Abstract
The importance of vanadium (V) in the functioning of land systems is extremely diverse, as this element may exert both positive and harmful effects on terrestrial organisms. It recently become considered an element of beneficial character with a range of applications for human welfare. The health-ameliorative properties of this transition element depend on its degree of oxidation and on optimal concentration in the target cells. It was found that a similar relationship applies to vascular plants. However, excessive amounts of vanadium in the environment contaminate the soil and negatively affect the majority of living organisms. A significantly elevated level of V results in the destabilization of plant physiological balance, slowing down the growth of biomass which significantly reduces yield. In turn, low doses of the appropriate vanadium ions can stimulate plant growth and development, exert cytoprotective effects, and effectively enhance the synthesis of some biologically active compounds. We present the scientific achievements of research teams dealing with such topics. The issues discussed concern the role of vanadium in the environment, particular organisms, and highlight its dualistic influence on plants. Achievements in the field of V bioremediation, with the use of appropriately selected microorganisms and plant species, are emphasized.
1. Introduction
Many elements that appear in the lithosphere provide nutrients necessary for sustaining living organisms. Vanadium (V) is included as one among elements relatively widespread in the environment. Its concentration in the upper crust of the Earth in some areas may sometimes be similar to that of zinc and nickel. However, due to uneven distribution, the abundance of vanadium in the crust is difficult to estimate. Usually, it is assumed to be approximately 150 mg kg−1 (c.a. 0.019% of crust) so V is relatively common in parental rock, soil, groundwater, fossil fuels, and living organisms [1,2,3,4,5]. Volcanic areas with basaltic layers or gabbros are extremely rich in vanadium (c.a. 200–300 mg V per kg of mafic rock) [6,7,8]. The V content in carbonaceous sedimentary rocks was even treated as guideline for petroleum exploration [9,10]. Anthropogenic emissions from fossil fuels, especially as a result of long-term combustion of hard coal or crude oil, contribute to an increase in the level of vanadium to a much greater extent than is the case with any known natural sources [11,12]. Moreover, during coal combustion, elevated levels of vanadium pentoxide (V2O5) are emitted into atmosphere along with fine particles. The V2O5 becomes toxic to higher animals, including humans in concentrations exceeding 1 mg L−1 [13,14,15,16]. It was ascertained that the concentration of vanadium oxides in an urban environment with a large number of inhabitants can achieve 10 ng per cubic meter of air, while in sparsely populated rural areas is about 1 ng m−30, and above the eastern Pacific Ocean the mean vanadium air concentration is as low as 0.1 ng m−3 [17,18,19].
This paper provides insights into the results of researches conducted by scientific teams working around the world. Featured results concern both innovative ways of using vanadium in human economic activity and, on the other hand, the environmental threats resulting from the growing demand for the extraction of vanadium-rich ores. Bearing in mind the constantly increasing content of vanadium in the environment, we have undertaken a review of the most important reports on its effects on human health and plant welfare, the latter of which is known to form the basis of the trophic chain. We mainly intended to shed a light on the possible, still insufficiently researched, environmental relationships of this element.
Thus, we were focused on the current research on the interaction of plants and humans with some compounds or ions of this 21st century element, as vanadium is currently termed. In particular, we have noticed a significant gap in the progress of research on the phytoremediation of V-contaminated areas.
2. The Economic Use of Vanadium Compounds, the Basic Geochemistry and Elements of Biochemistry of V
In land environments, vanadium compounds are found in over fifty different ores [20,21]. This redox-sensitive metal exists in various minerals in different forms such as, among others, V(II), V(III), V(IV) and V(V), and is found in, for example, vanadates containing sulfides such as patronite, silicates e.g., roscoelite and cavansite or phosphates. In addition naturally occurring vanadium-containing ores include vanadite, carnotite, goethite, and birnessite [22,23,24]. These resources are currently highly exploited, mainly because of the extensive applications of this metal in some important modern industries [25,26,27]. Therefore, continuous mining and smelting activities, especially for vanadium-titanium magnetite, carnotite, vanadinite and other V-containing ores contributes to contamination of vast areas in many countries on most inhabited continents. Canada, Brazil, China, Russia, Australia, South Africa, and some European countries deal with extraction or processing of vanadium compounds [20,26,28,29,30,31], since it is a relevant element in several important industrial fields. The main current applications are the production of high-grade steel metal alloys [32], the building of redox flow batteries (VRFBs) [26], nanomaterials [25], and catalysis [26,33]. In particular, V-catalyzed processes have attracted the attention of scientists [33,34,35,36] because those metal-catalyzed reactions are a powerful tool used to design new sustainable chemical synthetic processes according to Green Chemistry principles [32,37]. Vanadium and zircon are also routinely used in the process of ceramic pigment production [32,33,38].
Therefore, this important transition metal is gradually becoming more widespread in the lithosphere and soil in the form of ores or different types of compounds, respectively. In recent years, increased content of V in soil has been found more and more frequently [10,12,21,39,40]. Aside from iron-bearing minerals, some mafic rock with V content (from 600 to 4100 mg kg−1) or rock phosphates (50−2000 mg kg−1) are exploited, and bring measurable economic benefits [23,25,26,38]. Thus, it is not surprising that vanadium is found to be present in soils within a range of 10−220 mg kg−1, depending on the considered location [3,12,28,31,41,42]. Moreover, it is additionally released to the soil matrix as a result of applying V-bearing fertilizers in agriculture, or in the case of leachates from mine tailings, land-filled municipal sewage sludge, or slag from steel manufacturing [5,30,43]. The presence of vanadium compounds in the soil is currently considered a serious toxicological problem, taking into account the effect that excessive vanadium levels exert on plants and animals.
The biochemical properties of vanadium are also interesting due to the complexity of the chemical behavior of this metallic element, and the great versatility of the coordination sphere around the metal center. Moreover, since V belongs to the transition series, it has a rich redox chemistry and is a typical compound with various degrees of oxidation. From the point of view of biological systems, it is essential that all important valences of this element, i.e., III, IV, and V (that is, from −III to +V) should be examined [44,45,46]. Vanadium(III) and V(IV) compounds are rather unstable in the presence of oxygen at physiological pH. Although V in the oxidation state +III may be found in some minerals, it is virtually absent in environmental solutions because of limited solubility. In turn, despite the fact that vanadium(IV) compounds are easily oxidized to vanadium(V), the stability of the second form is highly dependent on the presence of specific ligands [45]. In biological systems, vanadium(V) is mainly found as vanadate(−V) anions. For organisms, the toxicity of the pentavalent cationic form (+V) is considered six to ten times greater than that of V(+IV) (i.e., vanadyl(+IV)), as it is connected with inhibitions of phosphatases, ATPases, and other important enzymes [47,48,49].
