Influence of Land Use on the C and N Status of a C4 Invasive Grass in a Semi-Arid Region: Implications for Biomonitoring
Abstract
1. Introduction
2. Results
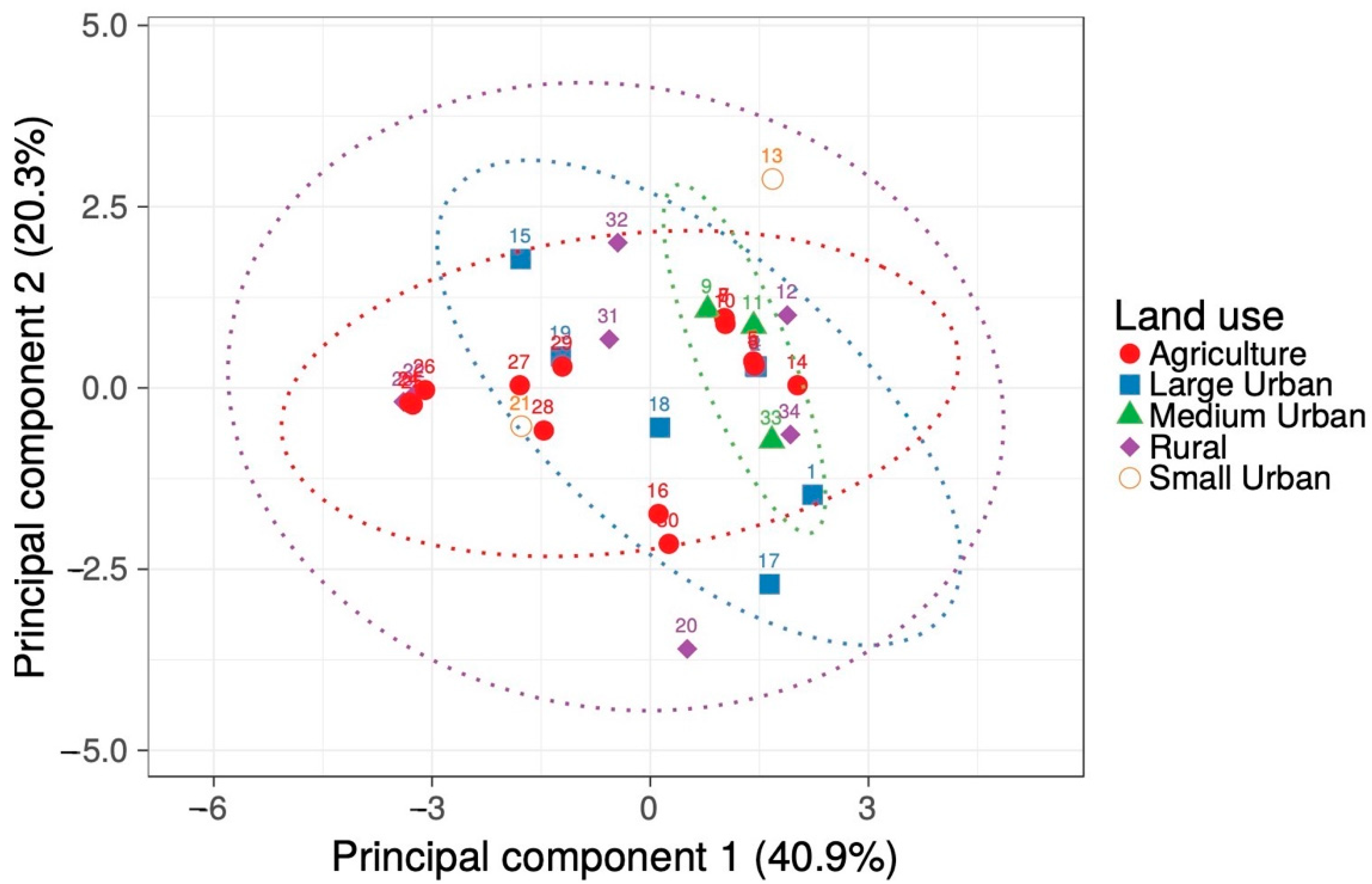
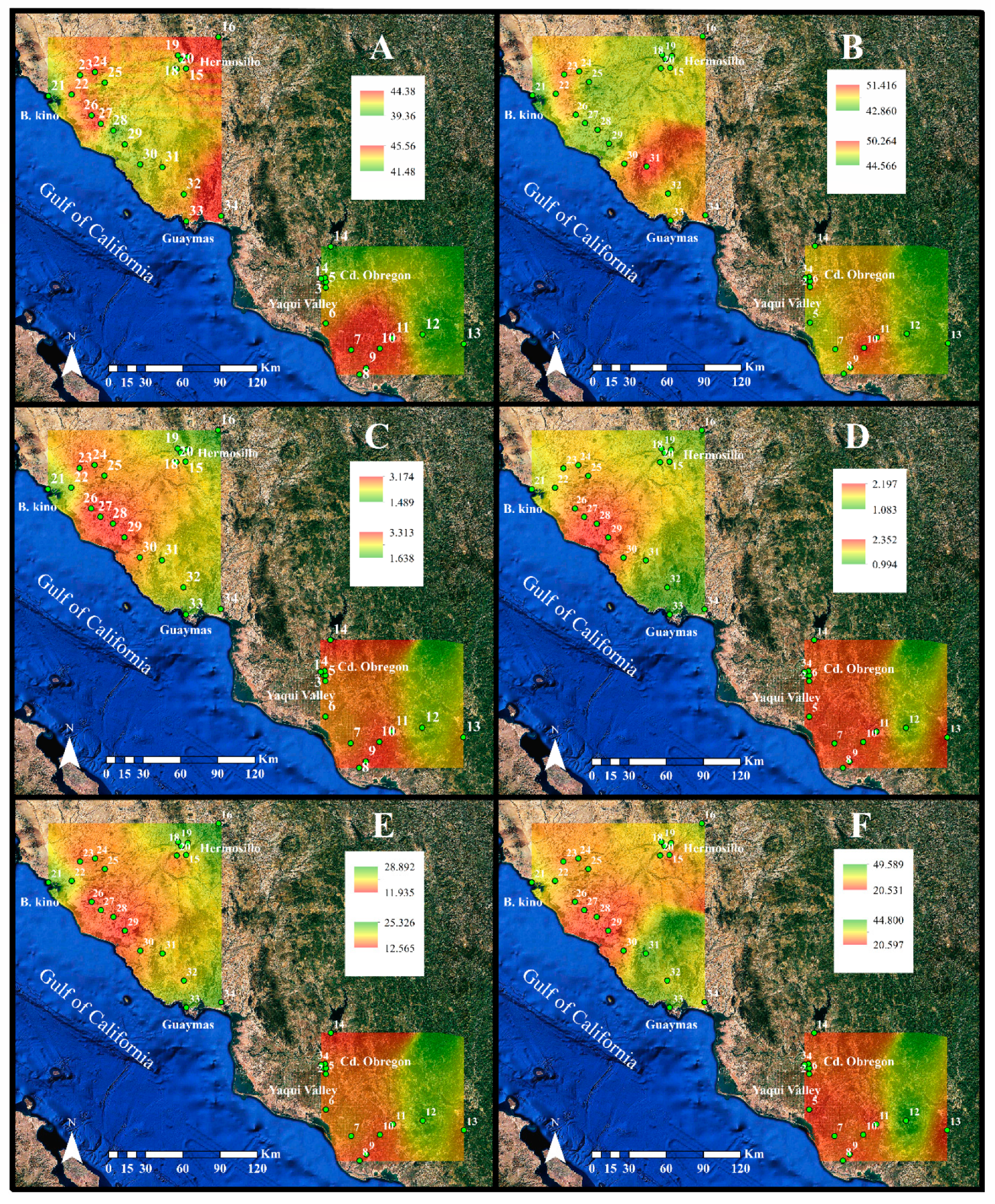
2.1. Environmental Characteristics
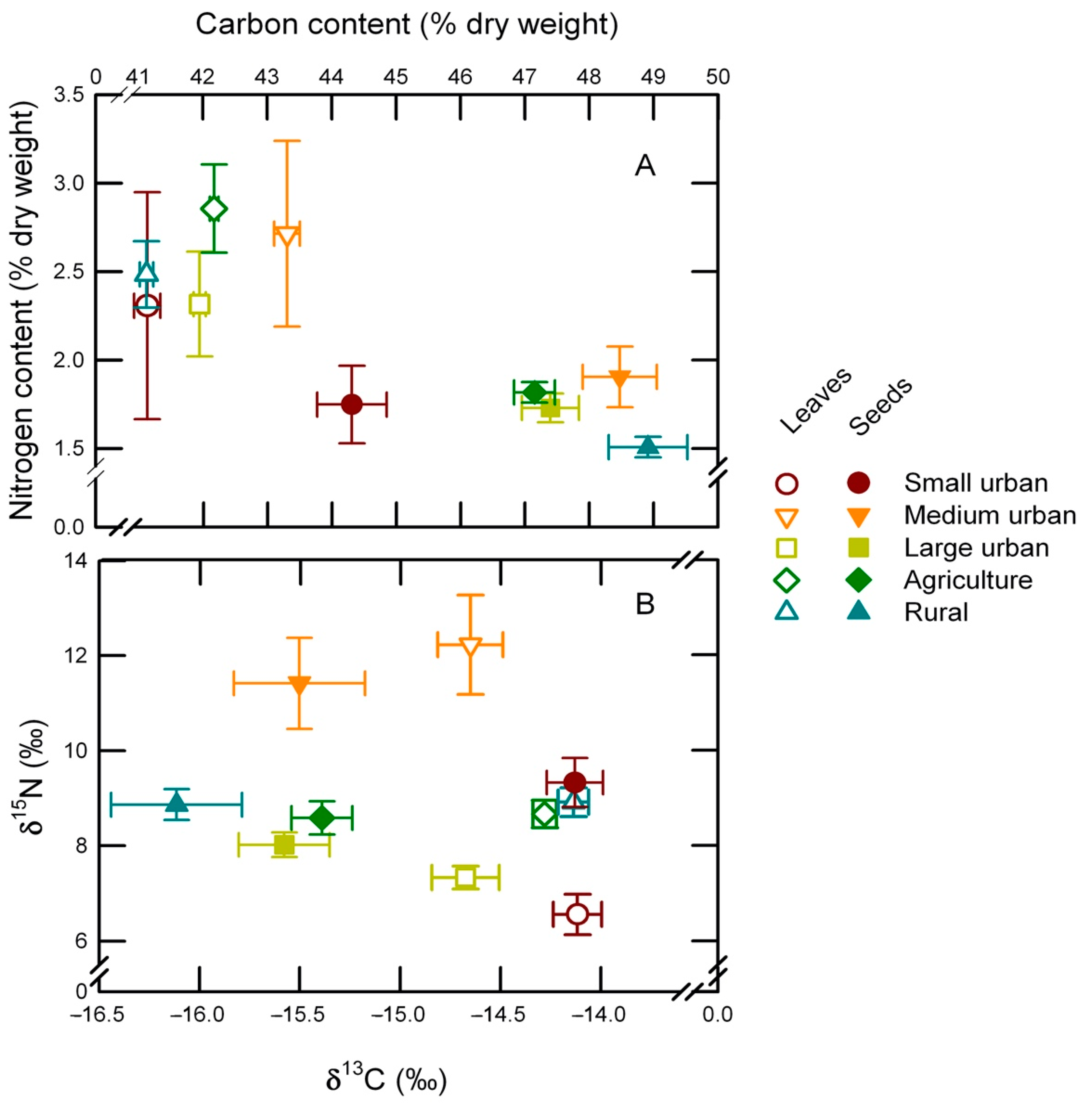
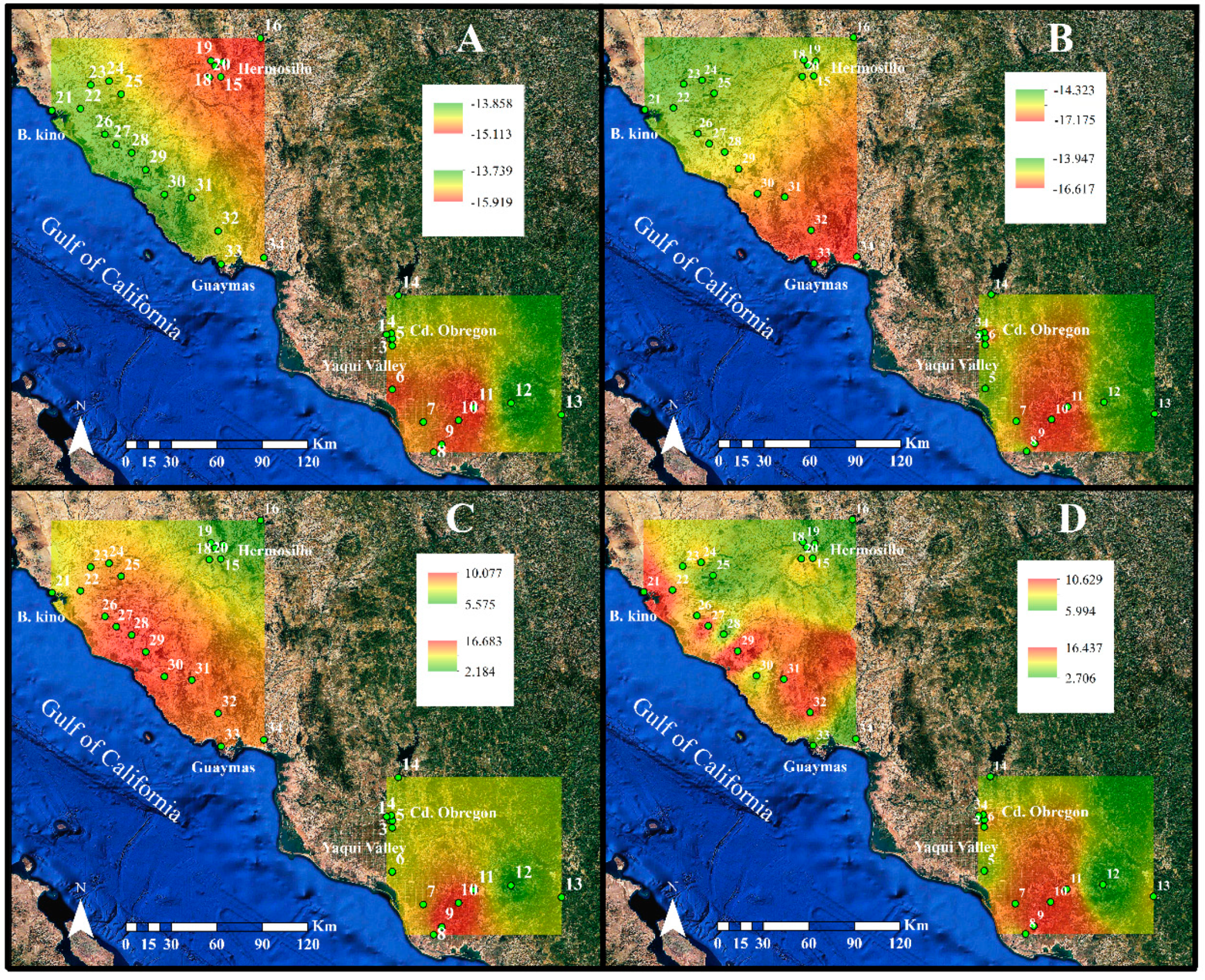
2.2. Elemental and Isotopic Composition for Buffelgrass
3. Discussion
4. Materials and Methods
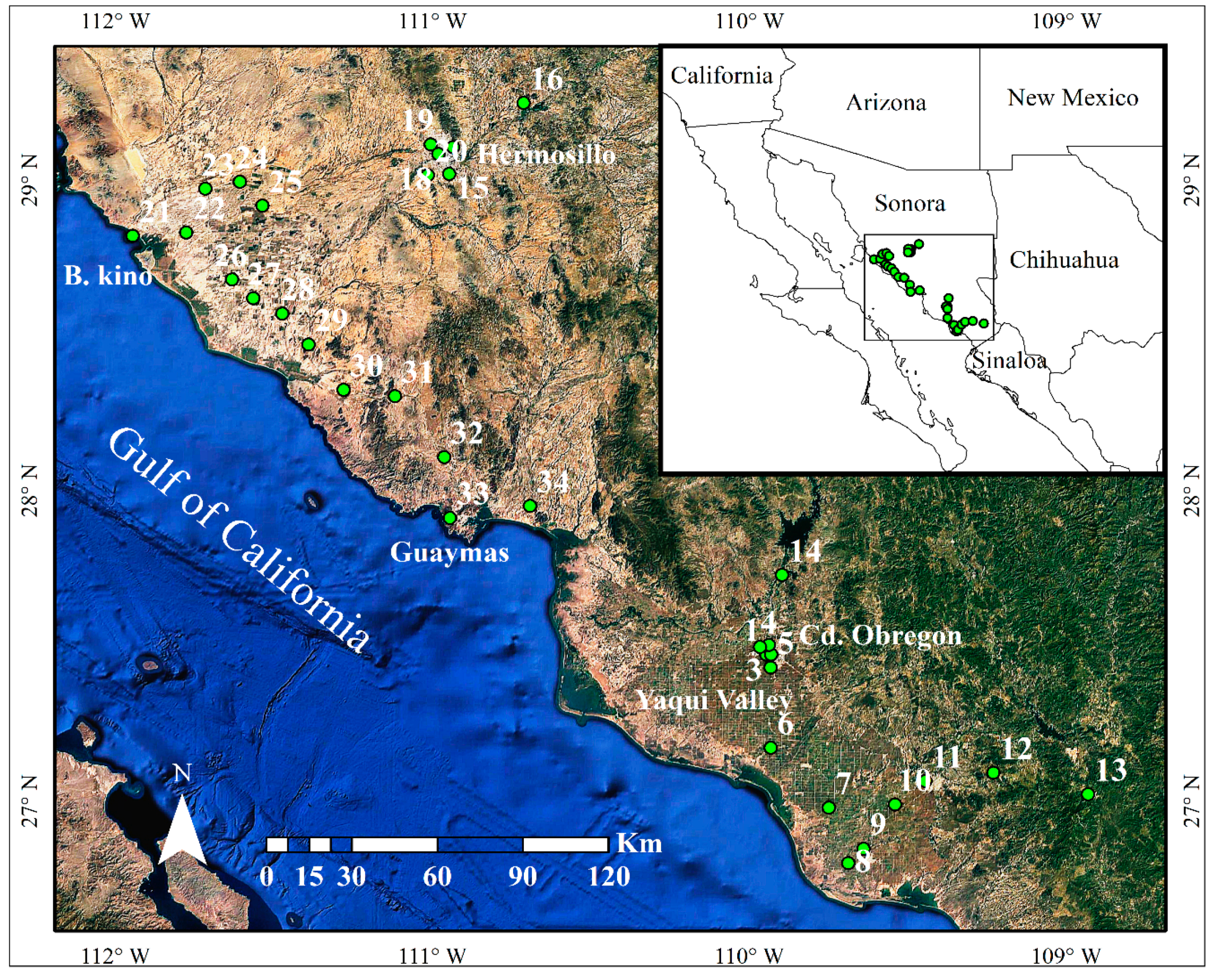
4.1. Study Region
4.2. Sampling of Plant Material
4.3. Elemental and Isotopic Analysis
4.4. Statistical Analyses
4.5. Geostatistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Land-Use Class | Carbon Content (% Dry Mass) | Nitrogen Content (% Dry Mass) | C:N (Adimensional) | ∂13C (‰) | ∂15N (‰) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Seeds | Leaves | Seeds | Leaves | Seeds | Leaves | Seeds | Leaves | Seeds | |
| Small urban | 41.13 ± 0.64 | 44.31 ± 0.54 | 2.31 ± 0.20 | 1.75 ± 0.22 | 18.96 ± 1.43 | 29.05 ± 3.31 | −14.11 ± 0.12 | −14.13 ± 0.14 | 6.56 ± 0.42 | 9.32 ± 0.52 |
| Medium urban | 43.31 ± 0.53 | 48.48 ± 0.58 | 2.71 ± 0.20 | 1.90 ± 0.17 | 17.30 ± 1.33 | 29.81 ± 3.41 | −14.65 ± 0.16 | −15.51 ± 0.33 | 12.23 ± 1.04 | 11.40 ± 0.96 |
| Large urban | 41.95 ± 0.30 | 47.15 ± 0.32 | 2.32 ± 0.09 | 1.73 ± 0.08 | 19.34 ± 0.91 | 29.39 ± 1.27 | −14.67 ± 0.17 | −15.58 ± 0.23 | 7.33 ± 0.24 | 8.01 ± 0.26 |
| Agriculture | 42.17 ± 0.25 | 47.15 ± 0.32 | 2.86 ± 0.07 | 1.81 ± 0.06 | 15.66 ± 0.57 | 27.68 ± 0.85 | −14.28 ± 0.06 | −15.39 ± 0.15 | 8.67 ± 0.29 | 8.57 ± 0.35 |
