Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis
Abstract
1. Introduction
2. Results and Discussion
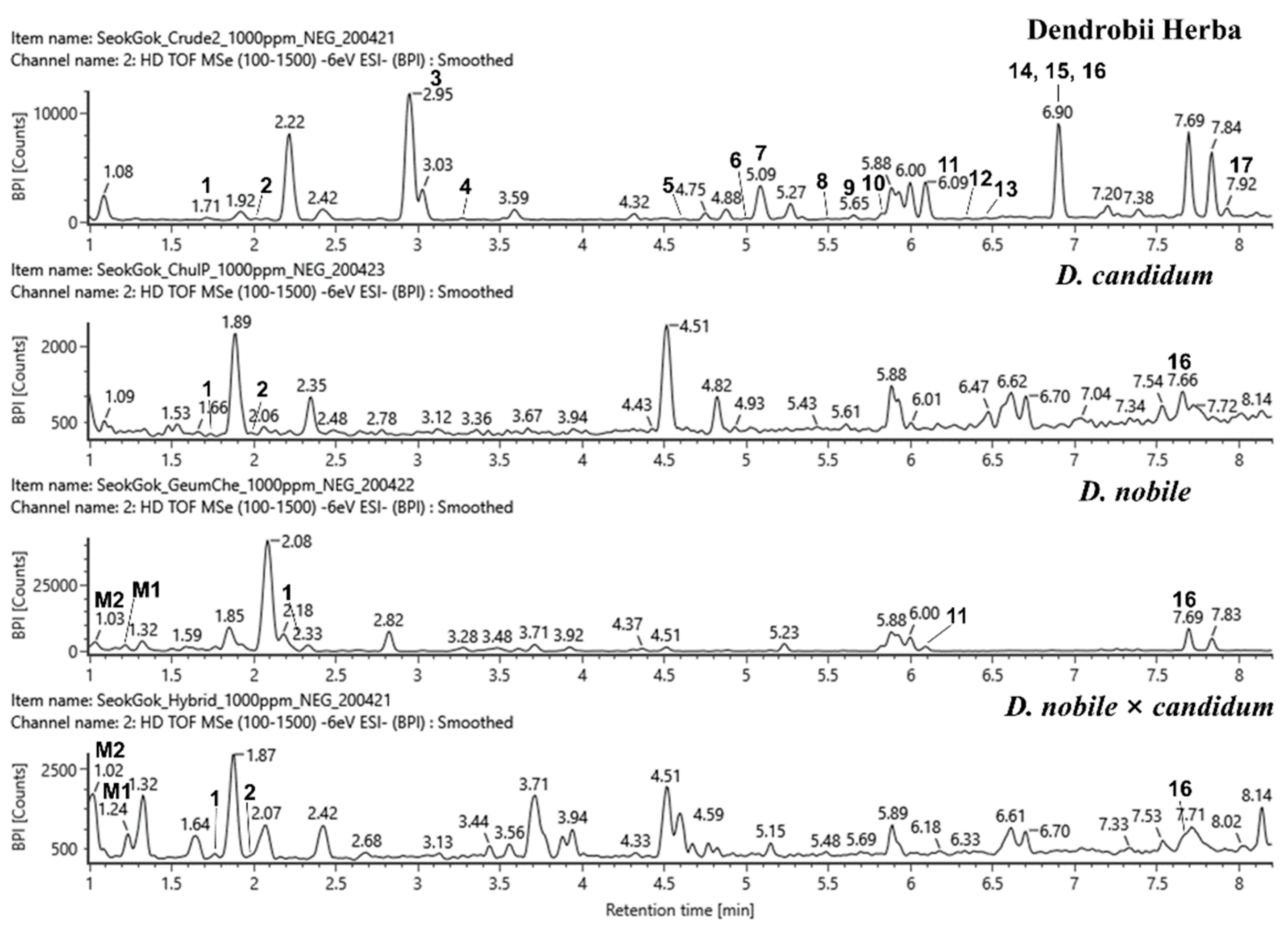
2.1. Identification of the Compounds in Dendrobium Samples and Their Cytotoxic Activities
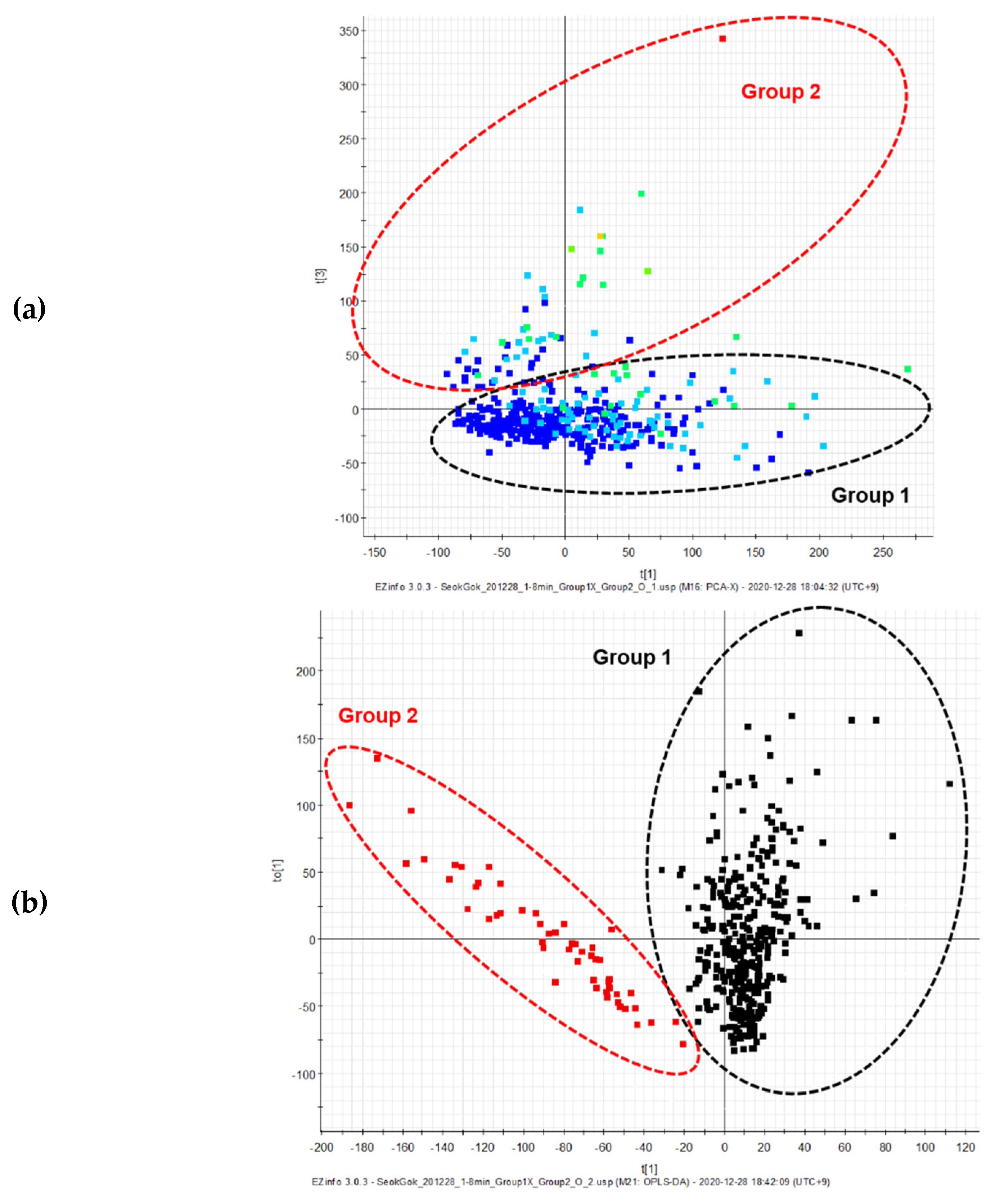
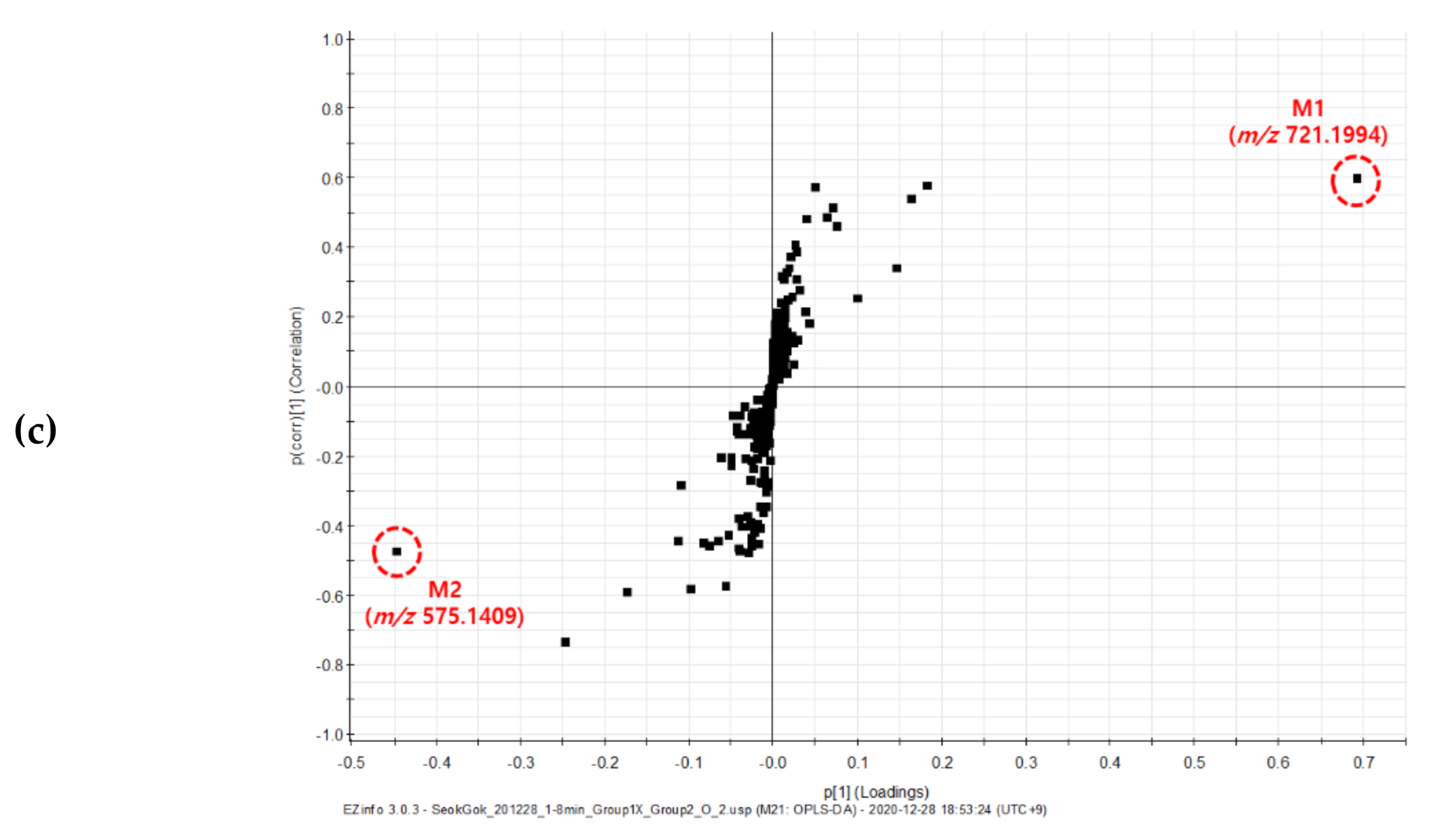
2.2. Untargeted UPLC-QToF MS Analysis of Gamma-Irradiated Mutant Lines of D. Nobile × Candidum
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Sample Preparation
3.4. UPLC-QTof MS Analysis
3.5. Cytotoxicity Assay
3.6. Chemometric Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, Y.; Ng, T.B.; Yao, R.M.; Shi, J.; Xu, K.; Sze, S.; Zhang, K.Y. Evaluation of chemical constituents and important mechanism of pharmacological biology in Dendrobium plants. Evid. Based Complement. Alternat. Med. 2015, 2015, 841752. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, X.; Wu, L.; Jiang, X.; Huang, L. Quality evaluation of Dendrobium based on ultra-performance liquid chromatography (UPLC) and chemometrics. J. Appl. Pharm. Sci. 2017, 7, 17–23. [Google Scholar] [CrossRef][Green Version]
- Ng, T.B.; Liu, J.; Wong, J.H.; Ye, X.; Sze, S.C.W.; Tong, Y.; Zhang, K.Y. Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biot. 2012, 93, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-S.; Lu, Y.-L.; Nie, J.; Xu, Y.-Y.; Zhang, W.; Yang, W.-J.; Gong, Q.-H.; Lu, Y.-F.; Lu, Y.; Shi, J.-S. Dendrobium nobile Lindl alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Aβ25-35 in hippocampus neurons in vitro. CNS Neurosci. Ther 2017, 23, 329–340. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, Q.; Wu, Q.; Shi, J. Neuroprotective effects of Dendrobium alkaloids on rat cortical neurons injured by oxygen-glucose deprivation and reperfusion. Phytomedicine 2010, 17, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.-W.; He, Y.-L.; Peng, C.; Ding, X.-J.; Guo, L.; Xiong, L. Picrotoxane sesquiterpenoids from the stems of Dendrobium nobile and their absolute configurations and angiogenesis effect. Fitoterapia 2017, 121, 206–211. [Google Scholar] [CrossRef]
