Biotechnological Resources to Increase Disease-Resistance by Improving Plant Immunity: A Sustainable Approach to Save Cereal Crop Production
Abstract
:1. Introduction
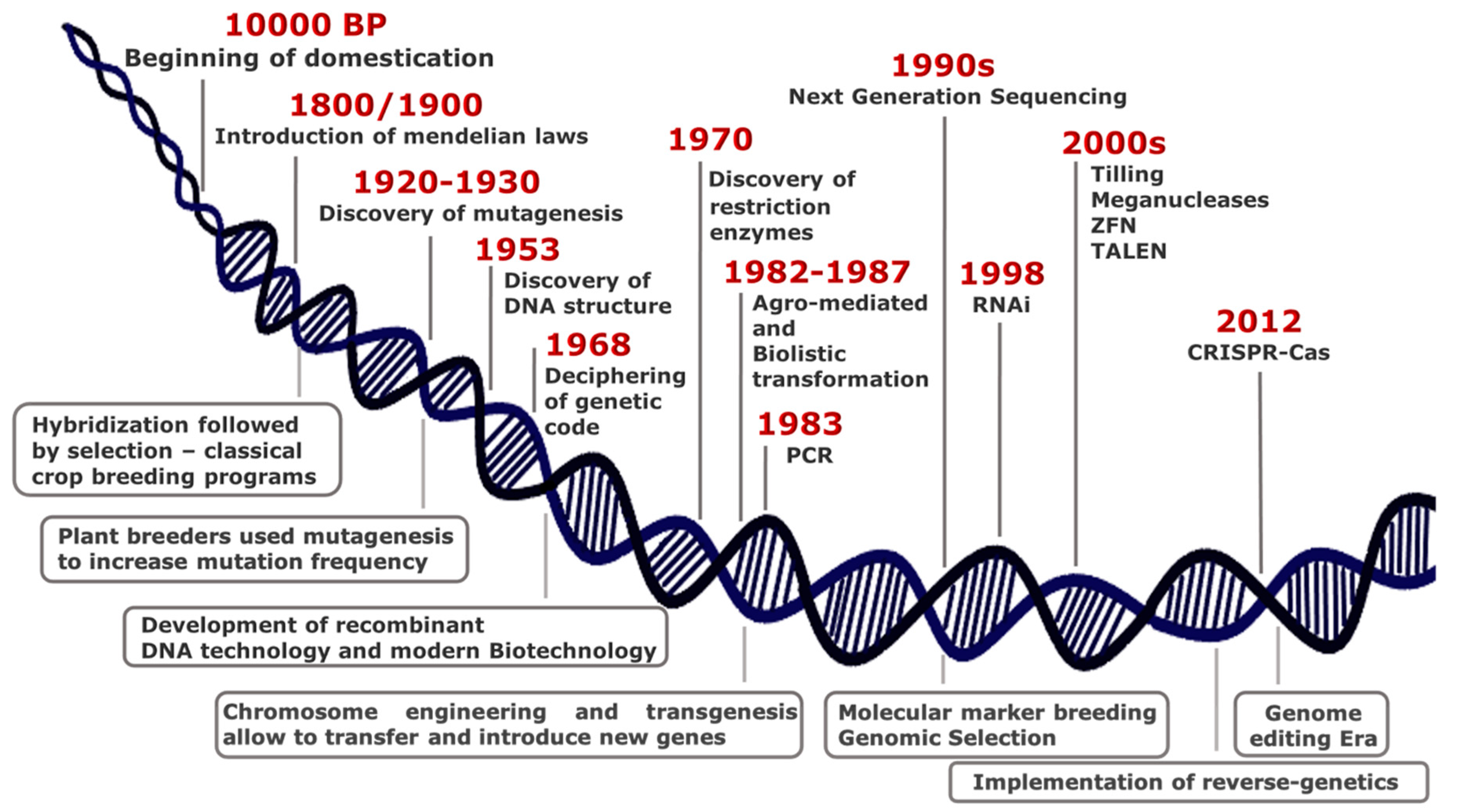
2. Plant Biotechnology: From Random to Directed, Precise and Safe Mutagenesis
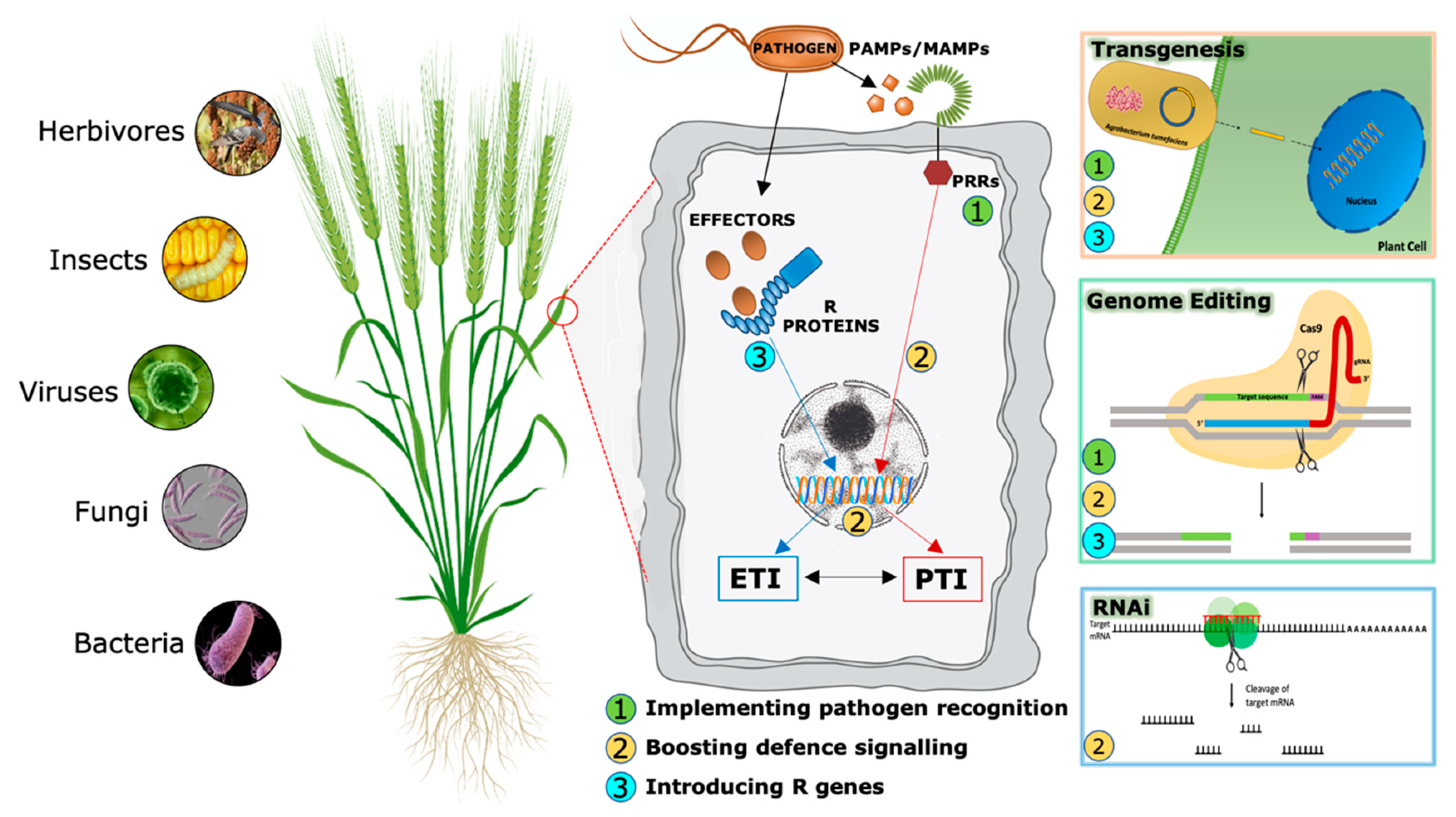
3. Increasing Disease-Resistance in Cereals by Implementing Plant Immunity Through Transgenesis
3.1. Pathogen Detection
3.2. Boosting the Immune Signaling
3.3. R Gene Transfer
4. Increasing Disease-Resistance in Cereals by Using Gene Expression or Editing Techniques
4.1. RNA Interference (RNAi)
4.2. CRISPR/Cas9 Mediated Genome Editing
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The Challenge of Feeding the World. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [Green Version]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.P.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, H.K.; Garg, H. Pesticide: Environmental Impacts and Management Strategies. In Pesticides—Toxic Effects; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2014; pp. 187–230. [Google Scholar]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. 2019, 94, 135–155. [Google Scholar] [CrossRef]
- Bargués-Ribera, M.; Gokhale, C.S. Eco-evolutionary agriculture: Host-pathogen dynamics in crop rotations. PLoS Comput. Biol. 2020, 16, e1007546. [Google Scholar] [CrossRef] [PubMed]
- Poland, J.; Rutkoski, J. Advances and Challenges in Genomic Selection for Disease Resistance. Annu. Rev. Phytopathol. 2016, 54, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.-F.; Ser, H.-L.; Khan, T.M.; Chuah, L.-H.; Pusparajah, P.