| Rural | 41.12 ± 0.19 | 48.91 ± 0.61 | 2.48 ±0.10 | 1.51 ± 0.08 | 17.29 ± 0.74 | 35.38 ± 2.47 | –14.14 ± 0.08 | −16.12 ± 0.33 | 8.92 ± 0.30 | 8.85 ± 0.33 |
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Houghton, R.A.; House, J.I.; Pongratz, J.; van der Werf, G.R.; DeFries, R.S.; Hansen, M.C.; Ramankutty, N. Carbon emissions from land use and land-cover change. Biogeosciences 2012, 9, 5125–5142. [Google Scholar] [CrossRef]
- Gasser, T.; Ciais, P. A theoretical framework for the net land-to-atmosphere CO2 flux and its implications in the definition of “emissions from land-use change”. Earth Syst. Dyn. 2013, 4, 171–186. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzing, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Xie, X.; Semanjski, I.; Gautama, S.; Tsiligianni, E.; Deligiannis, N.; Rajan, R.; Pasveer, F.; Philips, W. A Review of Urban Air Pollution Monitoring and Exposure Assessment Methods. ISPRS Int. J. Geo. Inf. 2017, 6, 389. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; Lindig-Cisneros, R.; de la Barrera, E. Biomonitors of atmospheric nitrogen deposition: Potential uses and limitations. Conserv. Physiol. 2018, 6, coy011. [Google Scholar] [CrossRef] [PubMed]
- Górecki, T.; Namieśnik, J. Passive sampling. TrAC Trends Analyt. Chem. 2002, 21, 276–291. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; de la Barrera, E. Characterization of nitrogen deposition in a megalopolis by means of atmospheric biomonitors. Sci. Rep. 2018, 8, 13569. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; de la Barrera, E. Isotopic biomonitoring of anthropic carbon emissions in a megalopolis. PeerJ 2020, 8, e9283. [Google Scholar] [CrossRef] [PubMed]