- Morita, H.; Fujiwara, M.; Yoshida, N.; Kobayashi, J. New picrotoxinin-type and dendrobine-type sesquiterpenoids from Dendrobium snowflake ‘Red Star’. Tetrahedron 2000, 56, 5801–5805. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, C.; Yang, H.; Zhang, M.; Wang, Z.; Xu, L. Two new alkaloids from Dendrobium chrysanthum. Heterocycles-Sendai Inst. Heterocycl. Chem. 2005, 65, 633–636. [Google Scholar]
- Liu, G.-Y.; Tan, L.; Cheng, L.; Ding, L.-S.; Zhou, Y.; Deng, Y.; He, Y.-Q.; Guo, D.-L.; Xiao, S.-J. Dendrobine-type alkaloids and bibenzyl derivatives from Dendrobium findlayanum. Fitoterapia 2020, 142, 104497. [Google Scholar] [CrossRef]
- Song, T.-H.; Chen, X.-X.; Lee, C.K.-F.; Sze, S.C.-W.; Feng, Y.-B.; Yang, Z.-J.; Chen, H.-Y.; Li, S.-T.; Zhang, L.-Y.; Wei, G. Dendrobine targeting JNK stress signaling to sensitize chemotoxicity of cisplatin against non-small cell lung cancer cells in vitro and in vivo. Phytomedicine 2019, 53, 18–27. [Google Scholar] [CrossRef]
- Charoenrungruang, S.; Chanvorachote, P.; Sritularak, B.; Pongrakhananon, V. Gigantol, a bibenzyl from Dendrobium draconis, inhibits the migratory behavior of non-small cell lung cancer cells. J. Nat. Prod. 2014, 77, 1359–1366. [Google Scholar] [CrossRef]
- Bhummaphan, N.; Pongrakhananon, V.; Sritularak, B.; Chanvorachote, P. Cancer stem cell–suppressing activity of chrysotoxine, a bibenzyl from Dendrobium pulchellum. J. Pharmacol. Exp. Ther. 2018, 364, 332–346. [Google Scholar] [CrossRef]
- Pinkhien, T.; Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Batatasin III inhibits migration of human lung cancer cells by suppressing epithelial to mesenchymal transition and FAK-AKT signals. Anticancer Res. 2017, 37, 6281–6289. [Google Scholar]
- Chanvorachote, P.; Kowitdamrong, A.; Ruanghirun, T.; Sritularak, B.; Mungmee, C.; Likhitwitayawuid, K. Anti-metastatic activities of bibenzyls from Dendrobium pulchellum. Nat. Prod. Commun. 2013, 8, 1934578X1300800127. [Google Scholar] [CrossRef]
- Pai, H.-C.; Chang, L.-H.; Peng, C.-Y.; Chang, Y.-L.; Chen, C.-C.; Shen, C.-C.; Teng, C.-M.; Pan, S.-L. Moscatilin inhibits migration and metastasis of human breast cancer MDA-MB-231 cells through inhibition of Akt and Twist signaling pathway. J. Mol. Med. 2013, 91, 347–356. [Google Scholar] [CrossRef]
- Gong, Y.-Q.; Fan, Y.; Wu, D.-Z.; Yang, H.; Hu, Z.-B.; Wang, Z.-T. In vivo and in vitro evaluation of erianin, a novel anti-angiogenic agent. Eur. J. Cancer. 2004, 40, 1554–1565. [Google Scholar] [CrossRef]
