873. [Google Scholar] [CrossRef] [Green Version]
- Baack, E.J.; Whitney, K.D.; Rieseberg, L.H. Hybridization and genome size evolution: Timing and magnitude of nuclear DNA content increases in Helianthus homoploid hybrid species. New Phytol. 2005, 167, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Janes, J.K.; Hamilton, J.A. Mixing it up: The role of hybridization in forest management and conservation under climate change. Forests 2017, 8, 237. [Google Scholar] [CrossRef] [Green Version]
- Pellicer, J.; Powell, R.F.; Leitch, I.J. The Application of flow cytometry for estimating genome size, ploidy level, endopolyploidy, and reproductive modes in plants BT. In Molecular Plant Taxonomy: Methods and Protocols; Besse, P., Ed.; Springer: New York, NY, USA, 2021; pp. 325–361. ISBN 978-1-0716-0997-2. [Google Scholar]
- Doležel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Hidalgo, O.; Pellicer, J.; Liu, H.; Marquardt, J.; Robert, Y.; Christenhusz, M.; Zhang, S.; Gibby, M.; Leitch, I.J.; et al. Genome evolution of ferns: Evidence for relative stasis of genome size across the fern phylogeny. New Phytol. 2016, 210, 1072–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayer, R.; Leitch, I.J.; Hanson, L.; Bennett, M.D. Nuclear DNA C-values in 30 species double the familial representation in pteridophytes. Ann. Bot. 2002, 90, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loureiro, J.; Rodriguez, E.; Doležel, J.; Santos, C. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]

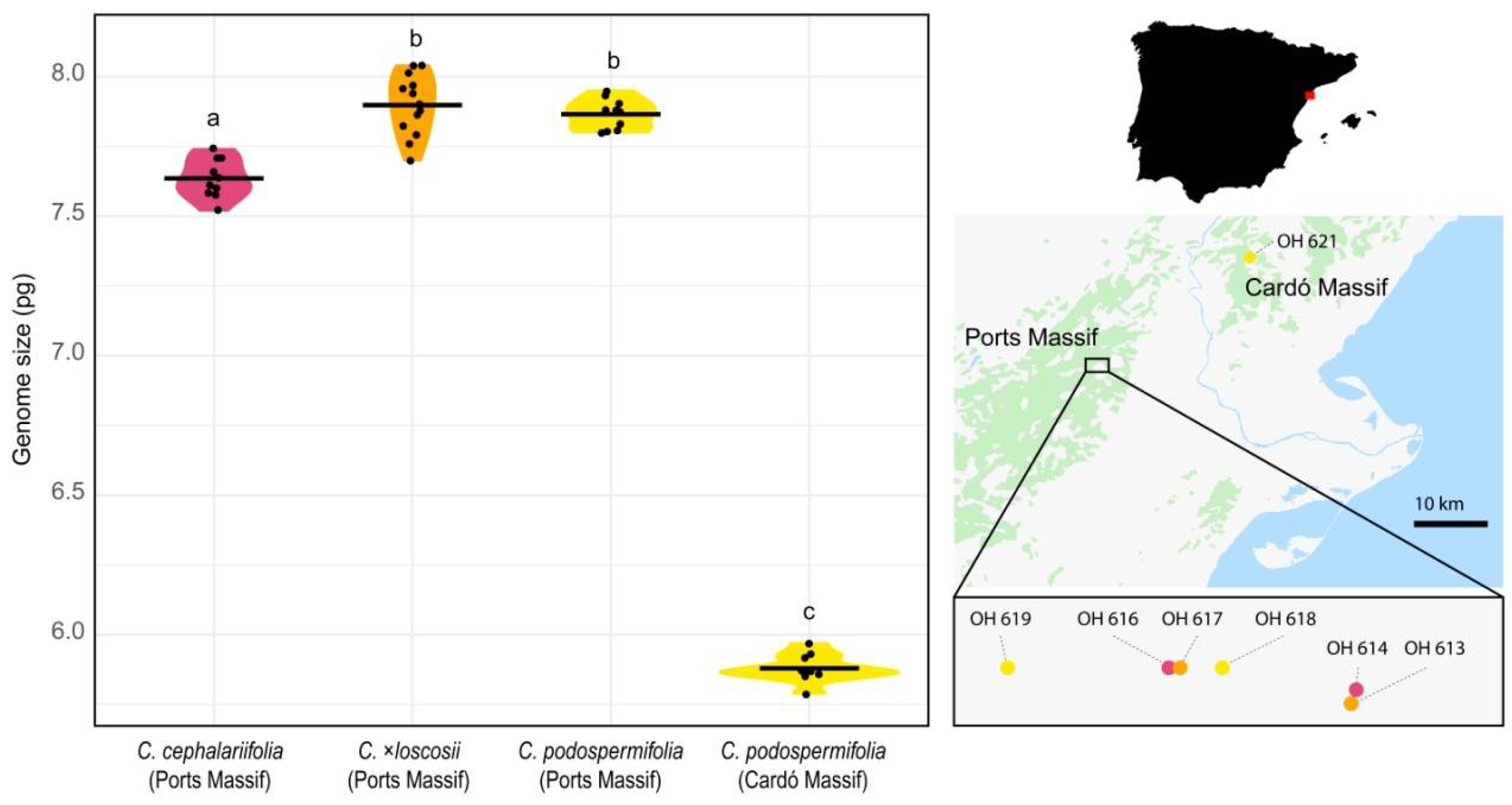
| Massif | Centaurea Species (Population) | 2C in pg (SD) 1 | N 2 | CVplt 3 | CVstd 4 | Geographical Coordinates |
|---|---|---|---|---|---|---|