- Lichtfouse, E.; Lichtfouse, M.; Jaffrézic, A. δ13C Values of Grasses as a Novel Indicator of Pollution by Fossil-Fuel-Derived Greenhouse Gas CO2 in Urban Areas. Environ. Sci. Technol. 2003, 37, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pataki, D.E. Spatial patterns of plant isotope tracers in the Los Angeles urban region. Landsc. Ecol. 2010, 25, 35–52. [Google Scholar] [CrossRef]
- Wang, W.; Pataki, D.E. Drivers of spatial variability in urban plant and soil isotopic composition in the Los Angeles basin. Plant Soil 2011, 350, 323–338. [Google Scholar] [CrossRef]
- Redling, K.; Elliot, E.; Bain, D.; Sherwell, J. Highway contributions to reactive nitrogen deposition: Tracing the fate of vehicular NOx using stable isotopes and plant biomonitors. Biogeochem. 2013, 116, 261–274. [Google Scholar] [CrossRef]
- Sulzman, E.W. Stable Isotope Chemistry and Measurement: A Primer. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Malden, MA, USA, 2007; pp. 1–121. [Google Scholar]
- Maricle, B.R.; Caudle, K.L.; Adler, P.B. Influence of water Availability on Photosynthesis, Water Potential, Leaf δ13C, and Phenology in Dominant C4 Grasses In Kansas, USA. Trans. Kans. Acad. Sci. 2015, 118, 173–193. [Google Scholar] [CrossRef]
- Williams, D.G.; Gempko, V.; Fravolini, A.; Leavitt, S.W.; Wall, G.W.; Kimball, B.A.; Pinter, P.J., Jr.; LaMorte, R.; Ottman, M. Carbon isotope discrimination by Sorghum bicolorunder CO2 enrichment and drought. New Phytol. 2001, 150, 285–293. [Google Scholar] [CrossRef]