- Lee, E.; Han, A.-R.; Nam, B.; Kim, Y.-R.; Jin, C.H.; Kim, J.-B.; Eun, Y.-G.; Jung, C.-H. Moscatilin induces apoptosis in human head and neck squamous cell carcinoma cells via JNK signaling pathway. Molecules. 2020, 25, 901. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.-K.; Wang, J.; Wang, N.-L.; Kurihara, H.; Kitanaka, S.; Yao, X.-S. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J. Nat. Prod. 2007, 70, 24–28. [Google Scholar] [CrossRef]
- Zhou, X.-M.; Zheng, C.-J.; Gan, L.-S.; Chen, G.-Y.; Zhang, X.-P.; Song, X.-P.; Li, G.-N.; Sun, C.-G. Bioactive phenanthrene and bibenzyl derivatives from the stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Yang, D.; Liu, L.-Y.; Cheng, Z.-Q.; Xu, F.-Q.; Fan, W.-W.; Zi, C.-T.; Dong, F.-W.; Zhou, J.; Ding, Z.-T.; Hu, J.-M. Five new phenolic compounds from Dendrobium aphyllum. Fitoterapia 2015, 100, 11–18. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.-K.; Wang, N.-L.; Kurihara, H.; Yao, X. Antioxidant phenanthrenes and lignans from Dendrobium nobile. J. Chin. Pharm. Sci. 2008, 17, 314–318. [Google Scholar]
- Kim, J.H.; Oh, S.-Y.; Han, S.-B.; Uddin, G.M.; Kim, C.Y.; Lee, J.K. Anti-inflammatory effects of Dendrobium nobile derived phenanthrenes in LPS-stimulated murine macrophages. Arch. Pharmacal Res. 2015, 38, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, S.A.; Hong, S.S.; Han, X.H.; Lee, C.; Kang, S.J.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K. Phenanthrenes from Dendrobium nobile and their inhibition of the LPS-induced production of nitric oxide in macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2010, 20, 3785–3787. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Matsuzaki, K.; Wang, J.; Daikonya, A.; Wang, N.-L.; Yao, X.-S.; Kitanaka, S. New phenanthrenes and stilbenes from Dendrobium loddigesii. Chem. Pharm. Bull. 2010, 58, 628–633. [Google Scholar] [CrossRef]
- Bhummaphan, N.; Petpiroon, N.; Prakhongcheep, O.; Sritularak, B.; Chanvorachote, P. Lusianthridin targeting of lung cancer stem cells via Src-STAT3 suppression. Phytomedicine 2019, 62, 152932. [Google Scholar] [CrossRef]
- Yang, H.; Sung, S.H.; Kim, Y.C. Antifibrotic phenanthrenes of Dendrobium nobile stems. J. Nat. Prod. 2007, 70, 1925–1929. [Google Scholar] [CrossRef]