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. The Potential of Streptomyces as Biocontrol Agents against the Rice Blast Fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. Abiotic Biot. Stress Plants 2019. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.J. Advances in Plant Health Management in the Twentieth Century. Annu. Rev. Phytopathol. 2000, 38, 95–116. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Groenewald, J.; Elliott, M.; Canning, G.; McMillan, V.; Crous, P. Take-all or nothing. Stud. Mycol. 2016, 83, 19–48. [Google Scholar] [CrossRef] [Green Version]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Bonjean, A.P.; Angus, W.J.; Sági, F. The World Wheat Book: A History of Wheat Breeding. Cereal Res. Commun. 2001, 29, 459. [Google Scholar] [CrossRef] [Green Version]
- Briggs, F.N. The Use of the Backcross in Crop Improvement. Am. Nat. 1938, 72, 285–292. [Google Scholar] [CrossRef]
- Borlaug, N.E. Contributions of Conventional Plant Breeding to Food Production. Science 1983, 219, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadler, L.J. Genetic Effects of X-Rays in Maize. Proc. Natl. Acad. Sci. USA 1928, 14, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitao, J.M. Chemical mutagenesis. In Plant Mutation Breeding and Biotechnology; CABI Publishing: Vienna, Austria, 2012; pp. 135–158. [Google Scholar]
- Mba, C.; Afza, R.; Shu, Q.Y. Mutagenic Radiations: X-Rays, Ionizing Particles and Ul-traviolet. Plant Mutat. Breed. Biotechnol. 2012, 83–90. [Google Scholar] [CrossRef]
- Bhatia, S.; History, Scope and Development of Biotechnology. Introduction to Pharmaceutical Biotechnology. 2018. Available online: https://iopscience.iop.org/chapter/978-0-7503-1299-8/bk978-0-7503-1299-8ch1.pdf (accessed on 3 June 2021).