3. Vanadium in the Environmental Systems
3.1. Vanadium in the Soil and Possibilities of the Use of Microorganisms to Reduce Its Toxicity
Soil contamination with vanadium is currently an important concern. Due to complex V environmental chemistry, there is no simple solution allowing the limitation of soil pollution and enhancing its cleanup. Vanadium toxicity varies considerably with the nature of the compound under consideration. As explained above, vanadium occur in nature in different oxidation states (mainly from −1 to +5), but the most common are its trivalent, tetravalent, and pentavalent forms [28,49]. However, most published works concern V in the oxidation states V(IV) and V(V) i.e., assessment of vanadyl cations VO2+(IV) and vanadate(V) anions, HVO4−, and H2VO4− [28,29,49]. In aerobic conditions, the dominant forms are vanadate(V) compounds, while in reducing environments VO2+(IV) is much more frequent. As with other trace elements (TEs), vanadium may be sorbed in or adsorbed on different soil components [31,41,42]. For example, the cation VO2+ can be strongly adsorbed on soil particles, and in different pH ranges it may form complexes with humic acids [29]. Therefore, promoting reduction of V(V) forms to lower redox states may be treated as reasonable remediation strategy (Figure 1). The level of soil contamination may be verified, among other ways, on the basis of geoaccumulation index (GI), enrichment factor (EF) or pollution load index (PLI) [50,51,52]. Larson et al. [53] used XANES spectroscopy and HPLC-ICP-MS to study the speciation of vanadium in the soil of a pine forest stand localized in southern Sweden. A field experiment was started there in 1978. At that time, different liming levels were used, i.e., 0.2, 0.7 and 1. kg m−2. The authors of the aforementioned paper [53] found that the pentavalent vanadium form was largely accumulated in the mor layer, and they concluded that after 26 years the content of V in studied soil was below toxic the threshold according to Swedish regulations. Using speciation techniques, they determined that the speciation of vanadium in soil depends on properties such as V concentration, particle size distribution, sand, clay and silt content, soil pH, cation exchange capacity (CEC), organic matter content (SOM), and iron and aluminum hydroxide content, as the latter hydroxides have been proven to be important sorbents of V2O.
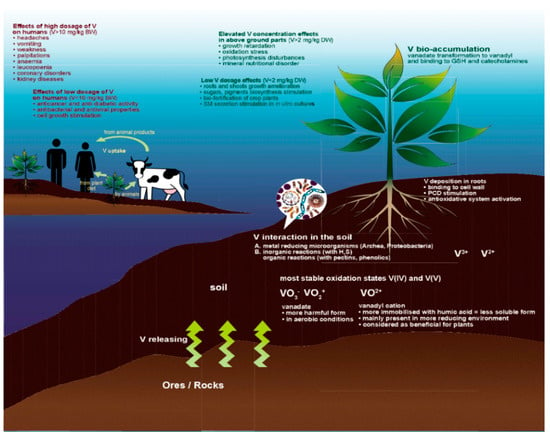
Figure 1.
Vanadium circulation in the environment and main beneficial and detrimental effects on plants and humans. (1) V transformation within the soil and roots—marked with white font; (2) V translocation to stems and leaves and its effects on plant physiology—marked with green font; (3) V uptake and its effect on human organism—marked with yellow and brown font; SM—secondary metabolites.
Vanadate is almost a structural and electronic analog of phosphate, especially in the tetrahedral trianionic forms, that is, VO43− and PO43−. In most living organisms, vanadate ions negatively influence the phosphate-metabolizing system by inhibiting phosphatases, phosphodiesterases, ribonucleases, ATPases and other enzymes [54,55]. It is therefore important that vanadium(V) may be reduced to a less soluble V(IV) form by inorganic reactions such as with H2S [56] or by some metal-reducing microorganisms (Figure 1). The microbiota may reduce vanadium through detoxification or to use this element as an electron acceptor during respiration. A second pathway was found for Anabena azotica and Azotobacter vinelandii (which have vanadium-dependent nitrogenases for the conversion of dinitrogen to ammonium anions) [52,53]. Geobacter metallireducens from the Geobacteriaceae family have been found to be effective vanadium-reducing bacteria [57]. Shewanella oneidensis and some strains of Pseudomonas have also been reported to be almost comparably effective [58,59,60,61]. Zhang et al. [62] proved that thermophilic methanogen archeons are capable of reducing pentavalent vanadium (vanadate) to vanadyl forms under a variety of conditions. This approach is currently treated as anaerobic vanadium remediation. Thus, both chemical and biological treatments allowing the immobilization of soluble vanadium forms and forming reduced forms should be applied in order to remediate either contaminated ground/waters or the soil water fraction [57,61].
Relatively recently, a sustainable low-cost technology that uses anaerobic microbes that form an electroactive biofilm, the so-called microbial fuel cells (MFCs), was developed. On this basis, very promising instruments for the purification of the fraction of water contaminated with vanadium and other heavy metals (HMs) were constructed [63,64]. The bio-electrochemical device has anode and cathode compartments, which are usually separated by the proton exchange membrane (PEM). In the anode compartment, the appropriate bacterial strains break down different types of contaminated media with organic matter, while generating electrons and protons (which must diffuse through the PEM). After the remediation process, electrochemically reduced metals can be recovered from the cathode surface [65]. In vanadium-MFCs with Rhodoferax ferriducens inoculated to the anode chamber, V reduction efficiency reached 67.9% after 240 h. The device could work with a maximum power density of 970.2 mW m2 [66,67]. MFCs also have great potential as biosensors for water quality assessment [67].
3.2. Vanadium in Soil-Plant Continuity: The Effect of Vanadium on Plants
Plants, to grow and develop, must take up minerals from the soil solution in an ionic form. The uptake of needed elements from the soil by individuals of a given population, and in polluted habitats also of some ballast elements, should be treated as a form of geochemical circulation of elements [68,69,70,71]. Excessive doses of micronutrients or ultra-elements often have a negative or sometimes even lethal effect on plant cells [72,73]. The question of the dose and harmfulness threshold is definitely important here, but in the case of vanadium this parameter has not been established for most species. Long-term exposure to excessive doses of HMs has a negative effect on the processes taking place in individual tissues and organs. Thus, the effect of exposure can ultimately be observed at the level of the whole organism.
As a result of uptake of vanadium ions with further accumulation in the aerial parts, some plant species indicate the current level of V in the soil. The content of V in shoots of indicator species should be directly proportional to the content of soluble forms of this element in soil, and these values should be reflected by a linear function. Also, the concentration in the plant root system increases with leachable vanadium content in soil [74]. Wild-growing species such as Astragalus confertiflorus, A. lentiginosus, A preussi, A. thompsonae, Trifolium pratense (Fabaceae), Castillega angustifolia synon. C. chromosa (Orobanchaceae), Chrysothamnus viscidiflorus, Cichorium intybus var. foliosum, Eupatorium capilifolium (Asteraceae), and Allium macropetalum (Amaryllidaceae) were mentioned as indicator plants for vanadium concentration in soils [75,76,77].
In the majority of cultivated plants, the internal V concentration is about 10 times lower than that in the rhizosphere. When vanadium is already present in plant cells, it tends to be converted to vanadyl forms that are bound either to glutathione, catecholamines, or some low molecular-weight peptides [77,78]. In most tested species, vanadium concentration in the tissues varied with their shoot-root ratio. As for the aboveground organs, generally, leaves (shoots) contain higher vanadium content than fruits and seeds because the plants actively protect the generative progeny from the toxic effects of heavy metals [79].
Somewhat similarly to the case of the use of different species in bio-prospection, it is possible to use plant material to remediate polluted sites [80,81,82]. Examples of species that can be used on vanadium-contaminated surfaces are shown in Table 1. The representatives presented in the table are quite often used in this type of application, such as the most commonly used species from the Brassicaceae family [83,84] as well as from Fabaceae, Chenopodiaceae, Asteraceae [85,86,87,88], and Poaceae [74,89]. As intended, Table 1 contains interesting field-scale studies and shows that successful attempts can be made to biologically clean up V-contaminated soils using higher plants. Laboratory and field experiments have also been conducted to elucidate the effects on plant functioning. The results broaden basic knowledge and facilitate the development of phytoremediation protocols for V-contaminated soils.
