Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species
Abstract
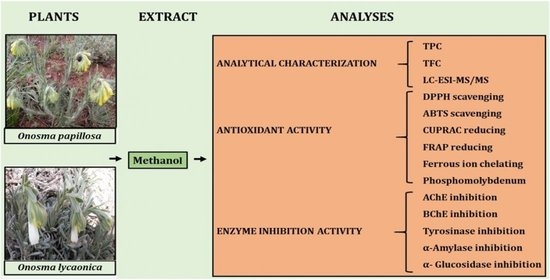
:1. Introduction
2. Results and Discussion
2.1. Yield, Total Phenolics and Flavonoids
2.2. Phytochemical Composition
2.3. Antioxidant Properties
2.4. Enzyme Inhibition Assay
3. Materials and Methods
3.1. Standard Phytochemicals and Chemicals
3.2. Plant Material and Extract Preparation
3.3. Analysis of Total Phenolics and Flavonoids
3.4. Analysis of Phytochemical Composition by LC–ESI–MS/MS
3.5. Antioxidant, and Enzyme Inhibition Assays
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J. Complex of shikonin and β-cyclodextrins by using supercritical carbon dioxide. J. Incl. Phenom. Macrocycl. Chem. 2008, 63, 249–255. [Google Scholar] [CrossRef]
- Noula, E.; Samanidou, V.F.; Assimopoulou, A.N.; Papageorgiou, V.P.; Papadoyannis, I.N. Solid-phase extraction for purification of alkannin/shikonin samples and isolation of monomeric and dimeric fractions. Anal. Bioanal. Chem. 2010, 397, 2221–2232. [Google Scholar] [CrossRef]
- Pal, M.; Chaudhury, A. High frequency direct plant regeneration, micropropagation and Shikonin induction in Arnebia hispidissima. J. Crop. Sci. Biotechnol. 2010, 13, 13–19. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, V.; Pandit, K.; Tuli, H.S.; Sak, K.; Jain, S.K.; Kaur, S. Antioxidant Phytoconstituents From Onosma bracteata Wall. (Boraginaceae) Ameliorate the CCl4 Induced Hepatic Damage: In Vivo Study in Male Wistar Rats. Front. Pharmacol. 2020, 11, 1301. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Cheng, W.; Wei, Y.; Zhang, L. Phosphorylated modification and in vitro antioxidant activity of Radix Hedysari polysaccharide. Glycoconj. J. 2012, 29, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Zhang, Q.; Zhao, T.; Chen, R.; Zhang, H.; Niu, X.; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol. 2005, 37, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sarikurkcu, C.; Sarikurkcu, R.T.; Wang, M.-H. A comparative study on the phenolic composition, antioxidant and enzyme inhibition activities of two endemic Onosma species. Ind. Crop. Prod. 2019, 142, 111878. [Google Scholar] [CrossRef]
- Stanković, J.S.K.; Ceylan, R.; Zengin, G.; Matić, S.; Jurić, T.; Diuzheva, A.; Jeko, J.; Cziáky, Z.; Aktumsek, A. Multiple biological activities of two Onosma species (O. sericea and O. stenoloba) and HPLC-MS/MS characterization of their phytochemical composition. Ind. Crop. Prod. 2020, 144, 112053. [Google Scholar] [CrossRef]
- Baltaci, N.; Aydogdu, N.; Sarikurkcu, C.; Tepe, B. Onosma gracilis (Trautv.) and O. oreodoxa (Boiss. & Heldr.): Phytochemistry, in silico docking, antioxidant and enzyme inhibitory activities. S. Afr. J. Bot. 2021. [Google Scholar] [CrossRef]
- Prochazkova, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Son, S.; Yun, H.Y.; Kim, H.; Chun, P.; Moon, H.R. Tyrosinase inhibitors: A patent review (2011–2015). Expert Opin. Ther. Pat. 2016, 26, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Binzet, R.; Eren, Ö. Onosma erzincanica (Boraginaceae: Lithospermeae), a new scree species from Turkey. Phytotaxa 2018, 356, 117–130. [Google Scholar] [CrossRef]