- Wattanathamsan, O.; Treesuwan, S.; Sritularak, B.; Pongrakhananon, V. Cypripedin, a phenanthrenequinone from Dendrobium densiflorum, sensitizes non-small cell lung cancer H460 cells to cisplatin-mediated apoptosis. J. Nat. Med. 2018, 72, 503–513. [Google Scholar] [CrossRef]
- Zhao, G.-Y.; Deng, B.-W.; Zhang, C.-Y.; Cui, Y.-D.; Bi, J.-Y.; Zhang, G.-G. New phenanthrene and 9, 10-dihydrophenanthrene derivatives from the stems of Dendrobium officinale with their cytotoxic activities. J. Nat. Med. 2018, 72, 246–251. [Google Scholar] [CrossRef]
- Chen, X.-J.; Mei, W.-L.; Zuo, W.-J.; Xeng, J.-B.; Guo, Z.-K.; Song, X.-Q.; Dai, H.-F. A new antibacterial phenanthrenequinone from Dendrobium sinense. J. Asian Nat. Prod. Res. 2013, 15, 67–70. [Google Scholar] [CrossRef]
- Zhang, G.-N.; Zhong, L.-Y.; Bligh, S.A.; Guo, Y.-L.; Zhang, C.-F.; Zhang, M.; Wang, Z.-T.; Xu, L.-S. Bi-bicyclic and bi-tricyclic compounds from Dendrobium thyrsiflorum. Phytochemistry 2005, 66, 1113–1120. [Google Scholar] [CrossRef]
- Kyokong, N.; Muangnoi, C.; Thaweesest, W.; Kongkatitham, V.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A new phenanthrene dimer from Dendrobium palpebrae. J. Asian Nat. Prod. Res. 2019, 21, 391–397. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, H.-W.; Gao, H.; Han, H.-Y.; Wang, N.-L.; Wu, H.-M.; Yao, X.-S.; Wang, Z. Nine new sesquiterpenes from Dendrobium nobile. Helv. Chim. Acta 2007, 90, 2386–2394. [Google Scholar] [CrossRef]
- Zhao, W.; Ye, Q.; Dai, J.; Martin, M.-T.; Zhu, J. Allo-aromadendrane-and picrotoxane-type sesquiterpenes from Dendrobium moniliforme. Planta Med. 2003, 69, 1136–1140. [Google Scholar]
- Park, B.-Y.; Oh, S.-R.; Ahn, K.-S.; Kwon, O.-K.; Lee, H.-K. (–)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis. Int. Immunopharmacol. 2008, 8, 967–973. [Google Scholar] [CrossRef]
- Zhou, C.; Xie, Z.; Lei, Z.; Huang, Y.; Wei, G. Simultaneous identification and determination of flavonoids in Dendrobium officinale. Chem. Cent. J. 2018, 12, 40. [Google Scholar] [CrossRef]
- Poudel, M.R.; Chand, M.B.; Karki, N.; Pant, B. Antioxidant activity and total phenolic and flavonoid contents of Dendrobium amoenum Wall. ex Lindl. Bot. Orient. J. Plant. Sci. 2015, 9, 20–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Son, Y.-O.; Kim, S.-S.; Jang, Y.-S.; Lee, J.-C. Antioxidant and anti-hyperglycemic activity of polysaccharide isolated from Dendrobium chrysotoxum Lindl. J. Biochem. Mol. Biol. 2007, 40, 670–677. [Google Scholar] [CrossRef]