Historically, however, vanadium was first identified as a plant growth disruptor. Only in the course of research in the 20th century was it found that V can have not only a negative but also a beneficial effect on plants [90]. There are several factors that influence effect of V on plants, both positive and negative, depending on their layout. Among them, the nutritional status of plants has been mentioned. It was found that higher sensitivity to vanadium pentoxide toxicity was ascertained in plants growing under nutrient stress [90,91]. The detrimental effect of vanadium becomes apparent when the concentration in the tissues exceeds 2 mg·kg−1 of dry weight [92]. As in other transition metals, vanadium-exposed plants suffer from growth retardation, resulting from severe nutritional and oxidative disorders. The level of V bioaccumulation in plant organs and the severity of negative symptoms increases with an increase in the V concentration in nutrient medium. A commonly observed response is overproduction of reactive oxygen species (ROS) leading to oxidative stress and cell death [92,93,94,95]. In such cases the efficiency of plant photosynthetic reactions decreases, which can be attributed to the disturbance of chloroplasts, namely the structure of thylakoids and photosystems [93,96,97].
Most plant species exposed to vanadium (in soil or nutrient medium) deposit large amounts of its ions in the roots as compared to the shoots [97,98]. In this case, the tolerance strategy involves restriction of V translocation to the shoot, and stabilization of the element in the root system within the soil-plant continuity. The fine roots gradually turn into organic matter. The ability of the roots to detoxify V can be increased by enlargement of intercellular spaces. Vanadium may bind to cell walls to further limit its mobility in the plant [98]. Tolerant species or ecotypes are able to intensify their antioxidant activity in order to counteract destructive effects of ROS [78,93]. This defense involves enhanced activity of antioxidant enzymes and efficient accumulation of non-enzymatic antioxidants, especially anthocyanins, phytochelatins and glutathione, compared to sensitive genotypes [93,98,99,100]. Research shows that it is possible to select V-tolerant genotypes within one species, as in case of rice [94,100]. Properly selected plant material that exhibits relative tolerance to V, and is able to accumulate it, is crucial for phytoremediation programs. Moreover, the detoxification of V-contaminated substrate can be facilitated by the use of various organic compounds, naturally present in the rhizosphere. Here, caffeic acid and pectins have been found to promote the reduction of vanadium(V) compounds to less toxic forms [101].

Table 1.
The assessment of the potential of the so far studied species representing various taxonomic origin for use during the reclamation schemes of the polluted substrate or bottom sediments from excessive vanadium content.
Table 1.
The assessment of the potential of the so far studied species representing various taxonomic origin for use during the reclamation schemes of the polluted substrate or bottom sediments from excessive vanadium content.
| Family | Studied Species | The Area under Study | Data on Vanadium Concentration | Phytoremediation Usefulness | Reference | |
|---|---|---|---|---|---|---|
| Phytoextraction | Phytostabilization | |||||
| Brassicaceae | Raphanus sativus | Field research in Panzhihua city- mining V-Ti magnetite | 26.6 mg kg−1 d.m. and 12.6 mg kg−1 d.m. in leaves and root respectively | may be useful | not surely | [84] |
| Fabaceae | Medicago sativa Trifolium alexandrinum | Field research near power station | Leaf V conc. 9.6 mg kg−1 d.m. and 13.6 mg kg−1 d.m. respectively | not fully not fully | useful fully useful | [87] |
| Glycine max | Field research in Panzhihua city- mining V-Ti magnetite | Leaf conc. 27.3 mg kg−1 d.m. | may be useful | useful | [84] | |
| Chenopodiaceae | Chenopodium album | Field research: Shian City, Hubei Province, China | 384.3 mg kg−1 d.m. in roots r | may be useful | fully useful | [5] |
| Spinacia oleracea | Field research near power station | Leaf conc. 9.1 mg kg−1 d.m. | not fully | not useful | [84] | |
| Beta vulgaris | Field research in Panzhihua city- mining V-Ti magnetite | Conc. in the leaf tissue 6.5–36.3 mg kg−1 d.m. | may be useful | not surely | [87] | |
| Lamiaceae | Mentha × piperata | Field research near power station | Leaf conc. 13.0.5 mg kg−1 d.m. | not fully | not surely | [87] |
| Solanaceae | Solanum tuberosum | Field research near power station | Leaf conc. 10.8 mg kg−1 d.m. | not useful | not useful | [87] |
| Asteraceae | Artemisia vulgaris | Field research at a brownfield site in New Jersey | 11.6 mg kg−1 d.m. and 113.0 mg kg−1 d.m. in leaves and roots respectively | may be useful | fully useful | [74] |
| Betulaceae | Betula populifolia | Field research at a brownfield site in New Jersey | 12.1 mg kg−1 d.m. and 280.0 mg kg−1 d.m. in leaves and roots respectively | may be useful | fully useful | [74] |
| Anacardiaceae | Rhus coppallinum | Field research at a brownfield site in New Jersey | 8.7 mg kg−1 d.m. and 118.0 mg kg−1 d.m. in leaves and roots respectively | not useful | fully useful | [74] |
| Poaceae | Phragmites australis | Field research at a brownfield site in New Jersey | 12.1 mg kg−1 d.m. and 280.0 mg kg−1 d.m. in leaves and rhizomes respectively | fully useful | fully useful | [74] |
| Settaria viridis | Field research: Shian City, Hubei Province, China | Up to 156.9 mg kg−1 d.m. and 142.4 mg kg−1 d.m. in shoots and roots respectively | fully useful | fully useful | [5,89] | |
| Rhizophoraceae | Cerriopis decandra | Field research: Swamp among Lothian Island and the Bengal Bay, India | 781.8 µg kg−1 d.m., 812.2 µg kg− d.m and 1439.61 µg kg− d.m. in leaves, wood and roots respectively | useful | useful | [102] |
| Fabaceae | Medicago sativa | Innovative plant/microbiota combined approach | Up to 500 mg kg −1 | useful | useful | [88] |
The stimulating effect of V occurs at low concentrations, not exceeding 2 mg·kg−1 of dry weight of plant tissue. The effect depends on the degree of oxidation state of the element. The tetravalent forms are considered to be beneficial to plant performance, while pentavalent forms are clearly harmful. Some plants, such as Ipomoea aquatica, are able to reduce V(V) to V(IV) in order to protect the tissues [103]. The positive effect of vanadium is reflected in the stimulation of growth and accumulation of biomass, especially in the formation of new leaves and flowers, as well as in the development of root systems [103,104,105]. It is often associated with an altered nutritional status of particular plant organs [106]. At the cellular level, the synthesis of chlorophylls, amino acids, sugars, and other metabolites, particularly non-enzymatic antioxidants, is enhanced. The enzymatic antioxidant system may be also activated, at low doses of vanadium. The benefits that were reported in plant functioning upon exposure to V are summarized in detail in Table 2.

Table 2.
Examples of beneficial effects of vanadium on plant physiology.
In agricultural and horticultural practice, vanadium salts can be used to manipulate plant secondary metabolism. It has recently been shown that V can facilitate the biofortification of crops with forms of iodine and that this favors the formation of its organic derivatives [106,107]. Vanadium compounds can also be applied to stimulate the production and secretion of secondary metabolites in suspension cultures for pharmaceutical purposes. Vanadium compounds can be further used to reduce the effects of stress and to improve the growth of plants under unfavorable conditions. Recent studies report on the protective effect of vanadium (IV) complexes during the treatment of plants with pro-oxidant H2O2. Arabidopsis thaliana facing oxidative stress performed better if it had been pretreated with oxidovanadium(IV) complexes [108]. Previously, organic V complexes, such as oxidovanad(IV) were found to possess cyto-protective properties when applied on animal and human cells suffering from oxidative stress [109,110]. Additionally, vanadium treatment can be used to reduce the detrimental effects or prevent accumulation of selected toxic trace metals, such as Cu, Hg, and Pb [104,105].