- Cecchi, L.; Coppi, A.; Selvi, F. Onosma juliae (Boraginaceae), a new species from southern Turkey, with remarks on the systematics of Onosma in the Irano-Turanian region. Phytotaxa 2016, 288, 201. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, R.; Kishore, K.; Onosma, L. A review of phytochemistry and ethnopharmacology. Pharmacogn. Rev. 2013, 7, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crop. Prod. 2020, 154, 112632. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Kirkan, B.; Sarikurkcu, C.; Ozer, M.S.; Cengiz, M.; Atılgan, N.; Ceylan, O.; Tepe, B. Phenolic profile, antioxidant and enzyme inhibitory potential of Onosma tauricum var. tauricum. Ind. Crop. Prod. 2018, 125, 549–555. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Kirkan, B.; Ozer, M.S.; Ceylan, O.; Atilgan, N.; Cengiz, M.; Tepe, B. Chemical characterization and biological activity of Onosma gigantea extracts. Ind. Crop. Prod. 2018, 115, 323–329. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Husunet, M.T.; Istifli, E.S.; Tepe, B. Two endemic Onosma species (O. sieheana and O. stenoloba): A comparative study including docking data on biological activity and phenolic composition. Ind. Crop. Prod. 2020, 154, 112656. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Tepe, B. Onosma aucheriana, O. frutescens, and O. sericea: Phytochemical profiling and biological activity. Ind. Crop. Prod. 2020, 154, 112633. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma ambigens: Phytochemical composition, antioxidant and enzyme inhibitory activity. Ind. Crop. Prod. 2020, 154, 112651. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.H.M.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Assessment of flavonoids contents and in vitro antioxidant activity of Launaea procumbens. Chem. Cent. J. 2012, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Guzmán, E.S.; Strong, F.C., III. Determination of tocopherols (vitamin E) by reduction of cupric ion. J. Assoc. Off. Anal. Chem. 1982, 65, 1215–1221. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Chapter 67-Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 625–635. [Google Scholar]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Morales, J.C.; Lucas, R. Chapter 97-Structure–Activity Relationship of Phenolic Antioxidants and Olive Components. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 905–914. [Google Scholar]
- Alagawany, M.; El-Hack, M.A.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Sathiyaseelan, A.; Kim, K.-N.; Cho, S.-H.; Mariadoss, A.V.A.; Wang, M.-H. Metabolite Profiling of Methanolic Extract of Gardenia jaminoides by LC-MS/MS and GC-MS and Its Anti-Diabetic, and Anti-Oxidant Activities. Pharmaceuticals 2021, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, L.; Singleton, K.; Enol, V.L. Viticult, 28. Anal. Abstr. 1977, 34, 49. [Google Scholar]
- Berk, S.; Tepe, B.; Arslan, S.; Sarikurkcu, C. Screening of the antioxidant, antimicrobial and DNA damage protection potentials of the aqueous extract of Asplenium ceterach DC. Afr. J. Biotechnol. 2011, 10, 8902–8908. [Google Scholar]