- Zambryski, P.; Depicker, A.; Kruger, K.; Goodman, H.M. Tumor Induction by Agro-bacterium Tumefaciens: Analysis of the Boundaries of T-DNA. J. Mol. Appl. Genet. 1982, 1, 361–370. [Google Scholar] [PubMed]
- Herrera-Estrella, L.; Depicker, A.; Van Montagu, M.; Schell, J. Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nat. Cell Biol. 1983, 303, 209–213. [Google Scholar] [CrossRef]
- De Block, M.; Herrera-Estrella, L.; Van Montagu, M.; Schell, J.; Zambryski, P. Expression of foreign genes in regenerated plants and in their progeny. EMBO J. 1984, 3, 1681–1689. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.M.; Wolf, E.D.; Wu, R.; Sanford, J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nat. Cell Biol. 1987, 327, 70–73. [Google Scholar] [CrossRef]
- Vasil, V.; Castillo, A.M.; Fromm, M.E.; Vasil, I.K. Herbicide Resistant Fertile Transgenic Wheat Plants Obtained by Microprojectile Bombardment of Regenerable Embryogenic Callus. Nat. Biotechnol. 1992, 10, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.G.; Redenbaugh, K. Commercialization of a tomato with an antisense polygalacturonase gene: The FLAVR SAVR? tomato story. Euphytica 1994, 79, 293–297. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Klöti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the Provitamin A (-Carotene) Biosynthetic Pathway into (Carotenoid-Free) Rice Endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Bizily, S.P.; Rugh, C.L.; Meagher, R.B. Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat. Biotechnol. 2000, 18, 213–217. [Google Scholar] [CrossRef]
- Wang, M.; Allefs, S.; Berg, R.G.V.D.; Vleeshouwers, V.G.A.A.; Van Der Vossen, E.A.G.; Vosman, B. Allele mining in Solanum: Conserved homologues of Rpi-blb1 are identified in Solanum stoloniferum. Theor. Appl. Genet. 2008, 116, 933–943. [Google Scholar] [CrossRef]
- Bhullar, N.K.; Zhang, Z.; Wicker, T.; Keller, B. Wheat gene bank accessions as a source of new alleles of the powdery mildew resistance gene Pm3: A large scale allele mining project. BMC Plant Biol. 2010, 10, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramkumar, G.; Srinivasarao, K.; Mohan, K.M.; Sudarshan, I.; Sivaranjani, A.K.P.; Gopalakrishna, K.; Neeraja, C.N.; Balachandran, S.M.; Sundaram, R.M.; Prasad, M.S.; et al. Development and validation of functional marker targeting an InDel in the major rice blast disease resistance gene Pi54 (Pik h). Mol. Breed. 2010, 27, 129–135. [Google Scholar] [CrossRef]
- Wang, D.; Guo, C.; Huang, J.; Yang, S.; Tian, D.; Zhang, X. Allele-mining of rice blast resistance genes at AC134922 locus. Biochem. Biophys. Res. Commun. 2014, 446, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Biselli, C.; Urso, S.; Tacconi, G.; Steuernagel, B.; Schulte, D.; Gianinetti, A.; Bagnaresi, P.; Stein, N.; Cattivelli, L.; Valè, G. Haplotype variability and identification of new functional alleles at the Rdg2a leaf stripe resistance gene locus. Theor. Appl. Genet. 2013, 126, 1575–1586. [Google Scholar] [CrossRef]
- McCallum, C.M.; Comai, L.; Greene, E.A.; Henikoff, S. Targeting Induced LocalLesions IN Genomes (TILLING) for Plant Functional Genomics. Plant Physiol. 2000, 123, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Acevedo-Garcia, J.; Spencer, D.; Thieron, H.; Reinstädler, A.; Hammond-Kosack, K.; Phillips, A.L.; Panstruga, R. mlo-based powdery mildew resistance in hexaploid bread wheat generated by a non-transgenic TILLING approach. Plant Biotechnol. J. 2016, 15, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hille, F.; Richter, H.; Wong, S.P.; Bratovič, M.; Ressel, S.; Charpentier, E. The Biology of CRISPR-Cas: Backward and Forward. Cell 2018, 172, 1239–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, C.S.; Mendonça, M.A.C.; Hassan, S.S.; Barh, D.; De Carvalho Azevedo, V.A. Biotechnology for improved crop productivity and quality. Appl. Mol. Biotechnol. 2016, 231. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the Four Yield-related Genes Gn1a, DEP1, GS3, and IPA1 in Rice Using a CRISPR/Cas9 System. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, D.; Zhang, D.; Zhao, X.; Cao, X.; Dong, L.; Liu, J.; Chen, K.; Zhang, H.; Gao, C.; et al. Analysis of the functions ofTaGW2homoeologs in wheat grain weight and protein content traits. Plant J. 2018, 94, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Sestili, F.; Pagliarello, R.; Zega, A.; Saletti, R.; Pucci, A.; Botticella, E.; Masci, S.; Tundo, S.; Moscetti, I.; Foti, S.; et al. Enhancing grain size in durum wheat using RNAi to knockdown GW2 genes. Theor. Appl. Genet. 2018, 132, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Xu, X.; Gong, Q.; Li, Z.; Li, Y.; Wang, S.; Yang, Y.; Ma, W.; Liu, L.; Zhu, B.; et al. Engineering Broad-Spectrum Bacterial Blight Resistance by Simultaneously Disrupting Variable TALE-Binding Elements of Multiple Susceptibility Genes in Rice. Mol. Plant 2019, 12, 1434–1446. [Google Scholar] [CrossRef] [Green Version]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nat. Cell Biol. 2016, 532, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Jiao, G.; Liu, Z.; Zhang, X.; Li, J.; Guo, X.; Du, W.; Du, J.; Francis, F.; Zhao, Y.; et al. Generation of High-Amylose Rice through CRISPR/Cas9-Mediated Targeted Mutagenesis of Starch Branching Enzymes. Front. Plant Sci. 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Kerr-Enskat, K. DuPont Pioneer Announces Intentions to Commercialize First CRISPR-Cas Product. Press Release. 2016. Available online: https://www.prweb.com/releases/dupont-pioneer-seed/crispr-cas-corn/prweb13349828.htm (accessed on 1 June 2021).