3.3. The Effect of Vanadium on Animals and Humans
Vanadium is usually present at ultra-trace or trace concentrations in higher plants and some animals respectively [110,111]. In humans the similarity between vanadate and phosphate accounts for the interplay between vanadate and phosphate-dependent enzymes, thus, vanadate may fulfill a regulatory function in metabolic processes depending on phosphate. However, for a long time, vanadium has not been considered a beneficial element for humans because food that we consume on a daily basis contains extremely low amounts of V (usually less than 1 ng g−1). Recently, vanadium has been recognized as an ultra trace element for animals including humans, so called occasionally beneficial element [112]. Parsley, and leafy vegetables such as lettuce and spinach, and spears of asparagus, some cereal products (e.g., rye flour), black pepper, and mushrooms are listed as good sources of vanadium in the diet [112,113]. They are now considered suitable sources of V in a well-balanced human diet.
Inorganic medicinal chemistry that uses the properties of metal ions to design compounds with therapeutic applications is currently a dynamically developing research area. The goal is an innovative treatment of several types of human diseases [47,48,114,115]. Vanadium compounds administered at pharmacological doses have shown interesting biological effects, such as anticancer activity [47,114,116,117,118,119], anti-amoebic activity, and beneficial effects in the treatment of some parasitic diseases [1,120]. These compounds have also been found to possess antibacterial and antiviral properties. There are reports of anti-tuberculosis activity [44,121] and activity against influenza and HIV viruses [44], but unfortunately, a toxic effect of V-therapy given at higher doses has sometimes been also found [122,123]. The risk of adverse health effects of vanadium occurs when V consumption exceeds 10 mg kg−1 of body weight (BW) for an adult person [122,124].
Some vanadium derivatives gained the term “growth factor mimetic agent” thanks to the work of Cortiso and Etcheverry’s [123]. Moreover, important results of Cortiso and Etcheverry [123] studies on osteoblast-like URM106 cells clearly show that even though lower doses of vanadate stimulated the growth of cells under in vitro conditions, supplementation with higher doses inhibited cell proliferation. Likewise, the application of vanadyl-stimulated cell proliferation in a dose-responsive manner. Additionally, it has been proven that vanadyl and pervanadate are stronger stimulants of cell growth than vanadate. Vanadium ions affect human cells through signal transduction pathways, including those involved in the action of new vanadium(IV)-trehalose complexes [125], and vanadium(IV) complexes, multiple oxygen donor ligands, such as oxadiacetate (oda) and related derivatives with 2,2′ bypiridine, and 1,10-phenanthroline. Regarding the therapeutic uses of vanadium compounds (VCs), research in medicinal chemistry has focused on improving the oral bioavailability, (bio)distribution, and tolerability of new vanadium therapeutic agents, such as insulin-like vanadium compounds, which have an antidiabetic (insulin-enhancing) effect [126]. Among the surveyed VCs, there are the so called insuline-mimetics or insuline-enhancers, which are usually organic vanadium derivatives, such as bis(ethylomalatolato)oxidovanadium(IV) (BEOV) [115,126]. They can be studied in detail in animal models [127], which may speed up studies and accelerate research progress.
In excessive doses V becomes seriously harmful for all living organisms, regardless of whether they are sessile like plants or mobile like animals [111,128]. For humans, vanadium poisoning may be very dangerous and cause dermatitis, green coloration of the tongue, vomiting, headache, weakness, palpitations, anemia, leucopoenia, leukocyte granulation or coronary insufficiency, as well as chronic kidney disease [3,44]. Since considerable vanadium amounts can be supplied to human organism with food cultivated on contaminated land (Figure 1), it has become crucial to monitor the content of this element in the soil and in crops grown for consumption. Moreover, it is very important to elucidate in detail how different plant organisms function in the presence of vanadium, and in particular, the availability and storage of V must be thoroughly investigated at various levels of biological organization.
4. Conclusions and Research Prospects
Vanadium is an important transition element. The degree of oxidation, and the concentration and duration of exposure determine the dual role that vanadium can play in living organisms. It is no longer considered just a toxic element, it became an ultra-element that can be widely exploited to improve both human health and plant performance. In nature, vanadium is usually present at ultratrace and trace concentrations in higher plants and animals, respectively. In the field of plant science, many of the presented reports support the dose-dependent effect of vanadium ions on plant physiology. Additionally, awareness of the toxic properties of excessive soil V content is growing, which in turn creates the need for the application of innovative bioremediation methods to prevent soil pollution. Novel data compiled in this review suggest V exploitation as a potent elicitor, a stimulant of secondary metabolite production, and a stress-protective compound that can be used for plant priming towards abiotic stress tolerance. These emerging areas of research broaden our understanding on the conditions of beneficial effects of vanadium on plants, which enables the application of vanadium compounds to facilitate production of agricultural plants. On the other hand, it is necessary to further progress in application research related to the reclamation of polluted environments in accordance with the principles of sustainable development.
While significant progress has been made in the field of vanadium biogeochemistry, the metabolomics of vascular plants capable of detoxifying various types of V ions is currently at a very early stage. To approach this problem, the first challenge would be to identify the appropriate plant material. This is not an easy task because the information required for this identification is scattered across different types of publications. Further knowledge should then focus on the specific effects of vanadium in different models and in experiments conducted at different levels of plant organization. In particular, we need to explain the interrelationships between V and other elements/metabolites in some herbaceous and tree models. In our opinion, the next step should be to standardize the methodology which would favor the integration of data obtained by different research teams. This would be especially important for comparing results obtained from different metabolomics studies.
In addition, another important issue is to address model aquatic organisms. In this particular case, both the correct selection of material and the development of an appropriate methodological approach would allow for an effective diagnosis of ecological threats in various water reservoirs.
The long-term task is to reproduce clones (lines) useful in projects aimed at effective remediation of different vanadium-contaminated environmental compartments.