- Cittan, M.; Çelik, A. Development and Validation of an Analytical Methodology Based on Liquid Chromatography–Electrospray Tandem Mass Spectrometry for the Simultaneous Determination of Phenolic Compounds in Olive Leaf Extract. J. Chromatogr. Sci. 2018, 56, 336–343. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC–DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Tepe, B.; Sarikurkcu, C.; Berk, S.; Alim, A.; Akpulat, H.A. Chemical composition, radical scavenging and antimicrobial activity of the essential oils of Thymus boveii and Thymus hyemalis. Rec. Nat. Prod. 2011, 5, 208–220. [Google Scholar]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E.; Erçağ, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.S.; Sarikurkcu, C.; Cengiz, M.; Kocak, S.; Uren, M.C.; Tepe, B. Salvia cadmica: Phenolic composition and biological activity. Ind. Crop. Prod. 2016, 85, 204–212. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Uyar, P.; Aktumsek, A.; Uysal, S.; Kocak, M.S.; Ceylan, R. Crepis foetida L. subsp. rhoeadifolia (Bieb.) Celak. as a source of multifunctional agents: Cytotoxic and phytochemical evaluation. J. Funct. Foods 2015, 17, 698–708. [Google Scholar] [CrossRef]

| Assays | O. lycaonica | O. papillosa |
|---|---|---|
| Yield (%) | 3.52 | 4.02 |
| Total flavonoids (mg QEs/g extracts) | 26.0 ± 0.5 b | 32.9 ± 0.3 a |
| Total phenolics (mg GAEs/g extracts) | 43.5 ± 1.5 a | 33.9 ± 0.4 b |
| Compound | O. lycaonica | O. papillosa |
|---|---|---|
| Gallic acid | 12.6 ± 0.4 b | 15.2 ± 0.1 a |
| Protocatechuic acid | 162.2 ± 3.6 b | 249 ± 1.9 a |
| 3,4-Dihydroxyphenylacetic acid | 11.1 ± 0.6 a | 7.4 ± 0.1 b |
| (+)-Catechin | 30.4 ± 4.2 | nd |
| Pyrocatechol | nd | nd |
| Chlorogenic acid | 847.5 ± 19.5 b | 14088 ± 115 a |
| 2,5-Dihydroxybenzoic acid | 209.5 ± 4.5 b | 265.8 ± 11.0 a |
| 4-Hydroxybenzoic acid | 953.4 ± 7.6 a | 935.3 ± 2.7 a |
| (−)-Epicatechin | nd | nd |
| Caffeic acid | 863.0 ± 43.2 a | 222.2 ± 2.8 b |
| Vanillic acid | 1260 ± 25 a | 622.1 ± 12.1 b |
| Syringic acid | 30.1 ± 1.6 b | 74.3 ± 0.4 a |
| 3-Hydroxybenzoic acid | 21.0 ± 1.2 a | 17.6 ± 0.1 a |
| Vanillin | 80.7 ± 3.9 a | 70.2 ± 6.8 a |
| Verbascoside | nd | nd |
| Taxifolin | nd | nd |
| Sinapic acid | 26.8 ± 2.8 b | 83.1 ± 2.1 a |
| p-Coumaric acid | 189.5 ± 1.6 b | 226.7 ± 0.3 a |
| Ferulic acid | 1204 ± 8 a | 495.6 ± 21.6 b |
| Luteolin 7-glucoside | 20,846 ± 522 a | 789.1 ± 10.4 b |
| Hesperidin | 15,417 ± 288 b | 54,123 ± 239 a |
| Hyperoside | 2853 ± 64 b | 4555 ± 100 a |
| Rosmarinic acid | 65,632± 1418 a | 6312 ± 110 b |
| Apigenin 7-glucoside | 21,416 ± 361 a | 1217 ± 129 b |
| 2-Hydroxycinnamic acid | nd | nd |
| Pinoresinol | 3567 ± 8 a | 2756 ± 49 b |
| Eriodictyol | 4.3 ± 0.2 | nd |
| Quercetin | 11.0 ± 0.2 b | 49.6 ± 0.3 a |
| Luteolin | 2559 ± 46 a | 277.4 ± 20.4 b |
| Kaempferol | nd | 42.2 ± 2.6 |
| Apigenin | 1623 ± 34 a | 319.6 ± 18.9 b |
| Antioxidant Activity | O. lycaonica | O. papillosa | Trolox | BHA | BHT | EDTA |
|---|---|---|---|---|---|---|