- Liang, J.; Wu, Y.; Yuan, H.; Yang, Y.; Xiong, Q.; Liang, C.; Li, Z.; Li, C.; Zhang, G.; Lai, X. Dendrobium officinale polysaccharides attenuate learning and memory disabilities via anti-oxidant and anti-inflammatory actions. Int. J. Biol. Macromol. 2019, 126, 414–426. [Google Scholar] [CrossRef]
- Xia, L.; Liu, X.; Guo, H.; Zhang, H.; Zhu, J.; Ren, F. Partial characterization and immunomodulatory activity of polysaccharides from the stem of Dendrobium officinale (Tiepishihu) in vitro. J. Funct. Foods 2012, 4, 294–301. [Google Scholar] [CrossRef]
- Nam, B.; Ryu, S.M.; Lee, D.; Jung, C.-H.; Jin, C.H.; Kim, J.-B.; Lee, I.-S.; Han, A.-R. Identification of two new phenanthrenes from Dendrobii Herba and their cytotoxicity towards human hypopharynx squamous carcinoma cell (FaDu). Molecules 2019, 24, 2339. [Google Scholar] [CrossRef]
- Ali, H.; Ghori, Z.; Sheikh, S.; Gul, A. Effects of gamma radiation on crop production. In Crop Production and Global Environmental Issues; Hakeem, K., Ed.; Springer: Cham, Switzerland, 2016; pp. 27–78. [Google Scholar]
- Jankowicz-Cieslak, J.; Mba, C.; Till, B.J. Mutagenesis for crop breeding and functional genomics. In Biotechnologies for Plant Mutation Breeding; Jankowicz-Cieslak, J., Tai, T., Kumlehn, J., Till, B., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]
- Jo, Y.D.; Kim, Y.-S.; Ryu, J.; Choi, H.-I.; Kim, S.W.; Kang, H.S.; Ahn, J.-W.; Kim, J.-B.; Kang, S.-Y.; Kim, S.H. Deletion of carotenoid cleavage dioxygenase 4a (CmCCD4a) and global up-regulation of plastid protein-coding genes in a mutant chrysanthemum cultivar producing yellow petals. Sci. Hortic. 2016, 212, 49–59. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, D.Y.; Nam, B.; Ahn, J.-W.; Kwon, S.-J.; Seo, Y.W.; Kim, J.-B. Characterization of novel mutants of hexaploid wheat (Triticum aestivum L.) with various depths of purple grain color and antioxidant capacity. J. Sci. Food Agric. 2019, 99, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, X.; Wang, H.-Y.; Yang, J.; Kuang, Y.; Ji, K.-Y.; Yang, Y.; Pang, K.; Yang, S.-Z.; Qin, J.