Author Contributions
Conceptualization, E.H.-F., I.K. and A.W.; supervision E.H.-F.; bibliography query, E.H.-F. and A.W.; writing—original draft preparation, E.H.-F. and A.W.; writing—editing, E.H.-F. and I.K.; visualization I.K.; writing—responding to reviewers’ comments, E.H.-F. and I.K.; funding acquisition, E.H.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ministry of Science and Higher Education of the Republic of Poland, grant no. 050012-D011.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef]
- Costa Pessoa, J.; Etcheverry, S.B.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301, 24–48. [Google Scholar] [CrossRef]
- Jayawardana, D.T.; Pitawala, H.M.G.A.; Ishiga, H. Geochemical evidence for the accumulation of vanadium in soils of chronic kidney disease areas in Sri Lanka. Environ. Earth Sci. 2015, 73, 5415–5424. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Yan, H.; Cheng, Y.; Wang, S.; He, Z. Microbial reduction of vanadium (V) in groundwater: Interactions with coexisting common electron acceptors and analysis of microbial community. Environ. Pollut. 2017, 231, 1362–1369. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Jiang, J.; Li, D.; Liu, N.; Yang, N.; Meng, Y. The interactions of metal concentrations and soil properties on toxic metal accumulation of native plants in vanadium mining area. J. Environ. Manag. 2018, 222, 216–226. [Google Scholar] [CrossRef]
- Mathieu, L. Origin of the vanadiferous serpentine-magnetite rocks of the Mt. Sorcerer area, Lac Doré layered intrusion, Chibougamau, Quebec. Geosciences 2019, 9, 110. [Google Scholar] [CrossRef]
- Ottens, B.; Götze, J.; Schuster, R.; Krenn, K.; Hauzenberger, C.; Zsolt, B.; Vennemann, T. Exceptional multi stage mineralization of secondary minerals in cavities of flood basalts from the Decan Volcanic Province, India. Minerals 2019, 9, 351. [Google Scholar] [CrossRef]
- Sanilot, N.; Vlastélic, S.; Moune, S.; Rose-Koga, E.F.; Schiavi, F.; Valade, S.; Aguilera, F. Uptake of gaseous thallium, vanadium and molybdenum into anhydrous alum, Lascar volcano fumaroles, Chile. Geochim. Cosmochim. Acta 2020, 15, 64–82. [Google Scholar] [CrossRef]
- Breit, G.N.; Wanty, R.B. Vanadium accumulation in carbonaceous rocks: A review of geochemical controls during deposition and diagenesis. Chem. Geol. 1991, 91, 83–97. [Google Scholar] [CrossRef]
- de Caritat, P.; Reimann, C.; Smith, D.B.; Wang, X. Chemical elements in the environment: Multi-elemental geochemical datasets from continental—to national-scale surveys on four continents. Appl. Geochem. 2018, 89, 150–159. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, M.; Jiang, Z.; Liu, Y.; Cao, X. Soil vanadium pollution and microbial response characteristics from stone coal smelting district. T. Nonfer. Metals Soc. 2015, 25, 1271–1278. [Google Scholar] [CrossRef]
- Li, J.; Sun, C. Evaluation of migration of thallium, cadmium, vanadium, and chromium from thermal power plant. Environ. Earth Sci. 2016, 75, 388. [Google Scholar] [CrossRef]
- Moreno, T.; Querol, M.; Alastuey, A.; de la Rosa, J.; Sánchez de la Campa, A.M.; Minguillón, M.; Pandofili, M.; González-Castanedo, Y.; Monford, E.; Gibbons, W. Variations in vanadium, nickel and lantanoid element concentrations in urban air. Sci. Total Environ. 2010, 20, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Qi, C.; Cheng, S.; Sun, R. Chemical speciation and combustion behavior of chromium and vanadium in coals. Fuel 2016, 184, 42–49. [Google Scholar] [CrossRef]
- Rodríguez-Lara, V.; Morales-Rivero, A.; Muñiz Rivera-Cambas, A.; Fortoul, T.I. Vanadium inhalation induces actin changes in mice testicular cells. Toxicol. Ind. Health 2016, 32, 367–374. [Google Scholar] [CrossRef]
- Gallardo-Vera, F.; Tapia-Rodrigues, M.; Dias, D.; van der Goes, T.V.; Montaño, L.F.; Rendón-Hueta, E.P. Vanadium pentoxide increased PTEN and decreased SHP1 expression in NK-92MI cells, affecting PI3K-AKT-mTOR and Ras-MAPK pathways. J. Immunol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Janssen, N.A.H.; Hoek, G.; Simic-Lawson, M.; Fisher, P.; van Bree, L.; ten Brink, H.; Kekuen, M.; Atkinson, R.W.; Anderson, R.H.; Brunkreef, B.; et al. Black carbon as an additional indicator of the adverse health effects of airborne particles compared with PM10 and PM2.5. Environ. Health Perspect. 2011, 119, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.A.; Zhang, K.; Schroeck, N.J.; McCoy, B.; Mc Elmurry, S.P. Petroleum Coke in The Urban Environment: A Review of Potential Health Effects. Int. J. Environ. Res. Public Health 2015, 12, 6218–6231. [Google Scholar] [CrossRef] [PubMed]
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, S.; Hunter, R.P. Health Outcomes of Exposure to Biological and Chemical Components of Inhalable and Respirable Particulate Matter. Int. J. Environ. Res. Public Health 2016, 13, 529. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, R.; Nikoloski, A.N. The extraction of vanadium from titanomagnetic and other sources. Miner. Eng. 2020, 146, 106. [Google Scholar] [CrossRef]
- Silin, I.; Hahn, K.M.; Gürsel, D.; Kremer, D.; Gronen, L.; Stopić, S.; Friedrich, B.; Wotruba, H. Mineral processing and metallurgical Treatment of Laed Vanade Ores. Minerals 2020, 10, 197. [Google Scholar] [CrossRef]
- Kurt, M.; Çelik, F. Investigation of single crystal and polycrystaline forms of copper(Ii) and vanadium(II) doped beta potassium sulfate complex by electron spin resonance technique. Int. J. Pure Appl. Sci. IJPAS 2017, 3, 33–39. [Google Scholar]
- Schuth, S.; Horn, I.; Brüske, A.; Wolf, P.E.; Weyer, S. First vanadium isotope analyses of V-rich minerals by femtosecond laser ablation and solution-nebulization MC-ICP-MS. Ore Geol. Rev. 2017, 81, 1271–1286. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, X.; Guo, Z.; Peng, C.; Wang, X.; Yang, A. Characteristic and behaviour of vanadium(V) adsorption on goethite and birnessite. Environ. Earth Sci. 2020, 79, 240. [Google Scholar] [CrossRef]
- Ojelere, O.; Graf, D.; Ludwig, T.; Vogt, N.; Klein, A.; Mathur, S. Reductive transformation of V(III) precursors into vanadium(II) oxide nanowires. Dalton Trans. 2018, 47, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Roznyatovskaya, N.; Noack, J.; Pinkwart, K.; Tübk, J. Aspect of electron transfer processes in vanadium redox-flow batteries. Cur. Opin. Electrochem. 2020, 19, 42–48. [Google Scholar] [CrossRef]
- Preziozi, E.; Guliano, G.; Vivona, R. Natural background levels and threshold values derivation for naturally As, V abd F rich groundwater bodies: A methodological case study in Central Italy. Environ. Earth Sci. 2010, 61, 885–897. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Shaheen, S.M. Distribution of Total and Ammonium Bicarbonate-DTPA-extractable Soil Vanadium from Greece and Egypt and their Correlation to Soil Properties. Soil Sci. 2010, 175, 535–543. [Google Scholar] [CrossRef]
- Yang, J.; Tengng, Y.; Wu, J.; Chen, H.; Wang, G.; Song, L.; Yue, W.; Zuo, R.; Zhai, Y. Current status and associated human health risk of vanadium in soil in China. Chemosphere 2017, 171, 635–643. [Google Scholar] [CrossRef]
- Blake, J.; Brown, J.E.; Fergusson, K.; Bixby, R.J.; Delay, N.T. Sediment record of mining legacy and water quality from drinking-water reservoir, Aztec, New Mexico, USA. Environ. Earth Sci. 2020, 79, 404. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Liu, Z.; Wang, Z.; Yao, J.; Bortwick, A.L. Vanadium contamination and associated health risk of framland soil near smelters throughout China. Environ. Pollut. 2020, 263, 114540. [Google Scholar] [CrossRef]
- Pickering, L.; Reed, D.; Bevan, A.I.; Book, D. Ti−V−Mn based metal hydrides for hydrogen compression applications. J. Alloy. Compd. 2015, 645, S400–S403. [Google Scholar] [CrossRef]
- Hartung, J. Steroselective syntheses of functionalized cyclic ethers via (Schiff-base)vanadium(V)-catalysed oxidations. Pure Appl. Chem. 2005, 77, 1559–1574. [Google Scholar] [CrossRef]
- Licini, G.; Conte, V.; Coletti, A.; Mba, M.; Zonta, C. Recent advances in vanadium catalysed oxygen transfer reactions. Coord. Chem. Rev. 2011, 255, 2345–2357. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Coletti, A.; Conte, V. Sustainable vanadium-catalysed oxidation of organic substrates with H2O2. Catal. Today 2017, 285, 49–56. [Google Scholar] [CrossRef]