| Phosphomolybdenum (EC50: mg/mL) | 2.05 ± 0.07 c | 1.90 ± 0.07 c | 1.16 ± 0.06 b | 0.31 ± 0.01 a | 0.38 ± 0.03 a | |
| DPPH scavenging (IC50: mg/mL) | 2.69 ± 0.10 c | 3.41 ± 0.05 d | 0.26 ± 0.02 a | 0.22 ± 0.02 a | 1.00 ± 0.03 b | |
| ABTS scavenging (IC50: mg/mL) | 2.18 ± 0.01 b | 2.50 ± 0.16 c | 0.32 ± 0.03 a | 0.21 ± 0.01 a | 0.29 ± 0.02 a | |
| CUPRAC reducing (EC50: mg/mL) | 1.10 ± 0.01 c | 1.32 ± 0.02 d | 0.28 ± 0.02 b | 0.14 ± 0.01 a | 0.18 ± 0.02 a | |
| FRAP reducing (EC50: mg/mL) | 0.69 ± 0.01 c | 0.88 ± 0.02 d | 0.10 ± 0.01 a | 0.09 ± 0.01 a | 0.18 ± 0.01 b | |
| Ferrous ion chelating (IC50: mg/mL) | 2.32 ± 0.16 b | 3.65 ± 0.07 c | 0.051 ± 0.003 a | |||
| Phosphomolybdenum (mg TEs/g extracts) | 540.6 ± 19.6 a | 584.3 ± 20.4 a | ||||
| DPPH scavenging (mg TEs/g extracts) | 92.6 ± 3.6 a | 73.1 ± 1.0 b | ||||
| ABTS scavenging (mg TEs/g extracts) | 130.6 ± 0.4 a | 115.1 ± 7.1 a | ||||
| CUPRAC reducing (mg TEs/g extracts) | 249.5 ± 1.4 a | 207.5 ± 3.9 b | ||||
| FRAP reducing (mg TEs/g extracts) | 144.4 ± 0.1 a | 113.1 ± 0.1 b | ||||
| Ferrous ion chelating (mg EDTAEs/g extracts) | 21.6 ± 1.5 a | 14.0 ± 0.3 b |
| Enzyme Inhibitory Activity | O. lycaonica | O. papillosa | Galanthamine | Acarbose | Kojic Acid |
|---|---|---|---|---|---|
| AChE inhibition (IC50: mg/mL) | 1.32 ± 0.02 b | 1.47 ± 0.06 c | 0.0035 ± 0.0004 a | - | - |
| BChE inhibition (IC50: mg/mL) | 8.94 ± 0.08 c | 4.96 ± 0.07 b | 0.0058 ± 0.0003 a | - | - |
| α-Amylase inhibition (IC50: mg/mL) | 2.57 ± 0.11 b | 2.40 ± 0.07 b | - | 0.97 ± 0.03 a | - |
| α-Glucosidase inhibition (IC50: mg/mL) | 2.60 ± 0.14 b | 2.61 ± 0.04 b | - | 1.74 ± 0.03 a | - |
| Tyrosinase inhibition (IC50: mg/mL) | 2.20 ± 0.03 b | 2.05 ± 0.08 b | - | - | 0.31 ± 0.01 a |
| AChE inhibition (mg GALAEs/g extracts) | 2.31 ± 0.04 a | 2.07 ± 0.08 a | |||
| BChE inhibition (mg GALAEs/g extracts) | 0.63 ± 0.01 b | 1.13 ± 0.02 a | |||
| α-Amylase inhibition (mgACEs/g extracts) | 402.9 ± 18.1 a | 430.7 ± 13.5 a | |||
| α-Glucosidase inhibition (mgACEs/g extracts) | 670.4 ± 36.7 a | 666.7 ± 10.7 a | |||
| Tyrosinase inhibition (mg KAEs/g extracts) | 139.0 ± 1.9 a | 149.0 ± 5.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravanakumar, K.; Sarikurkcu, C.; Sahinler, S.S.; Sarikurkcu, R.B.; Wang, M.-H. Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species. Plants 2021, 10, 1373. https://doi.org/10.3390/plants10071373
Saravanakumar K, Sarikurkcu C, Sahinler SS, Sarikurkcu RB, Wang M-H. Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species. Plants. 2021; 10(7):1373. https://doi.org/10.3390/plants10071373
Chicago/Turabian StyleSaravanakumar, Kandasamy, Cengiz Sarikurkcu, Saliha Seyma Sahinler, Rabia Busra Sarikurkcu, and Myeong-Hyeon Wang. 2021. "Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species" Plants 10, no. 7: 1373. https://doi.org/10.3390/plants10071373
APA StyleSaravanakumar, K., Sarikurkcu, C., Sahinler, S. S., Sarikurkcu, R. B., & Wang, M.-H. (2021). Phytochemical Composition, Antioxidant, and Enzyme Inhibition Activities of Methanolic Extracts of Two Endemic Onosma Species. Plants, 10(7), 1373. https://doi.org/10.3390/plants10071373