Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds
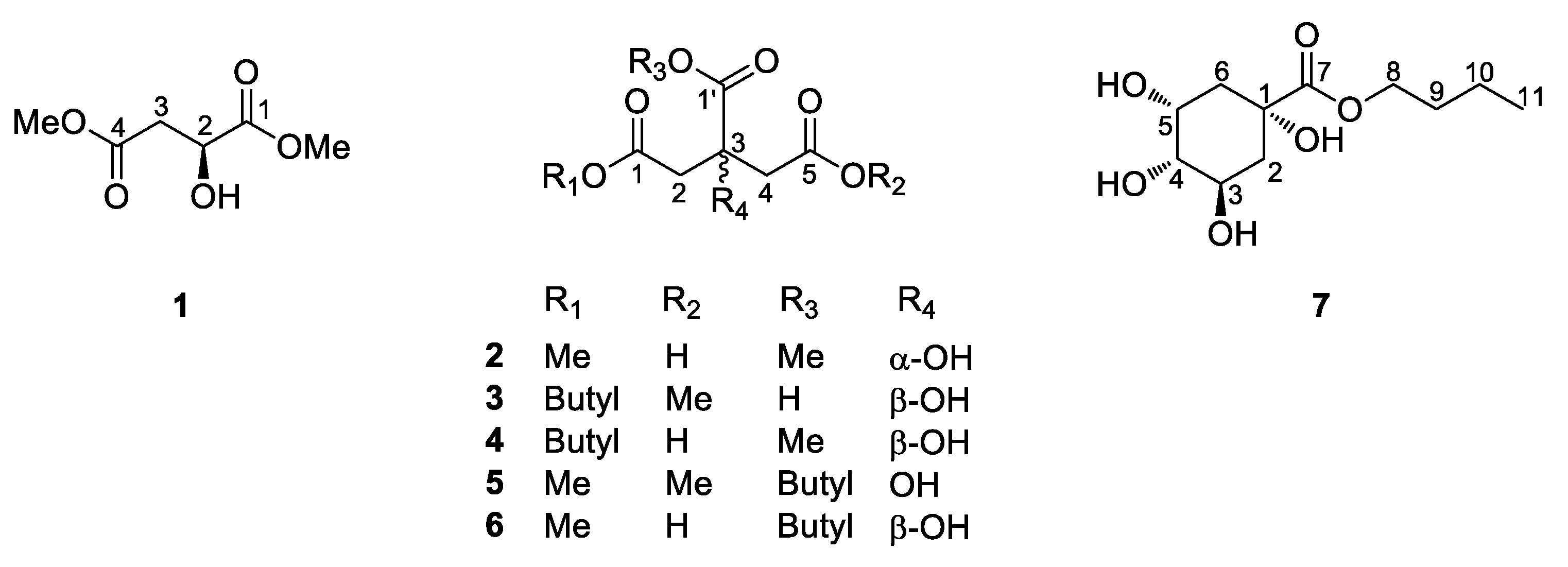
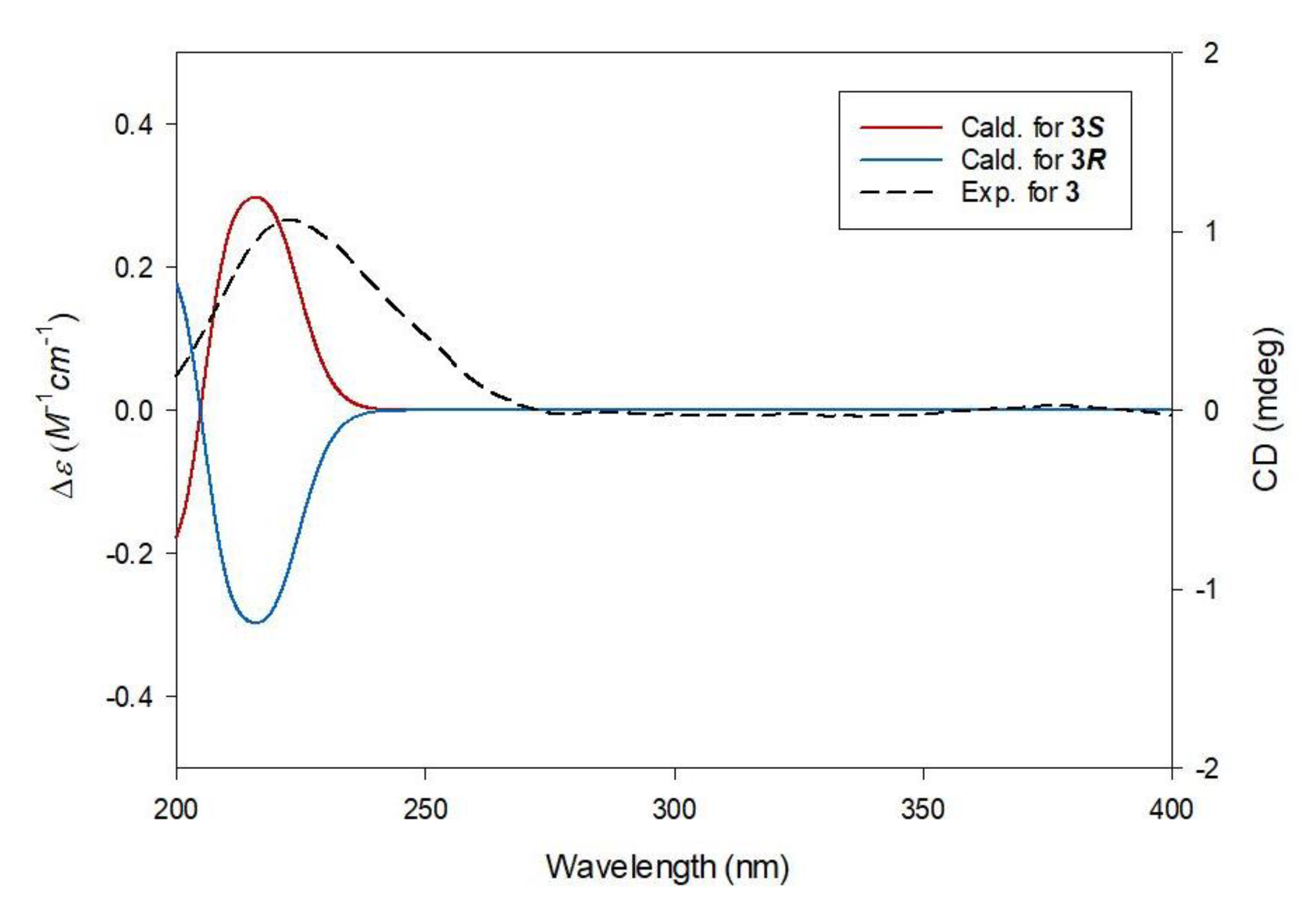
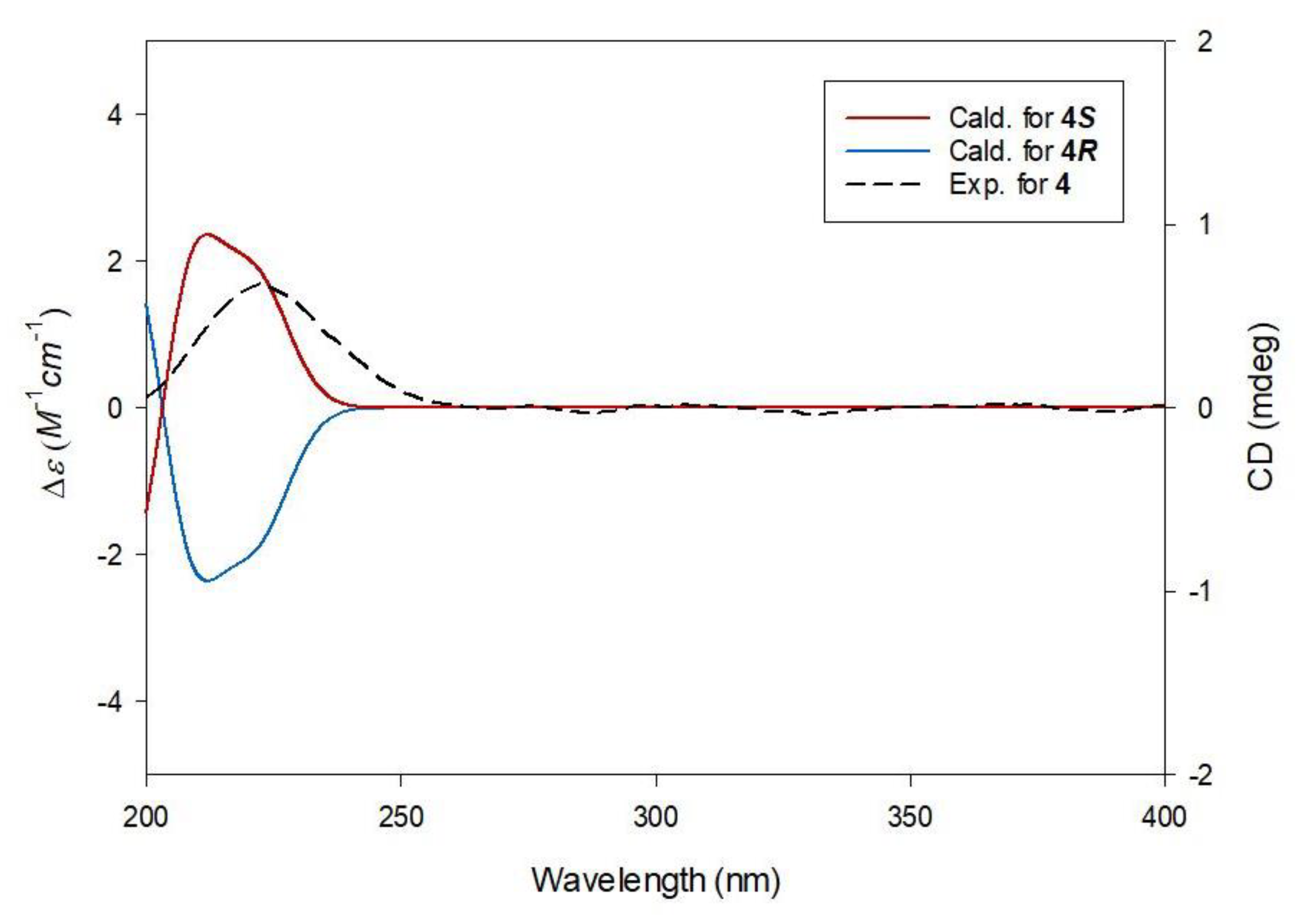
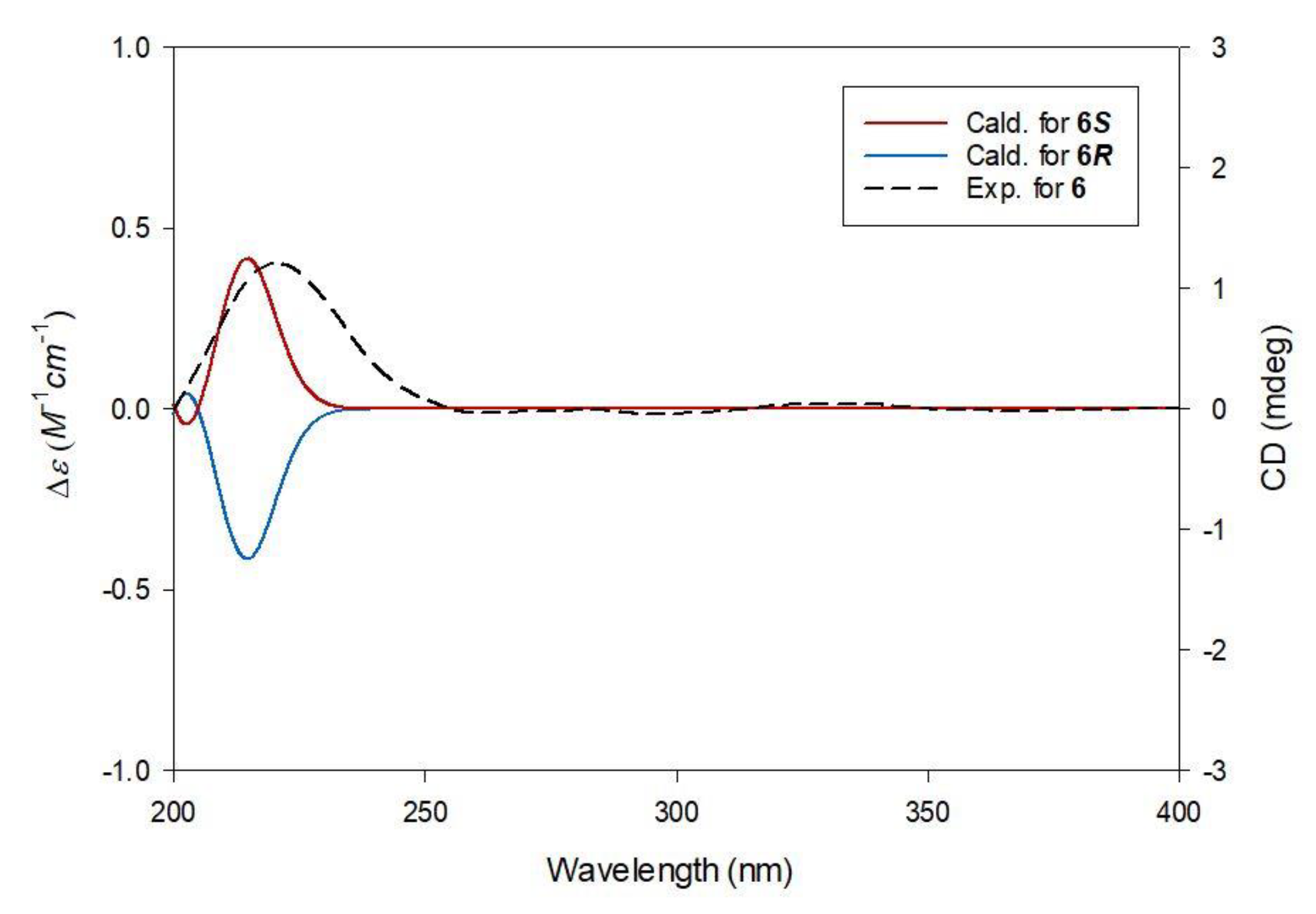
2.2. Elucidation of Compound Structures
2.3. Evaluation of Biological Activities of Compounds 1–7
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.3.1. (S)-1-Butyl-5-Methyl Citrate (3)
3.3.2. (S)-1-Butyl-1′-Methyl Citrate (4)
3.3.3. (S)-1-Methyl-1′-Butyl Citrate (6)
3.4. Computational Analysis
3.5. Cell Culture and Differentiation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mingyu, X.; Xiaoxuan, S.; Jinhua, C. The medicinal research and development of seabuckthorn. J. Water Soil Conserv. 1991, 1–11. [Google Scholar]
- Panossian, A.; Wagner, H. From traditional to evidence-based use of Hippophae rhamnoides L.: Chemical composition, experimental, and clinical pharmacology of sea buckthorn berries and leaves extracts. In Evidence and Rational Based Research on Chinese Drugs; Springer: Vienna, Austria, 2013; pp. 181–236. [Google Scholar]
- Watanabe, T.; Rajbhanddari, K.R.; Malla, K.J.; Yahara, S. A handbook of medicinal plants of Nepal. Banko Janakari 2005, 15, 106–107. [Google Scholar]
- Chauhan, A.S.; Negi, P.S.; Ramteke, R.S. Antioxidant and antibacterial activities of aqueous extract of Seabuckthorn (Hippophae rhamnoides) seeds. Fitoterapia 2007, 78, 590–592. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef]