- Coletti, A.; Sabuzi, F.; Floris, B.; Galloni, P.; Conte, V. Efficient and sustainable V-catalysed oxidative desulfurization of fuels assisted by ionic liquids. J. Chem. Technol. 2018, 46, 1121–1129. [Google Scholar] [CrossRef]
- Ulmer, U.; Asano, K.; Patryk, A.; Enoki, H.; Nakamura, Y.; Pohl, A.; Dittmeyer, R.; Fichtner, M. Cost reduction possibilities of vanadium-based solutions—Microstructural, thermodynamic, cyclic and environmental effects of ferrovanadium substitution. J. Alloy. Compd. 2015, 648, 1024–1030. [Google Scholar] [CrossRef]
- Llustar, M.; Vincent, J.B.; Badenes, J.; Tena, M.A.; Monrós, G. Environmental optimization of Blue Vanadium Zircon Ceramic Pigment. J. Eur. Ceram. Soc. 1999, 19, 2647–2657. [Google Scholar] [CrossRef]
- Zhizhong, C.; Xuejing, X.; Wensheng, Y.; Jizhou, F.; Qin, Z.; Jindong, F. Multi-element geochemical mapping in Southern China. J. Geochem. Explor. 2014, 139, 183–192. [Google Scholar] [CrossRef]
- Prytulak, J.; Nielsen, S.G.; Ionov, D.A.; Halliday, A.N.; Harvey, J.; Kelley, K.A.; Niu, Y.L.; Peate, D.W.; Shimizu, K.; Sims, K.W.W. The stable vanadium isotope composition of mantle and mafic lavas. Earth Planet. Sci. Lett. 2013, 665, 177–189. [Google Scholar] [CrossRef]
- Gowd, S.S.; Reddy, R.; Govil, P.K. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Uannao industrial areas of the Ganga Plain, Uttar, Pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinkelbe, J. A review of the distribution coefficient of trace elements in soils: Influence of soil system, elemental characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef]
- Reijonen, I.; Metzler, M.; Hartikainen, H. Impact of soil pH and organic matter on chemical availability of vanadium species: The underlying basis for risk assessment. Environ. Pollut. 2016, 210, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. Vanadium. Its role for humans. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer Science: Dordrecht, The Netherlands, 2013; Volume 13, pp. 139–167. [Google Scholar]
- Rudbari, H.A.; Iravani, M.R.; Moazam, V.; Askari, B.; Khorshudifard, M.; Habibi, N.; Bruno, G. Synthesis, characterization, Xray crystal structures and antibacterial activities of Schiff base ligands derived from allyamine and their vanadium(IV), cobalt(III), nickel(II), copper(II), zinc(II), and palladium(II) complexes. J. Mol. Struct. 2016, 1125, 113–120. [Google Scholar] [CrossRef]
- Jakusch, T.; Kiss, T. In vitro study of the antidiabetic behavior of vanadium compounds. Coord. Chem. Rev. 2017, 351, 118–126. [Google Scholar] [CrossRef]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 249, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D. Implications of vanadium in technical applications and pharmaceutical issues. Inorganica Chim. Acta 2017, 455, 378–389. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Alessi, D.S.; Tack, F.M.G.; Ok, Y.S.; Kim, K.H.; Gustafsson, J.P.; Sparks, D.L.; Rinklebe, J. Redox chemistry of vanadium in soils: Interactions with colloidal materials, mobilization, speciation, and relevant environmental implications—A review. Adv. Colloid Interface Sci. 2019, 265, 1–13. [Google Scholar] [CrossRef]
- Hashin, M.A.; Mukhopadhyay, S.; Narayan Sachu, J.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.A.; D’Amato, M.; Cudabba, F.; Raggi, A.; Öborn, I.; Kleja, D.B.; Gustafsson, J.P. Long-term fate and transformation of vanadium in a pine forest soil with added converter lime. Geoderma 2015, 259–260, 271–278. [Google Scholar] [CrossRef]
- Calliman, F.A.; Robu, B.M.; Samandra, C.; Pavel, V.L.; Gavrilescu, M. Soil and groundwater cleanup: Benefits: And limits of emerging technologies. Clean Technol. Environ. Policy 2011, 13, 241–268. [Google Scholar] [CrossRef]
- Larsson, M.A.; Baken, S.; Gustafsson, J.P.; Hadialhejazi, G.; Smolders, E. Vanadium bioavailability and toxicity to soil microorganisms and plants. Environ. Toxicol. Chem. 2013, 32, 2266–2273. [Google Scholar] [CrossRef]
- Rehder, D. The role of vanadium in biology. Metallomics 2015, 7, 730–742. [Google Scholar] [CrossRef]
- Stanić, M.; Cvetić-Antić, T.; Hadžibramović, M.; Živić, M.; Zakrzewska, J.; Žižić, M. Transport and metabolism of vanadium in filamentous fungi with emphasis on fungus Phycomyces blakesleeanus. In Proceedings of the Lecture published in Serbian Biochemical Society Eight Conference Materials “Coordination in Biochemistry and Life”, Novi Sad, Serbia, 16 November 2018; pp. 81–90. [Google Scholar]
- Zhang, R.; Levinskä, T.; Tansaken, J.; Gao, B.; Yue, Q. Utilization of ferric groundwater treatment residuals for inorganic-organic hybrid biosorbent preparation and its use in vanadium removal. Chem. Eng. J. 2019, 361, 680–689. [Google Scholar] [CrossRef]
- Ortiz-Bernard, I.; Anderson, R.P.; Virionis, H.A.; Lovley, D.R. Vanadium Respiration by Geobacter metallireducents: Novel Strategy for In Situ Removal of Vanadium from Groundwater. Appl. Environ. Microbiol. 2004, 70, 3091–3095. [Google Scholar] [CrossRef]
- Yurkowa, N.A.; Lyalikowa, N.N. New vanadate-reducing facultative chemolitotrophic bacteria. Microbiology 1991, 59, 672–677. [Google Scholar]
- Lyalikowa, N.N.; Yurkowa, N.A. Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol. J. 1992, 10, 15–26. [Google Scholar] [CrossRef]
- Carpentier, W.; Sandra, K.; De Smet, I.; Bridgé, A.; De Smet, L.; Van Beeumen, J. Microbial Reduction and Precipitation of vanadium by Shewanella oneidensis. Appl. Environ. Microbiol. 2003, 69, 3636–3639. [Google Scholar] [CrossRef]
- Lemaire, O.L.; Méjean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitious organisms sustaining and preserving aquatic ecosystems. Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, H.; Zhao, L.; McCarick, R.; Agraval, A. Microbial reduction and precipitation of vanadium by mesophilic and termophilic methanogens. Chem. Geol. 2014, 370, 29–39. [Google Scholar] [CrossRef]
- Chouler, J.; Padgett, G.A.; Cameroon, P.J.; Preuss, K.; Tritici, M.; Ireopulos, I.; Di Lorenzo, M. Towards effective small scale microbial fuel cells foe energy generation from urine. Electrochim. Acta 2016, 192, 89–98. [Google Scholar] [CrossRef]
- Ezziat, L.; Elabed, A.; Ibnsouda, S.; El Abed, S. Challenges of microbial Fuel Cell Architecture on Heavy Metal Recovery and removal From Wastewater. Front. Energy Res. 2019, 7, 1. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous reduction of vanadium(v) and chromium(VI) with enhanced energy recovery based on microbial fuel cell technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, X.; Luo, Y.; Sun, D.; Call, D.F.; Logan, B.E. Improving startup performance with carbon mesh anodes in separator electrode assembly microbial fuel cells. Bioresour. Technol. 2013, 133, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Maathius, F.J.M. Physiological functions of mineral micronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Soil-plant transfer of trace elements—An environmental issue. Geoderma 2004, 122, 143–149. [Google Scholar] [CrossRef]
- Semenova, L.N.; Semenov, Y.M. The migration ability of heavy metals in soils as the sensitivity indicator of geosystems. Geogr. Nat. Resour. 2010, 31, 116–123. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; Ji-Zheng, H.; Pinton, R.; Ceso, S. Review on iron availability in soil: Interaction of Fe minerals, plants and microbes. J. Soils Sediment. 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Sychta, K.; Słomka, A.; Kuta, E. Insights into Plant Programmed Cell Death Induced by Heavy Metals—Discovering a Terra Incognita. Cells 2021, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Gallagher, F.J.; Feng, H.; Wu, M.; Zhy, Q. Vanadium uptake and translocation in dominant plant species on an urban coastal brownfeld site. Sci. Total Environ. 2014, 476–477, 696–704. [Google Scholar] [CrossRef]
- Cannon, H.L. Geochemistry of Rocks and Related Soils and Vegetation in the Yellow Cat Area, Grand Country, Utach; Geological Survey Bulletin 1176; United States Government Publishing Office: Washington, DC, USA, 1964; pp. 71–75.