- León, S.S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2017, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Camerlengo, F.; Frittelli, A.; Sparks, C.; Doherty, A.; Martignago, D.; Larré, C.; Lupi, R.; Sestili, F.; Masci, S. CRISPR-Cas9 Multiplex Editing of the α-Amylase/Trypsin Inhibitor Genes to Reduce Allergen Proteins in Durum Wheat. Front. Sustain. Food Syst. 2020, 4, 104. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Yu, Y.; Si, X.; Zhai, X.; Zhang, H.; Dong, W.; Gao, C.; Xu, C. Domestication of wild tomato is accelerated by genome editing. Nat. Biotechnol. 2018, 36, 1160–1163. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Wu, C.; He, Y.; Ma, Y.; Hou, H.; Guo, X.; Du, W.; Zhao, Y.; Xia, L. Engineering Herbicide-Resistant Rice Plants through CRISPR/Cas9-Mediated Homologous Recombination of Acetolactate Synthase. Mol. Plant 2016, 9, 628–631. [Google Scholar] [CrossRef] [Green Version]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nat. Cell Biol. 2019, 576, 149–157. [Google Scholar] [CrossRef]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, K.; Minkenberg, B.; Yang, Y. Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc. Natl. Acad. Sci. USA 2015, 112, 3570–3575. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Crick, F. Central Dogma of Molecular Biology. Nat. Cell Biol. 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, D.; Shelake, R.M.; Kim, M.J.; Kim, J.-Y. CRISPR-Mediated Engineering across the Central Dogma in Plant Biology for Basic Research and Crop Improvement. Mol. Plant 2021, 14, 127–150. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, R.; Choudhury, S.; Hasanuzzaman, M.; Srivastava, S. Sustainable Agriculture in the Era of Climate Change; Springer Science and Business Media LLC: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- Malik, N.A.A.; Kumar, I.S.; Nadarajah, K. Elicitor and Receptor Molecules: Orchestrators of Plant Defense and Immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, C.G.; Guimarães, G.A.; Nogueira, S.R.; MacLean, D.; Cook, D.R.; Steuernagel, B.; Baek, J.; Bouyioukos, C.; Melo, B.D.V.A.; Tristão, G.; et al. A pigeonpea gene confers resistance to Asian soybean rust in soybean. Nat. Biotechnol. 2016, 34, 661–665. [Google Scholar] [CrossRef]
- Claus, L.A.N.; Savatin, D.V.; Russinova, E. The crossroads of receptor-mediated signaling and endocytosis in plants. J. Integr. Plant Biol. 2018, 60, 827–840. [Google Scholar] [CrossRef] [Green Version]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhou, J.; Shan, L.; Meng, X. Plant cell surface receptor-mediated signaling—A common theme amid diversity. J. Cell Sci. 2018, 131, jcs209353. [Google Scholar] [CrossRef] [Green Version]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395. [Google Scholar] [CrossRef] [Green Version]
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2007, 59, 501–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodds, P.N.; Rathjen, J. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; He, S.Y.; Zhou, J.M.; Xin, X.F. Pat-tern-Recognition Receptors Are Required for NLR-Mediated Plant Immunity. Nature 2020, 592, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Ngou, B.P.M.; Ahn, H.-K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nat. Cell Biol. 2021, 592, 1–6. [Google Scholar] [CrossRef]
- Weyers, J.D.B.; Paterson, N.W. Plant hormones and the control of physiological processes. New Phytol. 2001, 152, 375–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bianco, M.; Giustini, L.; Sabatini, S. Spatiotemporal changes in the role of cytokinin during root development. New Phytol. 2013, 199, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, S.; Hardtke, C.S. Hormone Signalling Crosstalk in Plant Growth Regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Bargmann, B.; Vanneste, S.; Krouk, G.; Nawy, T.; Efroni, I.; Shani, E.; Choe, G.; Friml, J.; Bergmann, D.C.; Estelle, M.; et al. A map of cell type-specific auxin responses. Mol. Syst. Biol. 2013, 9, 688. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garay-Arroyo, A.; Sánchez, M.D.L.P.; García-Ponce, B.; Azpeitia, E.; Álvarez-Buylla, E.R. Hormone symphony during root growth and development. Dev. Dyn. 2012, 241, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, H.; Wang, H.; Stefanato, F.L.; Craze, M.; Bowden, S.; Wallington, E.; Zipfel, C.; Ridout, C.J. Arabidopsis EF -Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol. 2015, 206, 606–613. [Google Scholar] [CrossRef]