- Li, T.S.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. HortTechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Skalski, B.; Kontek, B.; Rolnik, A.; Olas, B.; Stochmal, A.; Żuchowski, J. Anti-platelet properties of phenolic extracts from the leaves and twigs of Elaeagnus rhamnoides (L.) A. Nelson. Molecules 2019, 24, 3620. [Google Scholar] [CrossRef]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) A. Nelson, extracts as virulence modulators-in vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1498. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.; Li, T.S.; Oomah, B.D.; Smith, A. Sea buckthorn products: Manufacture and composition. J. Agri. Food Chem. 1999, 47, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kallio, H.P. Fatty acid composition of lipids in sea buckthorn (Hippophaë rhamnoides L.) berries of different origins. J. Agri. Food Chem. 2001, 49, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Pintea, A.; Dugo, P.; Torre, G.; Pop, R.M.; Mondello, L. Determination of Carotenoids and their Esters in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochem. Anal. 2012, 23, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.X.; Xu, X.D.; Tian, Z.; Yang, J.S. Chemical constituents from the fruits of Hippophae rhamnoides. Nat. Prod. Res. 2009, 23, 1451–1456. [Google Scholar] [CrossRef]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a Sterol Possessing a 6/5/6/5-Fused Ring System with a Contracted Tetrahydrofuran B-Ring, from the Fruiting Bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, H.S.; Yoo, M.J.; Yi, S.A.; Beemelmanns, C.; Lee, J.C.; Kim, K.H. Anti-adipogenic Pregnane Steroid from a Hydractinia-associated Fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharm. Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.A.; Park, E.J.; Lee, D.; Song, J.H.; Lee, H.L.; Kim, K.H.; Kim, Y.; Jung, K.; Kang, K.S.; Yoo, J.E. Estrogenic activity of sanguiin H-6 through activation of estrogen receptor α coactivator-binding site. Nat. Prod. Sci. 2019, 25, 28–33. [Google Scholar] [CrossRef][Green Version]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An Important Source of Natural Bioactive Compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Yu, J.S.; Li, C.; Kwon, M.; Oh, T.; Lee, T.H.; Kim, D.H.; Ahn, J.S.; Ko, S.K.; Kim, C.S.; Cao, S. Herqueilenone a, a unique rearranged benzoquinone-chromanone from the hawaiian volcanic soil-associated fungal strain Penicillium herquei FT729. Bioorg. Chem. 2020, 105, 104397. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal phenols from Woodfordia uniflora collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef]
- Baek, S.C.; Lee, D.; Jo, M.S.; Lee, K.H.; Lee, Y.H.; Kang, K.S.; Yamabe, N.; Kim, K.H. Inhibitory effect of 1,5-dimethyl citrate from sea buckthorn (Hippophae rhamnoides) on lipopolysaccharide-induced inflammatory response in RAW 264.7 Mouse Macrophages. Foods 2020, 9, 269. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Nagao, Y.; Toma, K.; Yoshikawa, Y.; Akiyama, T.; Nishioka, H.; Abe, H.; Harayama, T.; Yamamoto, S. Synthesis and siderophore activity of vibrioferrin and one of its diastereomeric isomers. Chem. Pharm. Bull. 1999, 47, 1284–1287. [Google Scholar] [CrossRef]