- Pataki, D.E.; Bowling, D.R.; Ehleringer, J.R. Seasonal cycle of carbon dioxide and its isotopic composition in an urban atmosphere: Anthropogenic and biogenic effects. J. Geophys. Res. 2003, 108, 4735. [Google Scholar] [CrossRef]
- Widory, D.; Javoy, M. The carbon isotope composition of atmospheric CO2 in Paris. Earth Planet. Sci. Lett. 2003, 215, 289–298. [Google Scholar] [CrossRef]
- Semmens, C.; Ketler, R.; Schwendenmann, L.; Nesic, Z.; Christen, A. Isotopic Composition of CO2 in Gasoline, Diesel and Natural Gas Combustion Exhaust in Vancouver, BC, Canada; Technical Report; The University of British Columbia: Columbia, BC, Canada, 2014; pp. 1–12. [Google Scholar]
- Molina-Freaner, F.; Gámez, R.C.; Tinoco-Ojanguren, C.; Castellanos, A.E. Vine species diversity across environmental gradients in northwestern México. Biodivers. Conserv. 2004, 13, 1853–1874. [Google Scholar] [CrossRef]
- Cox, J.R.; Martin-R., M.H.; Ibarra-F, F.A.; Fourie, J.H.; Rethman, N.F.G.; Wilcos, D.G. The influence of climate and soils on the distribution of four African grasses. J. Range Manag. 1988, 41, 127–139. [Google Scholar] [CrossRef]
- Ibarra-Flores, F.; Cox, J.R.; Martin, M.; Crowl, T.A.; Call, C.A. Predicting buffelgrass survival across a geographical and environmental gradient. J. Range Manag. 1995, 48, 53–59. [Google Scholar] [CrossRef]
- Tinoco-Ojanguren, C.; Reyes-Ortega, I.; Sánchez-Coronado, M.E.; Molina-Freaner, F.; Orozco-Segovia, A. Germination of an invasive Cenchrus ciliaris L. (buffel grass) population of the Sonoran Desert under various environmental conditions. S. Afr. J. Bot. 2016, 104, 112–117. [Google Scholar] [CrossRef]