- Schwessinger, B.; Bahar, O.; Thomas, N.; Holton, N.; Nekrasov, V.; Ruan, D.; Canlas, P.E.; Daudi, A.; Petzold, C.; Singan, V.R.; et al. Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses. PLoS Pathog. 2015, 11, e1004809. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.; Wang, H.; Wang, S.; Jiang, W.; Shan, C.; Li, B.; Yang, J.; Zhang, S.; Sun, W. Enhancement of innate immune system in monocot rice by transferring the dicotyledonous elongation factor Tu receptor EFR. J. Integr. Plant Biol. 2014, 57, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, Z.; Fang, Z.; Zhou, J.; Xia, Z.; Gao, L.; Chen, L.; Li, L.; Li, T.; Zhai, W.; et al. Rice Xa21 primed genes and pathways that are critical for combating bacterial blight infec-tion. Sci Rep. 2015, 5, 12165. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Xiao, J.; Xu, J.; Wan, W.; Qin, B.; Cao, A.; Chen, W.; Xing, L.; Du, C.; Gao, X.; et al. Two members of TaRLK family confer powdery mildew resistance in common wheat. BMC Plant Biol. 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaraman, J.; Douchkov, D.; Hensel, G.; Stefanato, F.L.; Gordon, A.; Ereful, N.; Caldararu, O.F.; Petrescu, A.-J.; Kumlehn, J.; Boyd, L.A.; et al. An LRR/Malectin Receptor-Like Kinase Mediates Resistance to Non-adapted and Adapted Powdery Mildew Fungi in Barley and Wheat. Front. Plant Sci. 2016, 7, 1836. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cheng, J.; Fan, A.; Zhao, J.; Yu, Z.; Li, Y.; Wang, X. LecRK-V, an L-type lectin receptor kinase in Haynaldia villosa, plays positive role in resistance to wheat powdery mildew. Plant Biotechnol. J. 2018, 16, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Holton, N.; Nekrasov, V.; Ronald, P.C.; Zipfel, C. The Phylogenetically-Related Pattern Recognition Receptors EFR and XA21 Recruit Similar Immune Signaling Components in Monocots and Dicots. PLoS Pathog. 2015, 11, e1004602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, N.C.; Oksenberg, N.; Liu, F.; Caddell, D.; Nalyvayko, A.; Nguyen, Y.; Schwessinger, B.; Ronald, P.C. The rice XA21 ectodomain fused to the Arabidopsis EFR cytoplasmic domain confers resistance to Xanthomonas oryzae pv. oryzae. PeerJ 2018, 6, e4456. [Google Scholar] [CrossRef] [Green Version]
- Boutrot, F.; Zipfel, C. Function, Discovery, and Exploitation of Plant Pattern Recognition Receptors for Broad-Spectrum Disease Resistance. Annu. Rev. Phytopathol. 2017, 55, 257–286. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, B.; Spalding, M.; Weeks, D.; Yang,, B. High-efficiency TALEN-based gene editing produces disease-resistant rice. Nat. Biotechnol. 2012, 30, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Hummel, A.W.; Doyle, E.L.; Bogdanove, A.J. Addition of transcription activator-like effector binding sites to a pathogen strain-specific rice bacterial blight resistance gene makes it effective against additional strains and against bacterial leaf streak. New Phytol. 2012, 195, 883–893. [Google Scholar] [CrossRef]
- Xu, G.; Yuan, M.; Ai, C.; Liu, L.; Zhuang, E.; Karapetyan, S.; Wang, S.; Dong, X. uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nat. Cell Biol. 2017, 545, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Wang, X.; Zhou, M.; Zhou, X.; Ye, X.; Wei, X. An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress-related genes. New Phytol. 2012, 196, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Shi, H.; Chern, M.; Yu, H.; Yi, H.; He, M.; Yin, J.; Zhu, X.; Li, Y.; et al. A single transcription factor promotes both yield and immunity in rice. Science 2018, 361, 1026–1028. [Google Scholar] [CrossRef] [Green Version]
- Risk, J.M.; Selter, L.L.; Chauhan, H.; Krattinger, S.G.; Kumlehn, J.; Hensel, G.; Viccars, L.A.; Richardson, T.M.; Buesing, G.; Troller, A.; et al. The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol. J. 2013, 11, 847–854. [Google Scholar] [CrossRef]
- Krattinger, S.G.; Sucher, J.; Selter, L.L.; Chauhan, H.; Zhou, B.; Tang, M.; Upadhyaya, N.M.; Mieulet, D.; Guiderdoni, E.; Weidenbach, D.; et al. The wheat durable, multipathogen resistance geneLr34confers partial blast resistance in rice. Plant Biotechnol. J. 2016, 14, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Schnippenkoetter, W.; Lo, C.; Liu, G.; Dibley, K.; Chan, W.L.; White, J.; Milne, R.; Zwart, A.; Kwong, E.; Keller, B.; et al. The wheat Lr34 multipathogen resistance gene confers resistance to anthracnose and rust in sorghum. Plant Biotechnol. J. 2017, 15, 1387–1396. [Google Scholar] [CrossRef]