- Vereshchagin, A.L.; Anikina, E.V.; Syrchina, A.I.; Lapin, M.F.; Azin, L.A.; Semenov, A.A. Chemical investigation of the bitter substances of the fruit of Lonicera caerulea. Chem. Nat. Compd 1989, 25, 289–292. [Google Scholar] [CrossRef]
- Lee, S.R.; Seok, S.; Ryoo, R.; Choi, S.U.; Kim, K.H. Macrocyclic trichothecene mycotoxins from a deadly poisonous mushroom, Podostroma cornu-damae. J. Nat. Prod. 2019, 82, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.C.; Lee, B.S.; Yi, S.A.; Yu, J.S.; Lee, J.; Ko, Y.J.; Pang, C.; Kim, K.H. Discovery of dihydrophaseic acid glucosides from the florets of Carthamus tinctorius. Plants 2020, 9, 858. [Google Scholar] [CrossRef]
- Rischer, M.; Lee, S.R.; Eom, H.J.; Park, H.B.; Vollmers, J.; Kaster, A.K.; Shin, Y.H.; Oh, D.C.; Kim, K.H.; Beemelmanns, C. Spirocyclic cladosporicin A and cladosporiumins I and J from a Hydractinia-associated Cladosporium sphaerospermum SW67. Org. Chem. Front. 2019, 6, 1084–1093. [Google Scholar] [CrossRef]
- Jin, T.Y.; Shen, T.; Zhou, M.X.; Li, A.L.; Feng, D.; Zheng, B.; Gong, J.; Sun, J.; Li, L.; Xiang, L. Chemical constituents from Portulaca oleracea and their bioactivities. J. Chin. Pharm. Sci. 2016, 25, 898–905. [Google Scholar]
- Yang, Y.B.; Li, X.; Yang, Q.; Wu, Z.J.; Sun, L.N. Study on chemical constituents of Papaya rugosa. Acad. J. Second Mil. Medi. Univ. 2009, 1195–1198. [Google Scholar] [CrossRef]