- Arriaga, L.; Castellanos, A.E.; Moreno, E.; Alarcón, J. Potential ecological distribution of alien invasive species and risk assessment: A case study for buffel grass in arid regions of Mexico. Conserv. Biol. 2004, 18, 1504–1514. [Google Scholar] [CrossRef]
- de la Barrera, E. Recent invasion of buffel grass (Cenchrus ciliaris) of a natural protected area from the southern Sonoran Desert. Rev. Mex. Biodivers. 2008, 79, 385–392. [Google Scholar]
- Marshall, V.M.; Lewis, M.M.; Ostendorf, B. Buffel grass (Cenchrus ciliaris) as an invader and threat to biodiversity in arid environments: A review. J. Arid Environ. 2012, 78, 1–12. [Google Scholar] [CrossRef]
- Williams, D.G.; Baruch, Z. African grass invasion in the Americas: Ecosystem consequences and the role of ecophysiology. Biol. Invasions 2000, 2, 123–140. [Google Scholar] [CrossRef]
- SEMARNAT. Norma Oficial Mexicana NOM-156-SEMARNAT-2012, Establecimiento y Operación de Sistemas de Monitoreo de la Calidad del Aire; Diario Oficial de la Federación: México D.F., Mexico, 2012.
- INEGI. Cuéntame, Información por Entidad. 2020. Available online: http://www.cuentame.inegi.org.mx/monografias/informacion/Son/Poblacion/default.aspx?tema=ME&e=26 (accessed on 23 March 2020).
- INECC, Instituto Nacional de Ecología y Cambio Climático. Informe Nacional de Calidad del Aire 2018, México; Coordinación General de Contaminación y Salud Ambiental, Dirección de Investigación de Calidad del Aire y Contaminantes Climáticos: Ciudad de México, Mexico, 2019; pp. 1–395.