- Sucher, J.; Boni, R.; Yang, P.; Rogowsky, P.; Büchner, H.; Kastner, C.; Kumlehn, J.; Krattinger, S.G.; Keller, B. The durable wheat disease resistance geneLr34confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol. J. 2016, 15, 489–496. [Google Scholar] [CrossRef]
- Rinaldo, A.; Gilbert, B.; Boni, R.; Krattinger, S.G.; Singh, D.; Park, R.F.; Lagudah, E.; Ayliffe, M. TheLr34adult plant rust resistance gene provides seedling resistance in durum wheat without senescence. Plant Biotechnol. J. 2017, 15, 894–905. [Google Scholar] [CrossRef] [Green Version]
- Milne, R.J.; Dibley, K.E.; Schnippenkoetter, W.; Mascher, M.; Lui, A.C.; Wang, L.; Lo, C.; Ashton, A.R.; Ryan, P.R.; Lagudah, E.S. The Wheat Lr67 Gene from the Sugar Transport Protein 13 Family Confers Multipathogen Resistance in Barley. Plant Physiol. 2019, 179, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- Albert, I.; Böhm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23–SOBIR1–BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Steuernagel, B.; Periyannan, S.K.; Hernández-Pinzón, I.; Witek, K.; Rouse, M.N.; Yu, G.; Hatta, A.; Ayliffe, M.; Bariana, H.; Jones, J.D.G.; et al. Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat. Biotechnol. 2016, 34, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Q.; He, F.; Akhunova, A.; Chao, S.; Trick, H.; Akhunov, E. Transgenerational CRISPR-Cas9 Activity Facilitates Multiplex Gene Editing in Allopolyploid Wheat. CRISPR J. 2018, 1, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzai, Y.; Kaku, H.; Shibuya, N.; Minami, E.; Nishizawa, Y. Expression of the chimeric receptor between the chitin elicitor receptor CEBiP and the receptor-like protein kinase Pi-d2 leads to enhanced responses to the chitin elicitor and disease resistance against Magnaporthe oryzae in rice. Plant Mol. Biol. 2012, 81, 287–295. [Google Scholar] [CrossRef]
- Bozkurt, T.O.; Richardson, A.; Dagdas, Y.F.; Mongrand, S.; Kamoun, S.; Raffaele, S. The Plant Membrane-Associated REMORIN1.3 Accumulates in Discrete Perihaustorial Domains and Enhances Susceptibility to Phytophthora infestans. Plant Physiol. 2014, 165, 1005–1018. [Google Scholar] [CrossRef] [Green Version]
- Boevink, P.C.; McLellan, H.; Gilroy, E.M.; Naqvi, S.; He, Q.; Yang, L.; Wang, X.; Turnbull, D.; Armstrong, M.R.; Tian, Z.; et al. Oomycetes Seek Help from the Plant: Phytophthora infestans Effectors Target Host Susceptibility Factors. Mol. Plant 2016, 9, 636–638. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-H.; Hagemann, S.; Mamareli, P.; Lauer, U.M.; Hoffmann, U.; Beckstette, M.; Fohse, L.; Prinz, I.; Pezoldt, J.; Suerbaum, S.; et al. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016, 9, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; He, Q.; Armstrong, M.; Giuliani, L.M.; Boevink, P.C.; Zhang, W.; Tian, Z.; Birch, P.R.J.; Gilroy, E.M. The Potato MAP3K StVIK Is Required for the Phytophthora infestans RXLR Effector Pi17316 to Promote Disease. Plant Physiol. 2018, 177, 398–410. [Google Scholar] [CrossRef] [Green Version]
- Ezhang, J.; Eyin, Z.; Ewhite, F. TAL effectors and the executor R genes. Front. Plant Sci. 2015, 6, 641. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Vleesschauwer, D.E.; Exu, J.; Hãfte, M. Making sense of hormone-mediated defense networking: From rice to Arabidopsis. Front. Plant Sci. 2014, 5, 611. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Macías, J.P.; García, Y.C.; Núñez, M.; Díaz, K.; Olea, A.F.; Espinoza, L. Plant Growth-Defense Trade-Offs: Molecular Processes Leading to Physiological Changes. Int. J. Mol. Sci. 2021, 22, 693. [Google Scholar] [CrossRef]
- Tsuda, K.; Somssich, I.E. Transcriptional networks in plant immunity. New Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.; Crespo-Herrera, L.A.; Villaseñor-Mir, H.E.; Rodriguez-Garcia, M.F.; Dreisigacker, S.; Barcenas-Santana, D.; Lagudah, E. Adult Plant Slow Rusting Genes Confer High Levels of Resistance to Rusts in Bread Wheat Cultivars from Mexico. Front. Plant Sci. 2020, 11, 824. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-espino, J.; Mcfadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. Pathogens in Wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böni, R.H. Functional Characterization of the Wheat Disease Resistance Gene Lr34 in Functional Characterization of the Wheat Disease Resistance Gene Lr34 in Heterologous Barley. Ph.D. Thesis, University of Zurich, Zürich, Switzerland, 2017. [Google Scholar]