| Ports | C. cephalariifolia (OH 614) | 7.66 (0.05) | 5 | 3.21 | 2.86 | 40°48′25″ N, 0°20′15″ E |
| C. cephalariifolia (OH 616) | 7.61 (0.08) | 5 | 2.51 | 2.35 | 40°48′28″ N, 0°19′40″ E | |
| C. ×loscosii (OH 613) | 7.94 (0.11) | 8 | 2.79 | 2.61 | 40°48′23″ N, 0°20′14″ E | |
| C. ×loscosii (OH 617) | 7.83 (0.06) | 5 | 2.67 | 2.64 | 40°48′28″ N, 0°19′42″ E | |
| C. podospermifolia (OH 618) | 7.85 (0.06) | 5 | 2.70 | 2.85 | 40°48′40″ N, 0°19′50″ E | |
| C. podospermifolia (OH 619) | 7.88 (0.04) | 5 | 2.92 | 2.87 | 40°48′28″ N, 0°19′10″ E | |
| Cardó | C. podospermifolia (OH 621) | 5.88 (0.05) | 10 | 2.91 | 2.84 | 40°56′28″ N, 0°35′06″ E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellicer, J.; López-Pujol, J.; Aixarch, M.; Garnatje, T.; Vallès, J.; Hidalgo, O. Detecting Introgressed Populations in the Iberian Endemic Centaurea podospermifolia through Genome Size. Plants 2021, 10, 1492. https://doi.org/10.3390/plants10081492
Pellicer J, López-Pujol J, Aixarch M, Garnatje T, Vallès J, Hidalgo O. Detecting Introgressed Populations in the Iberian Endemic Centaurea podospermifolia through Genome Size. Plants. 2021; 10(8):1492. https://doi.org/10.3390/plants10081492
Chicago/Turabian StylePellicer, Jaume, Jordi López-Pujol, Marc Aixarch, Teresa Garnatje, Joan Vallès, and Oriane Hidalgo. 2021. "Detecting Introgressed Populations in the Iberian Endemic Centaurea podospermifolia through Genome Size" Plants 10, no. 8: 1492. https://doi.org/10.3390/plants10081492
APA StylePellicer, J., López-Pujol, J., Aixarch, M., Garnatje, T., Vallès, J., & Hidalgo, O. (2021). Detecting Introgressed Populations in the Iberian Endemic Centaurea podospermifolia through Genome Size. Plants, 10(8), 1492. https://doi.org/10.3390/plants10081492