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and determinants of carbon content in plants: A global synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Castellanos-Villegas, A.E.; Yanes, G.; Valdez-Zamudio, D. Drought-tolerant exotic buffel-grass and desertification. In Weeds Across Borders; Tellman, B., Ed.; Proceedings of the North American Conference; The Arizona-Sonora Desert Museum: Tucson, Arizona, 2002; pp. 99–112. [Google Scholar]
- Bhatt, R.K.; Baig, M.J.; Tiwari, H.S. Growth, biomass production, and assimilatory characters in Cenchrus ciliaris L. Under elevated CO2 condition. Photosynthetica 2007, 45, 296–298. [Google Scholar] [CrossRef]
- Williams, D.G.; Black, R.A. Response to drought for a native and introduced Hawaiian grass. Oecologia 1994, 97, 512–519. [Google Scholar] [CrossRef]
- Ripley, B.S.; Abraham, T.I.; Osborne, C.P. Consequences of C4 photosynthesis for the partitioning of growth: A test using C3 and C4 subspecies of Alloteropsis semialata under nitrogen-limitation. J. Exp. Bot. 2007, 59, 1705–1714. [Google Scholar] [CrossRef]
- Buchmann, N.; Brooks, J.R.; Rapp, K.D.; Ehleringer, J.R. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 1996, 19, 392–402. [Google Scholar] [CrossRef]
- Millar, N.; Urrea, A.; Kahmark, K.; Shcherbak, I.; Robertson, G.P.; Ortiz-Monasterio, I. Nitrous oxide (N2O) flux responds exponentially to nitrogen fertilizer in irrigated wheat in the Yaqui Valley, Mexico. Agric. Ecosyst. Environ. 2018, 261, 125–132. [Google Scholar] [CrossRef]
- Cobley, L.A.E.; Pataki, D.E. Vehicle emissions and fertilizer impact the leaf chemistry of urban trees in Salt Lake Valley, UT. Environ. Pollut. 2019, 254, 112984. [Google Scholar] [CrossRef] [PubMed]
- Naus, S.; Röckmann, T.; Popa, M.E. The isotopic composition of CO in vehicle exhaust. Atmospheric Environ. 2018, 177, 132–142. [Google Scholar] [CrossRef]
- Winter, K.; Troughton, J.H.; Card, K.A. δ13C values of grass species collected in the northern Sahara desert. Oecologia 1976, 25, 115–123. [Google Scholar] [CrossRef]
- Börjesson, P.I.I. Emissions of CO2 from biomass production and transportation in agriculture and forestry. Energy Convers. Manag. 1996, 37, 1235–1240. [Google Scholar] [CrossRef]
- FAO. Agriculture, Forestry and Other Land Use Emissions by Sources and Removals by Sinks. 1990–2011 Analysis; FAO Statistics Division, Working Paper Series; FAO: Rome, Italy, 2014; pp. 1–89. [Google Scholar]
- Liu, X.; Zhang, S.; Bae, J. The nexus of renewable energy-agriculture-environment in BRICS. Appl. Energy 2017, 204, 489–496. [Google Scholar] [CrossRef]
- Gitz, V.; Ciais, P. Future Expansion Of Agriculture and Pasture Acts to Amplify Atmospheric CO2 Levels in Response to Fossil-Fuel and Land-Use Change Emissions. Clim. Change 2004, 67, 161–184. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; Lindig-Cisneros, R.; de la Barrera, E. Responses to simulated nitrogen deposition by the neotropical epiphytic orchid Laelia speciosa. PeerJ 2015, 3, e1021. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.F.; Schulze, E.D.; Gebauer, G.; Bruckner, G. Canopy Uptake and Utilization of Atmospheric Pollutant Nitrogen. In Carbon and Nitrogen Cycling in European Forest Ecosystems; Schulze, E.D., Ed.; Springer: Berlin, Germany, 2000; pp. 171–184. [Google Scholar]
- Ammann, M.; Siegwolf, R.; Pichlmayer, F.; Suter, M.; Saurer, M.; Brunold, C. Estimating the uptake of traffic-derived NO2 from 15N abundance in Norway spruce needles. Oecologia 1999, 118, 124–131. [Google Scholar] [CrossRef]
- Vallano, D.; Sparks, J. Quantifying foliar uptake of gaseous nitrogen dioxide using enriched foliar δ15N values. New Phytol. 2008, 177, 946–955. [Google Scholar] [CrossRef]
- Jung, K.; Gebauer, G.; Gehre, M.; Hofmann, D.; Weißflog, L.; Schüürmann, G. Anthropogenic impacts on natural nitrogen isotope variations in pinus sylvestris stands in an industrially polluted area. Environ. Pollut. 1997, 97, 175–181. [Google Scholar] [CrossRef]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiota, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, H.; Wu, D. Traffic-related dustfall and NOx, but not NH3, seriously affect nitrogen isotopic compositions in soil and plant tissues near the roadside. Environ. Pollut. 2019, 249, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hoering, T. The isotopic composition of the ammonia and the nitrate ion in rain. Geochim. Cosmochim. Acta 1957, 12, 97–102. [Google Scholar] [CrossRef]
- Gebauer, G.; Dietrich, P. Nitrogen isotope ratios in different compartments of a mixed stand of spruce, larch and beech trees and of understorey vegetation including fungi. Isot. Environ. Health Stud. 1993, 29, 35–44. [Google Scholar] [CrossRef]
- Gebauer, G.; Giesemann, A.; Schulze, E.-D.; Jäger, H.-J. Isotope ratios and concentrations of sulfur and nitrogen in needles and soils of Picea abies stands as influenced by atmospheric deposition of sulfur and nitrogen compounds. Plant Soil 1994, 164, 267–281. [Google Scholar] [CrossRef]
- Aber, J.; McDowell, W.; Nadelhoffer, K.; Magill, A.; Berntson, G.; Kamakea, M.; McNulty, S.; Currie, W.; Rustad, L.; Fernandez, I. Nitrogen saturation in temperate forest ecosystems. Bioscience 1998, 48, 921–934. [Google Scholar] [CrossRef]