- Meyer, M.B.; Benkusky, N.A.; Sen, B.; Rubin, J.; Pike, J.W. Epigenetic Plasticity Drives Adipogenic and Osteogenic Differentiation of Marrow derived Mesenchymal Stem Cells. J. Biol. Chem. 2016, 291, 17829–17847. [Google Scholar] [CrossRef]
- Ciuffreda, M.C.; Malpasso, G.; Musarò, P.; Turco, V.; Gnecchi, M. Protocols for in vitro Differentiation of Human Mesenchymal Stem Cells into Osteogenic, Chondrogenic and Adipogenic Lineages. Methods Mol. Biol. 2016, 1416, 149–158. [Google Scholar]
- Yi, S.A.; Lee, J.; Park, S.K.; Kim, J.Y.; Park, J.W.; Lee, M.G.; Nam, K.H.; Park, J.H.; Oh, H.; Kim, S.; et al. Fermented ginseng extract, BST204, disturbs adipogenesis of mesenchymal stem cells through inhibition of S6 kinase 1 signaling. J. Ginseng Res. 2020, 44, 58–66. [Google Scholar] [CrossRef]
- Kang, M.H.; Lee, S.J.; Lee, M.H. Bone remodeling effects of Korean Red Ginseng extracts for dental implant applications. J. Ginseng Res. 2020, 44, 823–832. [Google Scholar] [CrossRef]
 ) and HMBC (
) and HMBC ( ) correlations of compounds 3, 4, and 6.
) correlations of compounds 3, 4, and 6.

| Position | 3 | 4 | 6 | |||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |
| 1 | - | 170.2 | - | 170.0 | - | 170.5 |
| 2 | 2.92 m b 2.84 m b | 42.7 b | 2.92 d (15.5) 2.76 d (15.5) | 42.2 b | 2.93 d (15.5) 2.79 d (15.5) | 43.1 |
| 3 | - | 72.9 | - | 73.2 | 73.2 | |
| 4 | 2.92 m b 2.84 m b | 42.7 b | 2.88 d (15.5) 2.72 d (15.5) | 42.2 b | 2.85 d (15.5) 2.70 d (15.5) | 42.8 |