-X.; et al. Comparison of metabolomics of Dendrobium officinale in different habitats by UPLC-Q-TOF-MS. Biochem. Syst. Ecol. 2020, 89, 104007. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Avula, B.; Abe, N.; Wei, F.; Wang, M.; Ma, S.-C.; Ali, Z.; Elsohly, M.A.; Khan, I.A. Tandem Mass Spectrometry for Structural Identification of Sesquiterpene Alkaloids from the Stems of Dendrobium nobile Using LC-QToF. Planta Med. 2016, 82, 662–670. [Google Scholar] [CrossRef]
- Tao, Y.; Cai, H.; Li, W.; Cai, B. Ultrafiltration coupled with high-performance liquid chromatography and quadrupole-time-of-flight mass spectrometry for screening lipase binders from different extracts of Dendrobium officinale. Anal. Bioanal. Chem. 2015, 407, 6081–6093. [Google Scholar] [CrossRef]
- Zha, X.-Q.; Luo, J.-P.; Wei, P. Identification and classification of Dendrobium candidum species by fingerprint technology with capillary electrophoresis. S. Afr. J. Bot. 2009, 75, 276–282. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.-L.; Guo, S.-X.; Yang, J.-S.; Xiao, P.-G. Two new compounds from Dendrobium candidum. Chem. Pharm. Bull. 2008, 56, 1477–1479. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Wang, L.-Y.; Huang, B.; Xie, X.-L.; Wu, Q.; Shi, J.-S. Comparison of contents of polysaccharides and alkaloids in Dendrobium from different harvest time. Chin. Pharm. J. 2014, 29, 288–291. [Google Scholar]
- Chen, H.; Li, X.; Xu, Y.; Lo, K.; Zheng, H.; Hu, H.; Wang, J.; Lin, Y. Study on the polar extracts of Dendrobium nobile, D. officinale, D. loddigesii, and Flickingeria fimbriata: Metabolite identification, content evaluation, and bioactivity assay. Molecules 2018, 23, 1185. [Google Scholar] [CrossRef]
- Song, J.I.; Kang, Y.J.; Yong, H.; Kim, Y.C.; Moon, A. Denbinobin, a phenanthrene from Dendrobium nobile, inhibits invasion and induces apoptosis in SNU-484 human gastric cancer cells. Oncol. Rep. 2012, 27, 813–818. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Xin, W.; Liu, N.; Zhang, H. Nudol, a phenanthrene derivative from Dendrobium nobile, induces cell cycle arrest and apoptosis and inhibits migration in osteosarcoma cells. Drug Des Dev. Ther. 2019, 13, 2591–2601. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Y.; Fu, X.; Wang, Y.; Liu, Y.; Huo, B.; Sheng, J.; Hu, X. Dendrobium candidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers. OncoTargets Ther. 2016, 9, 21–30. [Google Scholar]