- Stewart, G.; Aidar, M.P.; Joly, C.A.; Schmidt, S. Impact of point source pollution on nitrogen isotope signatures (δ15N) of vegetation in SE Brazil. Oecologia 2002, 131, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Eugster, W.; Haeni, M. Nutrients or Pollutants? Nitrogen Deposition to European Forests. In Climate Change, Air Pollution and Global Challenges—Understanding and Perspectives from Forest Research; Matyssek, R., Clarke, N., Cudlin, P., Mikkelsen, T.N., Tuovinen, J.-P., Wieser, G., Paoletti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 13, pp. 37–56. [Google Scholar]
- Zech, M.; Bimüller, C.; Hemp, A.; Samimi, C.; Broesike, C.; Hörold, C.; Zech, W. Human and climate impact on 15N natural abundance of plants and soils in high-mountain ecosystems: A short review and two examples from the Eastern Pamirs and Mt. Kilimanjaro. Isot. Environ. Health Stud. 2011, 47, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Álvarez, E.A.; Reyes-García, C.; de la Barrera, E. A δ15N assessment of nitrogen deposition for the endangered epiphytic orchid Laelia speciosa from a city and an oak forest in Mexico. J. Plant Res. 2016, 129, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, J.A.; Sisk, T.D.; Hultine, K.R.; Sesnie, S.E.; Bowker, M.A.; Johnson, N.C. Indicators of vehicular emission inputs into semi-arid roadside ecosystems. J. Arid Environ. 2016, 134, 150–159. [Google Scholar] [CrossRef]
- Ahrens, T.; Harrison, J.; Berman, M.; Jewett, P.; Matson, P.A. Nitrogen in the Yaqui Valley: Sources, transfers, and consequences. In Seeds of Sustainability, Lessons from the Birthplace of the Green Revolution; Matson, P.A., Ed.; Island Press: Washington, DC, USA, 2012; pp. 171–195. [Google Scholar]
- Parra Suárez, S.; Peiffer, S.; Gebauer, G. Origin and fate of nitrate runoff in an agricultural catchment: Haean, South Korea—Comparison of two extremely different monsoon seasons. Sci. Total Environ. 2019, 648, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, P.; Johannisson, C. 15N abundance of forests is correlated with losses of nitrogen. Plant Soil 1993, 157, 147–150. [Google Scholar] [CrossRef]
- Pardo, L.H.; Templer, P.H.; Goodale, C.L.; Duke, S.; Groffman, P.M.; Adams, M.B.; Boeckx, P.; Boggs, J.; Campbell, J.; Colman, B.; et al. Regional assessment of N saturation using foliar and root. Biogeochemistry 2006, 80, 143–171. [Google Scholar] [CrossRef]
- Emmett, B.A. Nitrogen saturation of terrestrial ecosystems: Some recent findings and their implications for our conceptual framework. Water Air Soil Pollut. Focus 2007, 7, 99–109. [Google Scholar] [CrossRef]
- Panek, J.A.; Matson, P.A.; Ortíz-Monasterio, I.; Brooks, P. Distinguishing nitrification and denitrification sources of N2O in a Mexican wheat system using 15N. Ecol. Appl. 2000, 10, 506–514. [Google Scholar]
- Townsend-Small, A.; Pataki, D.E.; Czimczik, C.I.; Tyler, S.C. Nitrous oxide emissions and isotopic composition in urban and agricultural systems in southern California. J. Geophys. Res. 2011, 116, G01013. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliott, E.M. Isotopic composition of passively collected nitrogen dioxide emissions: Vehicle, soil and livestock source signatures. Atmos. Environ. 2014, 92, 359–366. [Google Scholar] [CrossRef]
- Felix, J.D.; Elliot, E.M.; Gay, D.A. Spatial and temporal patterns of nitrogen isotopic composition of ammonia at US ammonia monitoring network sites. Atmos. Environ. 2017, 150, 434–442. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Beman, J.M.; Harrison, J.A.; Jewett, P.K.; Matson, P.A. A synthesis of nitrogen transformations and transfers from land to the sea in the Yaqui Valley agricultural region of northwest Mexico. Water Resour. Res. 2008, 44, W00A05. [Google Scholar] [CrossRef]
- Billy, C.; Billen, G.; Sebilo, M.; Birgand, F.; Tournebize, J. Nitrogen isotopic composition of leached nitrate and soil organic matter as an indicator of denitrification in a sloping drained agricultural plot and adjacent uncultivated riparian buffer strips. Soil Biol. Biochem. 2012, 42, 108–117. [Google Scholar] [CrossRef]
- Payne, R.J.; Dise, N.B.; Field, C.D.; Dore, A.J.; Caporn, S.J.; Stevens, C.J. Nitrogen deposition and plant biodiversity: Past, present, and future. Front. Ecol. Environ. 2017, 15, 431–436. [Google Scholar] [CrossRef]
- Matson, P.; Luers, A.M.; Seto, K.C.; Naylor, R.L.; Ortiz-Monasterio, I. People, Land Use, and Environment in the Yaqui Valley, Sonora, Mexico. In Land Use, and Environment: Research Directions; Entwisle, B., Stern, P.C., Eds.; National Research Council: Washington, DC, USA, 2005; pp. 238–264. [Google Scholar]
- Díaz-Álvarez, E.A.; de la Barrera, E.; Barrios-Hernández, E.Y.; Arróniz-Crespo, M. Morphophysiological screening of potential organisms for biomonitoring nitrogen deposition. Ecol. Indic. 2020, 108, 105729. [Google Scholar] [CrossRef]
- Forman, R.T.T.; Godron, M. Landscape Ecology; Wiley: New York, NY, USA, 1986; pp. 1–619. [Google Scholar]
- Adams, A.S.; Vázquez, J.L.M.; Ley, A.N.L. Agricultura y manejo sustentable del acuífero de la Costa de Hermosillo. Rec. Soc. 2012, 3, 155–179. [Google Scholar]
- Research on Forest Climate Change: Potential Effects of Global Warming on Forests and Plant Climate Relationships in Western North America and Mexico. Available online: http://charcoal.cnre.vt.edu/climate/ (accessed on 9 April 2021).