- Mandalà, G.; Tundo, S.; Francesconi, S.; Gevi, F.; Zolla, L.; Ceoloni, C.; D’Ovidio, R. Deoxynivalenol Detoxification in Transgenic Wheat Confers Resistance to Fusarium Head Blight and Crown Rot Diseases. Mol. Plant-Microbe Interact. 2019, 32, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Tundo, S.; Kalunke, R.; Janni, M.; Volpi, C.; Lionetti, V.; Bellincampi, D.; Favaron, F.; D’Ovidio, R. Pyramiding PvPGIP2 and TAXI-III but Not PvPGIP2 and PMEI Enhances Resistance Against Fusarium graminearum. Mol. Plant-Microbe Interact. 2016, 29, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Tundo, S.; Janni, M.; Moscetti, I.; Mandalà, G.; Savatin, D.; Blechl, A.; Favaron, F.; D’Ovidio, R. PvPGIP2 Accumulation in Specific Floral Tissues But Not in the Endosperm Limits Fusarium graminearum Infection in Wheat. Mol. Plant-Microbe Interact. 2016, 29, 815–821. [Google Scholar] [CrossRef]
- Obbard, D.J.; Gordon, K.H.J.; Buck, A.; Jiggins, F.M. The evolution of RNAi as a defence against viruses and transposable elements. Philos. Trans. R. Soc. B Biol. Sci. 2008, 364, 99–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, C.; Kuo, Y.-W.; Wuriyanghan, H.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Sidahmed, A.M.E.; Wilkie, B. Endogenous Antiviral Mechanisms of RNA Interference: A Comparative Biology Perspective. Adv. Struct. Saf. Stud. 2010, 623, 3–19. [Google Scholar] [CrossRef]
- Gaffar, F.Y.; Koch, A. Catch Me If You Can! RNA Silencing-Based Improvement of Antiviral Plant Immunity. Viruses 2019, 11, 673. [Google Scholar] [CrossRef] [Green Version]
- Fahim, M.; Millar, A.; Wood, C.C.; Larkin, P.J. Resistance to Wheat streak mosaic virus generated by expression of an artificial polycistronic microRNA in wheat. Plant Biotechnol. J. 2011, 10, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Kis, A.; Tholt, G.; Ivanics, M.; Várallyay, É.; Jenes, B.; Havelda, Z. Polycistronic artificial miRNA-mediated resistance toWheat dwarf virusin barley is highly efficient at low temperature. Mol. Plant Pathol. 2015, 17, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Scorza, R.; Callahan, A.; Dardick, C.; Ravelonandro, M.; Polak, J.; Malinowski, T.; Zagrai, I.; Cambra, M.; Kamenova, I. Genetic engineering of Plum pox virus resistance: ‘HoneySweet’ plum—From concept to product. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 115, 1–12. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.-H. Host-induced gene silencing of cytochrome P450 lanosterol C14 -demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Song, X.-S.; Xiao-Li, Q.; Cao, L.-H.; Sun, K.; Qiu, X.-L.; Xu, Y.-B.; Yang, P.; Huang, T.; Zhang, J.-B.; et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015, 13, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; McCallum, B.; Bakkeren, G. Endogenous silencing of P uccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013, 73, 521–532. [Google Scholar] [CrossRef]
- Panwar, V.; McCallum, B.; Bakkeren, G. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Mol. Biol. 2013, 81, 595–608. [Google Scholar] [CrossRef]
- Chen, W.; Kastner, C.; Nowara, D.; Oliveira-Garcia, E.; Rutten, T.; Zhao, Y.; Deising, H.B.; Kumlehn, J.; Schweizer, P. Host-induced silencing of Fusarium culmorum genes protects wheat from infection. J Exp Bot. 2016, 67, 4979–4991. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Allah, E.F.A.; Bae, H. Genome Editing Tools in Plants. Genes 2017, 8, 399. [Google Scholar] [CrossRef] [Green Version]
- Arora, L.; Narula, A. Gene Editing and Crop Improvement Using CRISPR-Cas9 System. Front. Plant Sci. 2017, 8, 1932. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, Z.; Long, J.; Sosso, D.; Liu, B.; Eom, J.-S.; Huang, S.; Liu, S.; Cruz, C.V.; Frommer, W.; et al. Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J. 2015, 82, 632–643. [Google Scholar] [CrossRef]
- Wang, F.; Wang, C.; Liu, P.; Lei, C.; Hao, W.; Gao, Y.; Liu, Y.-G.; Zhao, K. Enhanced Rice Blast Resistance by CRISPR/Cas9-Targeted Mutagenesis of the ERF Transcription Factor Gene OsERF922. PLoS ONE 2016, 11, e0154027. [Google Scholar] [CrossRef]