| 5 | - | 170.5 | - | 172.6 | - | 173.9 b |
| 1′ | - | 175.3 | - | 174.1 | - | 173.9 b |
| 1′-OCH3 | - | - | 3.75 s | 51.5 | - | - |
| 1-OCH3 | - | - | - | - | 3.67 s | 50.2 |
| 5-OCH3 | 3.66 s | 50.7 | - | - | - | - |
| 1″ | 4.08 t (6.5) | 64.2 | 4.07 t (6.5) | 64.2 | 4.18 t (6.5) | 64.9 |
| 2″ | 1.61 m | 30.2 | 1.61 m | 30.2 | 1.67 m | 30.0 |
| 3″ | 1.40 m | 18.6 | 1.39 m | 18.6 | 1.43 m | 18.6 |
| 4″ | 0.94 t (7.5) | 12.5 | 0.94 t (7.5) | 12.5 | 0.97 t (7.5) | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.H.; Jang, H.J.; Park, K.H.; Kim, S.-H.; Kim, J.K.; Kim, J.-C.; Jang, T.S.; Kim, K.H. Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives. Plants 2021, 10, 860. https://doi.org/10.3390/plants10050860
Lee YH, Jang HJ, Park KH, Kim S-H, Kim JK, Kim J-C, Jang TS, Kim KH. Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives. Plants. 2021; 10(5):860. https://doi.org/10.3390/plants10050860
Chicago/Turabian StyleLee, Yong Hoon, Hee Joo Jang, Kun Hee Park, Seon-Hee Kim, Jung Kyu Kim, Jin-Chul Kim, Tae Su Jang, and Ki Hyun Kim. 2021. "Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives" Plants 10, no. 5: 860. https://doi.org/10.3390/plants10050860
APA StyleLee, Y. H., Jang, H. J., Park, K. H., Kim, S.-H., Kim, J. K., Kim, J.-C., Jang, T. S., & Kim, K. H. (2021). Phytochemical Analysis of the Fruits of Sea Buckthorn (Hippophae rhamnoides): Identification of Organic Acid Derivatives. Plants, 10(5), 860. https://doi.org/10.3390/plants10050860