- Peterson, P.J.; Girling, C.A. Other Trace Metals. In Effect of Heavy Metal Pollution on Plants. Volume 1. Effects of Trace Metals on Plant Function; Lepp, N.W., Ed.; Applied Science Publation: London, UK, 1981; pp. 213–278. ISBN 978-94-011-7339-1. [Google Scholar]
- Martin, H.W.; Young, T.R.; Kaplan, D.I.; Simon, L.; Adriano, D.C. Evaluation of three herbaceous species for availability of soil cadmium, chromium, nickel and vanadium. Plant Soil 1996, 182, 199–207. [Google Scholar] [CrossRef]
- Imtaiz, M.; Mushtaq, M.A.; Nawaz, M.A.; Ashraf, M.; Rizwan, M.S.; Mehmood, S.; Aziz, O.; Rizwan, M.; Virk, M.S.; Shakeel, Q.; et al. Physiological and anthocyanin biosynthesis genes response induced by vanadium stress in mustard genotypes with distinct photosynthetic activity. Environ. Toxicol. Pharmacol. 2018, 62, 20–29. [Google Scholar] [CrossRef]
- Babst-Kostecka, A.; Przybyłowicz, W.; van der Endt, A.; Ryan, C.; Dietrich, C.C.; Mesjasz-Przybyłowicz, J. Endosperm prevents toxic amounts of Zn from accumulating in the seed embryo—an adaptation to metalliferous sites in metal-tolerant Biscutella laevigata. Metallomics 2020, 12, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, K.E.; Grewing, P.D.; Greenberg, B.M. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Hanus-Fajerska, E.; Ciarkowska, K.; Muszyńska, E. Long-term field study on stabilization of contaminated wastes by growing clonally reproduced Silene vulgaris calamine ecotype. Plant Soil 2019, 439, 341–445. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Śliwińska, E.; Koźmińska, A. Distinct co-tolerance responses to combined salinity and cadmium exposure in metallicolous and non metallicolous ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 1–15. [Google Scholar] [CrossRef]
- Muszyńska, E.; Labudda, M.; Różańska, E.; Hanus-Fajerska, E.; Znojek, E. Heavy metal tolerance in contrasting ecotypes of Alyssum montanum. Ecotoxicol. Environ. Saf. 2018, 161, 305–3017. [Google Scholar] [CrossRef]
- Teng, Y.; Yang, J.; Sun, Z.; Wang, J.; Zuo, R.; Zheng, J. Environmental vanadium distribution, mobility and bioaccumulation in different land-use districts in Panzhihua Region, SW China. Environ. Monit. Assess. 2011, 176, 605–620. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Kamińska, I.; Koźmińska, A.; Hanus-Fajerska, E. Aspect of co-tolerance towards salt and heavy metal stresses in halophytic plant species. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fuita, M., Oku, H., Kamrun, N., Hawrylak-Nowak, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 447–498. [Google Scholar]
- Wiszniewska, A.; Koźmińska, A.; Hanus-Fajerska, E.; Dziurka, M.; Dziurka, K. Insight into mechanisms of multiple stress tolerance in a halophyte Aster tripolium subjected to salinity and heavy metal stress. Ecotoxicol. Environ. Saf. 2019, 180, 12–22. [Google Scholar] [CrossRef]
- Khan, S.; Kazi, T.G.; Kolachi, N.F.; Baig, J.A.; Afridi, H.I.; Shah, A.Q.; Kumar, S.; Shah, F. Hazardous impact and translocation of vanadium(V) species from soil to different vegetables and grasses grown in the vicinity of thermal power plant. J. Hazard. Mater. 2011, 1–3, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Chen, T.; Yang, J. Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacteria strain. Environ. Technol. Innov. 2020, 20, 101090. [Google Scholar] [CrossRef]
- Aihemaiti, A.; Jiang, J.; Gao, Y.; Meng, Y.; Zou, Q.; Yang, M.; Xu, Y.; Huan, S.; Yan, W.; Tuerhong, T. The effect of vanadium on essential element uptake of Setaria viridis seedlings. J. Environ. Manag. 2019, 237, 399–407. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Yang, J.Y.; Zhang, Y.X.; Wang, C.Q.; Guo, S.S.; Yu, Y.Q. Growth responses, accumulation, translocation and distribution of vanadium in tobacco and its potential in phytoremediation. Ecotoxicol. Environ. Saf. 2021, 207, 111297. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Chen, C.; Shireen, F.; Zheng, Z.; Jiao, Y.; Sohail, H.; Afzal, M.; Imtiaz, M.; Ali, M.A.; Huang, Y.; et al. Improving vanadium stress tolerance of watermelon by grafting onto bottle gourd and pumpkin rootstock. Plant Growth Regul. 2018, 85, 41–56. [Google Scholar] [CrossRef]
- Imtiaz, M.; Ashraf, M.; Rizwan, M.S.; Nawaz, M.A.; Rizwan, M.; Mehmood, S.; Yousaf, B.; Yuan, Y.; Ditta, A.; Mumtaz, M.A.; et al. Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: Effects on cell death, ROS and antioxidative systems. Ecotoxicol. Environ. Saf. 2018, 158, 139–144. [Google Scholar] [CrossRef]
- Altaf, M.M.; Diao, X.P.; Rehman, A.; Imtiaz, M.; Shakoor, A.; Altaf, M.A.; Ghani, M.U. Effect of Vanadium on Growth, Photosynthesis, Reactive Oxygen Species, Antioxidant Enzymes, and Cell Death of Rice. J. Soil Sci. Plant Nutr. 2020, 20, 2643–2656. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Saco, D.; Martín, S.; San Jose, P. Vanadium distribution in roots and leaves of Phaseolus vulgaris: Morphological and ultrastructural effects. Biol. Plant. 2013, 57, 128–132. [Google Scholar] [CrossRef]
- Tian, L.Y.; Yang, J.Y.; Huang, J.H. Uptake and speciation of vanadium in the rhizosphere soils of rape (Brassica juncea L.). Environ. Scie. Poll. Res. 2015, 22, 9215–9223. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Li, M.; Yang, X.; Pan, R. Responses of nonprotein thiols to stress of vanadium and mercury in maize (Zea mays L.) seedlings. Bull. Environ. Contam. Toxicol. 2019, 102, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Hu, C.; Xiong, L.; Lu, C. Tissue accumulation and subcellular distribution of vanadium in Brassica juncea and Brassica chinensis. Microchem. J. 2013, 110, 575–578. [Google Scholar] [CrossRef]
- Yuan, Y.; Imtiaz, M.; Rizwan, M.; Dong, X.; Tu, S. Effect of vanadium on germination, growth and activities of amylase and antioxidant enzymes in genotypes of rice. Int. J. Environ. Sci. Technol. 2020, 17, 383–394. [Google Scholar] [CrossRef]
- Garau, G.; Palma, A.; Lauro, G.P.; Mele, E.; Senette, C.; Manunza, B.; Deiana, S. Detoxification processes from vanadate at the root apoplasm activated by caffeic and polygalacturonic acids. PLoS ONE 2015, 10, e0141041. [Google Scholar] [CrossRef]