- Rahman, S.; Haq, F.U.; Ali, A.; Khan, M.N.; Shah, S.M.Z.; Adhikhari, A.; El-Seedi, H.R.; Musharraf, S.G. Combining untargeted and targeted metabolomics approaches for the standardization of polyherbal formulations through UPLC–MS/MS. Metabolomics 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Q.; Liu, Y.-L.; Hou, C.-Y.; Mabry, T.J. An acylated flavone C-glycoside from Glycyrrhiza eurycarpa. Phytochemistry 1994, 36, 1089–1090. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Keller, W.J.; Judd, R.L.; Bates, S.; Day, C. Hypoglycaemic activity of an HMG-containing flavonoid glucoside, chamaemeloside, from Chamaemelum nobile. Planta Med. 1998, 64, 612–614. [Google Scholar] [CrossRef]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Grdadolnik, S.G.; Markovic, D.; Sankovic-Babice, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar]
- Czemplik, M.; Mierziak, J.; Szopa, J.; Kulma, A. Flavonoid C-glucosides derived from Flax straw extracts reduce human breast cancer cell growth in vitro and induce apoptosis. Front. Pharmacol. 2016, 31, 282. [Google Scholar] [CrossRef] [PubMed]

| Peak No. | Identification | tR (min) | Observed m/z (Da) | Calculated m/z (Da) | Error (ppm) | Molecular Formula | Fragments |
|---|---|---|---|---|---|---|---|
| 1 | Gigantol | 1.73 | 275.12688 [M + H]+ | 275.1278 | −2.14 | C16H18O4 | 198 |
| 2 | (1R,2R)-1,7-Hydroxy-2,8-methoxy-2,3-dihydrophenanthrene-4(1H)-one | 1.98 | 287.09214 [M − H]− | 287.0914 | 4.44 | C16H14O5 | 272, 239 |
| 3 | Tristin | 2.98 | 259.09822 [M − H]‒ | 259.0965 | 5.17 | C15H16O4 | 243 |
| 4 | (-)-Syringaresinol | 3.32 | 417.15652 [M − H]‒ | 417.1555 | 5.21 | C22H26O8 | 387, 190 |
| 5 | Lusianthridin | 4.69 | 241.08710 [M − H]− | 241.087 | −4.67 | C15H14O3 | 116 |
| 6 | 2,7-Dihydroxy-phenanthrene-1,4-dione | 5.02 | 239.03168 [M − H]− | 239.0339 | −9.26 | C14H8O4 | 256 |
| 7 | Densiflorol B | 5.07 | 253.05075 [M − H]− | 253.0495 | 4.11 | C15H10O4 | 238 |
| 8 | Denthyrsinin | 5.49 | 299.09974 [M − H]− | 299.0925 | 22.73 | C17H16O5 | 284, 254 |
| 9 | Moscatilin | 5.63 | 305.13779 [M + H]+ | 305.1384 | −3.52 | C17H20O5 | 181 |