- Instituto Nacional de Geografía y Estadística. Conjunto de datos de perfiles de suelos. Escala 1:250,000. Serie II (Continuo nacional), Mexico. Available online: https://www.inegi.org.mx/temas/edafologia/#Descargas (accessed on 9 April 2021).
- Ehleringer, J.R.; Osmond, B.O. Stable isotopes. In Plant Physiological Ecology; Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., Eds.; Springer: Dordrecht, The Netherland; Berlin, Germany, 1989; pp. 281–300. [Google Scholar]
- Evans, R. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Cressie, N. Spatial prediction and ordinary Kriging. Math. Geol. 1988, 20, 405–421. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Mean temperature (°C) | 23.5 ± 0.1 |
| Minimum temperature (°C) | 7.5 ± 0.2 |
| Maximum temperature (°C) | 38.0 ± 0.1 |
| Annual precipitation (mm) | 287 ± 20 |
| Primary soil type | Cambisol (26% of sites), luvisol (26%), vertisol (24%), leptosol (12%), regosol (6%), phaeozem (6%) |
| Geological origin | Alluvial (85%), sedimentary (12%), igneous (3%) |
| Clay content (%) | 32.2 ± 3.0 |
| Silt content (%) | 22.5 ± 2.0 |
| Sand content (%) | 45.3 ± 3.6 |
| Electrical conductivity (mmho/cm) | 0.71 ± 0.14 |
| pH | 8.45 ± 0.14 |
| Principal Component | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Sand | 0.32 | 0.39 | −0.28 | 0.14 | −0.35 | −0.28 | 0.29 | −0.04 | 0.59 |
| Silt | 0.03 | 0.38 | 0.37 | −0.55 | 0.47 | 0.19 | 0.05 | 0.07 | 0.39 |
| Clay | −0.29 | −0.54 | 0.03 | 0.19 | 0.04 | 0.13 | −0.27 | −0.01 | 0.70 |
| Minimum temperature | −0.51 | 0.21 | 0.06 | −0.13 | −0.13 | −0.27 | −0.09 | −0.76 | −0.00 |
| Maximum temperature | 0.13 | −0.39 | −0.63 | −0.44 | 0.32 | −0.06 | 0.27 | −0.24 | −0.00 |
| Mean Temperature | −0.49 | 0.03 | −0.13 | −0.24 | −0.02 | −0.58 | −0.01 | 0.58 | 0.00 |
| Precipitation | −0.35 | 0.30 | −0.47 | −0.15 | −0.29 | 0.65 | −0.12 | 0.14 | 0.00 |
| Electrical conductivity | 0.40 | 0.03 | −0.13 | −0.31 | −0.16 | −0.18 | −0.82 | −0.02 | −0.00 |
| pH | −0.07 | 0.35 | −0.35 | 0.50 | 0.65 | −0.09 | −0.27 | −0.02 | −0.00 |
| Individual variance | 0.41 | 0.20 | 0.13 | 0.12 | 0.07 | 0.04 | 0.03 | 0.00 | 0.00 |
| Cumulative variance | 0.41 | 0.61 | 0.74 | 0.86 | 0.93 | 0.97 | 1.00 | 1.00 | 1.00 |
| p-value | 0.328 | 0.812 | 0.036 | 0.232 | 0.812 | 0.079 | 0.814 | 0.085 | 1.00 |
| C Content (% Dry Weight) | N Content (% Dry Weight) | C:N Ratio | δ13C (‰) | δ15N (‰) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | p | F | p | F | p | F | p | F | p | |
| Organ | 1 | 463.2 | 0.001 | 172.3 | 0.001 | 276.98 | 0.001 | 94.641 | 0.001 | 0.741 | 0.416 |
| Land use | 4 | 6.261 | 0.001 | 5.109 | 0.002 | 4.529 | 0.001 | 4.892 | 0.002 | 12.89 | 0.001 |
| Organ × Land use | 4 | 4.805 | 0.002 | 2.285 | 0.057 | 2.918 | 0.015 | 4.037 | 0.003 | 2.954 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Álvarez, E.A.; de la Barrera, E. Influence of Land Use on the C and N Status of a C4 Invasive Grass in a Semi-Arid Region: Implications for Biomonitoring. Plants 2021, 10, 942. https://doi.org/10.3390/plants10050942
Díaz-Álvarez EA, de la Barrera E. Influence of Land Use on the C and N Status of a C4 Invasive Grass in a Semi-Arid Region: Implications for Biomonitoring. Plants. 2021; 10(5):942. https://doi.org/10.3390/plants10050942
Chicago/Turabian StyleDíaz-Álvarez, Edison A., and Erick de la Barrera. 2021. "Influence of Land Use on the C and N Status of a C4 Invasive Grass in a Semi-Arid Region: Implications for Biomonitoring" Plants 10, no. 5: 942. https://doi.org/10.3390/plants10050942
APA StyleDíaz-Álvarez, E. A., & de la Barrera, E. (2021). Influence of Land Use on the C and N Status of a C4 Invasive Grass in a Semi-Arid Region: Implications for Biomonitoring. Plants, 10(5), 942. https://doi.org/10.3390/plants10050942