- Ray, R.; Dutta, B.; Mandal, S.K.; González, A.G.; Pokrovsky, O.S.; Jana, T.K. Bioaccumulation of vanadium (V) niobium (Nb) and tantalum (Ta) in diverse mangroves of the Indian Sundarbans. Plant Soil 2020, 448, 553–564. [Google Scholar] [CrossRef]
- Chen, T.; Li, T.Q.; Yang, J.Y. Damage suffered by swamp morning glory (Ipomoea aquatica Forsk) exposed to vanadium (V). Environ. Toxicol. Chem. 2016, 35, 695–701. [Google Scholar] [CrossRef]
- Akoumianaki-Ioannidou, A.; Barouchas, P.E.; Ilia, E.; Kyramariou, A.; Moustakas, N.K. Effect of vanadium on dry matter and nutrient concentration in sweet basil (Ocimum basilicum L.). Aust. J. Crop Sci. 2016, 10, 199–206. [Google Scholar]
- Wang, H.; Wang, T.; You, L.; Zhong, G.; Shi, G. Effects of vanadate supply on plant growth, Cu accumulation, and antioxidant capacities in Triticum aestivum L. Environ. Geochem. Health 2013, 35, 585–592. [Google Scholar] [CrossRef]
- Smoleń, S.; Kowalska, I.; Halka, M.; Ledwożyw-Smoleń, I.; Grzanka, M.; Skoczylas, Ł.; Czernicka, M.; Pitala, J. SelectedAspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy 2020, 10, 1. [Google Scholar] [CrossRef]
- Grzanka, M.; Smoleń, S.; Kováčik, P. Effect of Vanadium on the Uptake and Distribution of Organic and Inorganic Forms of Iodine in Sweetcorn Plants during Early-Stage Development. Agronomy 2020, 10, 1666. [Google Scholar] [CrossRef]
- Rojek, J.; Kozieradzka-Kiszkurno, M.; Kapusta, M.; Aksmann, A.; Jacewicz, D.; Drzeżdżon, J.; Tesmar, A.; Żamojć, K.; Wyrzykowski, D.; Chmurzyński, L. The effect of vanadium(IV) complexes on development of Arabidopsis thaliana subjected to H2O2-induced stress. Funct. Plant Biol. 2019, 46, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Wyrzykowski, D.; Inkielewicz-Stępniak, I.; Pranczk, J.; Żamojć, K.; Zięba, P.; Tesmar, A.; Jacewicz, D.; Ossowski, T.; Chmurzyński, L. Physicochemical properties of ternary oxovanadium (IV) complexes with oxydiacetate and 1, 10-phenanthroline or 2, 2′-bipyridine. Cytoprotective activity in hippocampal neuronal HT22 cells. Biometals 2015, 28, 307–320. [Google Scholar] [CrossRef]
- Schwartz, K.; Milne, D.B. Growth effect of vanadium in the rat. Science 1971, 174, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Yamaguchi, N.; Romaidi, R.; Isago, Y.; Tanahashi, H. Vanadium accumulation in ascidians: A system overview. Coord. Chem. Rev. 2015, 301–302, 300–308. [Google Scholar] [CrossRef]
- Gupta, P.K.; Vaswani, S. Basic information about vanadium ‘ultra-trace-element or occasionally beneficial element’ and its various functions in animals: A review article. J. Entomol. Zool. Stud. 2020, 8, 645–653. [Google Scholar]
- Concersa, G.; Miedico, O.; Charavale, E.; Elia, A. Heavy metal contents in green spears of asparagus (Asparagus officinalis L.) grown in southern Italy: Variability among farms, genotypes and effect of soil mycorrhizal inoculation. Sci. Hortic. 2019, 526, 108559. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–256. [Google Scholar] [CrossRef]
- Domingo, J.L.; Goméz, M. Vanadium compounds for the treatment of human diabetes mellitus: A scientific curiosity? A review of thirty years of research. Food Chem. Toxicol. 2016, 95, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Strianase, M.; Basile, A.; Mazzone, A.; Morello, S.; Turco, M.C.; Pellecchia, C. Therapeutic potential of a Pyridoxal−Based Vanadium(IV) Complex showing selective cytotoxicity for cancer versus healthy cells. J. Cell. Physiol. 2013, 228, 2202–2209. [Google Scholar] [CrossRef]
- Léon, I.E.; Butenko, N.; Di Virgillo, A.L.; Muglia, C.I.; Baran, E.J.; Cavaco, I.; Etcheverry, S.B. Vanadium and cancer treatment: Antitumoral mechanisms of tree oxivanadium(IV) complexes on a human osteosarcoma cell line. J. Inorg. Chem. 2014, 134, 106–117. [Google Scholar] [CrossRef]
- Wilk, A.; Szypulska-Koziarska, D.; Wiszniewska, B. The toxicity of vanadium on gastrointestinal urinary and reproductive system, and its influence on fertility and fetuses malformations. Post. Hig. Dos. 2017, 71, 850–859, e-ISSN 1732-2693. [Google Scholar] [CrossRef] [PubMed]
- Coreira, I.; Adão, P.; Roy, S.; Wahba, M.; Matos, C.; Maruya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.F.; Avecilla, F.; et al. Hydroxyquinoline derived vanadium (IV and V) and cooper(II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar] [CrossRef]
- Hopkins, L.L.; Cannon, H.L.; Miesch, A.T.; Welsch, R.M.; Nielsen, F.H. Vanadium. Geochem. Environ. 1973, 2, 93–109. [Google Scholar]
- Gambino, D. Potentiality of vanadium compounds as anti-parasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J. Oral bioaccessibility and health risk assessment of vanadium(IV) and vanadium(V) in a vanadium titanomagnetite mining region by a whole digestive system in vitro method (WDSM). Chemosphere 2019, 215, 294–304. [Google Scholar] [CrossRef]
- Cortiso, A.M.; Etcheverry, S.B. Vanadium derivatives act as a growth factor—mimetic compounds upon differentiation and proliferation of osteoblast-like URM106 cells. Mol. Cell. Biochem. 1995, 145, 97–102. [Google Scholar] [CrossRef]
- Marengo, M.; Durieux, E.D.H.; Terengo, S.; Ljeune, P.; Degrange, E.; Pasqualinu, V.; Gobert, S. Comparison of elemental composition in two wild and cultured marine fish and potential risks to human health. Ecotoxicol. Environ. Saf. 2018, 158, 204–212. [Google Scholar] [CrossRef]
- Barrio, D.A.; Williams, P.A.M.; Cortizo, A.M.; Etcheverry, S.B. Synthesis of a new vanadyl (IV) complex with trehalose (TreV0): Insulin-mimetic activities in osteoblast-like cells in culture. J. Biol. Inorg. Chem. 2003, 8, 459–468. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sanna, D.; Ugone, V.; Serra, M.; Garriba, E. Speciation of potential anti-diabetic vanadium complexes in real serum samples. J. Inorg. Chem. 2017, 173, 52–65. [Google Scholar] [CrossRef]
- Willsky, G.A.; Chi, L.H.; Godzala, M.; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 225, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Vanadium and Tungsten Derivatives as Antidiabetic Agents. A review of their toxic effects. Biol. Trace Elem. Res. 2002, 88, 97–112. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).