- Ma, J.; Chen, J.; Wang, M.; Ren, Y.; Wang, S.; Lei, C.; Cheng, Z. Sodmergen Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J. Exp. Bot. 2018, 69, 1051–1064. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Piffanelli, P.; Ramsay, L.; Waugh, R.; Benabdelmouna, A.; D’Hont, A.; Hollricher, K.; Jørgensen, J.H.; Schulze-Lefert, P.; Panstruga, R. A barley cultivation-associated polymorphism conveys resistance to powdery mildew. Nat. Cell Biol. 2004, 430, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze-Lefert, P.; et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Bai, Y.; Pavan, S.; Zheng, Z.; Zappel, N.F.; Reinstädler, A.; Lotti, C.; De Giovanni, C.; Ricciardi, L.; Lindhout, P.; Visser, R.; et al. Naturally Occurring Broad-Spectrum Powdery Mildew Resistance in a Central American Tomato Accession Is Caused by Loss of Mlo Function. Mol. Plant-Microbe Interact. 2008, 21, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Sedeek, K.E.M.; Mahas, A.; Mahfouz, M. Plant Genome Engineering for Targeted Improvement of Crop Traits. Front. Plant Sci. 2019, 10, 114. [Google Scholar] [CrossRef] [Green Version]
| Immunity Level of Intervention | Biotechnological Intervention | Gene | Species | Enhanced Resistance to | References |
|---|---|---|---|---|---|
| Pathogen sensing | Interspecies/interfamily transfer of known PRRs | AtEFR | Wheat | Pseudomonas syringae pv. oryzae | [79] |
| AtEFR | Rice | Xanthomonas oryzae pv. oryzae-derived elf18 | [80] | ||
| AtEFR | Rice | Acidovorax avenae subsp. avenae | [81] | ||
| OsXa21 | Rice | Xanthomonas oryzae pv. oryzae | [82] | ||
| TaRLK1 and TaRLK2 | Wheat | Blumeria graminis f. sp. tritici | [83] | ||
| HvLEMK1 | Barely, Wheat | Blumeria graminis f.sp. hordei; Blumeria graminis f. sp. tritici | [84] | ||
| HvLecRK-V | Wheat | Blumeria graminis f. sp. tritici | [85] | ||
| Production of chimeric receptor kinases and R genes | AtEFR-OsXa21 | Rice | Pseudomonas syringae pv. tomato; Agrobacterium tumefaciens; Xanthomonas oryzae pv. oryzae | [86,87] | |
| OsXa21-OsCEPiP | Rice | Magnaporthe oryzae | [88] | ||
| Effector detection | Deletion of effector binding sites | Os11N3/OsSWEET14 | Rice | Xanthomonas oryzae pv. oryzae | [89] |
| Addition of effector binding sites | OsXa27 | Rice | Xanthomonas oryzae pv. oryzae | [90] | |
| Immune signaling | Altered expression of signaling components | AtNPR1 | Rice | Broad-spectrum of pathogens | [91] |
| Altered expression of transcription factors | TaPIMP1 | Wheat | Bipolaris sorokiniana | [92] | |
| OsIPA1/OsSPL14 | Rice | Magnaporthe oryzae | [93] | ||
| R genes | Transfer of APR alleles | TaLr34 | Barely, Rice, Sorghum Maize, Durum wheat | Multiple biotrophic pathogens | [94,95,96,97,98] |
| TaLr67 | Barely | Multiple rusts and powdery mildew | [99] |
| Molecular Technique | Biotechnological Intervention | Gene | Species | Enhanced Resistance to | References |
|---|---|---|---|---|---|
| RNAi | Viral gene silencing | Wheat streak mosaic virus genes | Wheat | Wheat streak mosaic virus (WSMV) | [125] |
| Wheat dwarf virus genes | Barely | Wheat dwarf virus (WDV) | [126] | ||
| Host-induced gene silencing | FgCYP51A, FgCYP51B and FgCYP51C | Barely | Fusarium graminearum | [128] | |
| FgCh3b | Wheat | Fusarium graminearum | [129] | ||
| PtMAPK1, PtCYC1, PtCNB | Wheat | Puccinia triticina, P. graminis and P. striiformis | [130,131] | ||
| FcGls | Wheat | Fusarium culmorum | [132] | ||
| CRISPR/Cas9 | Silencing of host genes | TaMlo-A1 | Wheat | Blumeria graminis f. sp. tritici | [136] |
| OsSWEET13 | Rice | Xanthomonas oryzae pv. oryzae | [137] | ||
| OsERF922 | Rice | Magnaporthe oryzae | [138] | ||
| TaEDR1 | Wheat | Blumeria graminis f. sp. tritici | [43] | ||
| OsSEC3A | Rice | Magnaporthe oryzae | [139] | ||
| TaLpx-1 | Wheat | Fusarium graminearum | [102] | ||
| TaHRC | Wheat | Fusarium graminearum | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigini, V.; Camerlengo, F.; Botticella, E.; Sestili, F.; Savatin, D.V. Biotechnological Resources to Increase Disease-Resistance by Improving Plant Immunity: A Sustainable Approach to Save Cereal Crop Production. Plants 2021, 10, 1146. https://doi.org/10.3390/plants10061146
Bigini V, Camerlengo F, Botticella E, Sestili F, Savatin DV. Biotechnological Resources to Increase Disease-Resistance by Improving Plant Immunity: A Sustainable Approach to Save Cereal Crop Production. Plants. 2021; 10(6):1146. https://doi.org/10.3390/plants10061146
Chicago/Turabian StyleBigini, Valentina, Francesco Camerlengo, Ermelinda Botticella, Francesco Sestili, and Daniel V. Savatin. 2021. "Biotechnological Resources to Increase Disease-Resistance by Improving Plant Immunity: A Sustainable Approach to Save Cereal Crop Production" Plants 10, no. 6: 1146. https://doi.org/10.3390/plants10061146
APA StyleBigini, V., Camerlengo, F., Botticella, E., Sestili, F., & Savatin, D. V. (2021). Biotechnological Resources to Increase Disease-Resistance by Improving Plant Immunity: A Sustainable Approach to Save Cereal Crop Production. Plants, 10(6), 1146. https://doi.org/10.3390/plants10061146