| 10 | Lusianthridin dimer | 5.84 | 481.16393 [M − H]‒ | 481.1657 | −4.67 | C30H26O6 | 116 |
| 11 | Batatasin III | 6.05 | 243.11115 [M − H]‒ | 243.1027 | 31.18 | C15H16O3 | - |
| 12 | Ephemeranthol A | 6.31 | 273.11093 [M + H]+ | 273.1121 | 5.21 | C16H16O4 | 272, 241, 213 |
| 13 | Thunalbene | 6.43 | 241.08710 [M − H]‒ | 241.087 | 1.33 | C15H14O3 | - |
| 14 | Dehydroorchinol | 7.7 | 255.10002 [M + H]+ | 255.1016 | −2.21 | C16H14O3 | 240 |
| 15 | Dendrobine | 7.7 | 264.19496 [M + H]+ | 264.1958 | −2.03 | C16H25NO2 | - |
| 16 | Shihunine | 7.7 | 203.09751 [M]+ | 203.0952 | 11.49 | C12H13NO2 | 405, 203 |
| 17 | 1,5,7-Trimethoxy-2-phenanthrenol | 7.97 | 285.11172 [M + H]+ | 285.1121 | −5.40 | C17H16O4 | 253, 225 |
| Samples | Cytotoxicity 1 (IC50, μg/mL) | Peak No. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | ||
| Dendrobii Herba | 63.03 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| D. candidum | 84.47 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
| D. nobile | 89.22 | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | + | − |
| D. nobile × candidum | 91.93 | + | + | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, B.; Jang, H.-J.; Han, A.-R.; Kim, Y.-R.; Jin, C.-H.; Jung, C.-H.; Kang, K.-B.; Kim, S.-H.; Hong, M.-J.; Kim, J.-B.; et al. Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis. Plants 2021, 10, 1376. https://doi.org/10.3390/plants10071376
Nam B, Jang H-J, Han A-R, Kim Y-R, Jin C-H, Jung C-H, Kang K-B, Kim S-H, Hong M-J, Kim J-B, et al. Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis. Plants. 2021; 10(7):1376. https://doi.org/10.3390/plants10071376
Chicago/Turabian StyleNam, Bomi, Hyun-Jae Jang, Ah-Reum Han, Ye-Ram Kim, Chang-Hyun Jin, Chan-Hun Jung, Kyo-Bin Kang, Sang-Hoon Kim, Min-Jeong Hong, Jin-Baek Kim, and et al. 2021. "Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis" Plants 10, no. 7: 1376. https://doi.org/10.3390/plants10071376
APA StyleNam, B., Jang, H.-J., Han, A.-R., Kim, Y.-R., Jin, C.-H., Jung, C.-H., Kang, K.-B., Kim, S.-H., Hong, M.-J., Kim, J.-B., & Ryu, H.-W. (2021). Chemical and Biological Profiles of Dendrobium in Two Different Species, Their Hybrid, and Gamma-Irradiated Mutant Lines of the Hybrid Based on LC-QToF MS and Cytotoxicity Analysis. Plants, 10(7), 1376. https://doi.org/10.3390/plants10071376







