Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Treatments
2.2. Measurements of Plant Growth, Yield, and Yield Attributes
2.3. Measurements of Photosynthetic Efficiency and Stomatal Conductance
2.4. Membrane Stability Index and Relative Water Content Measurements
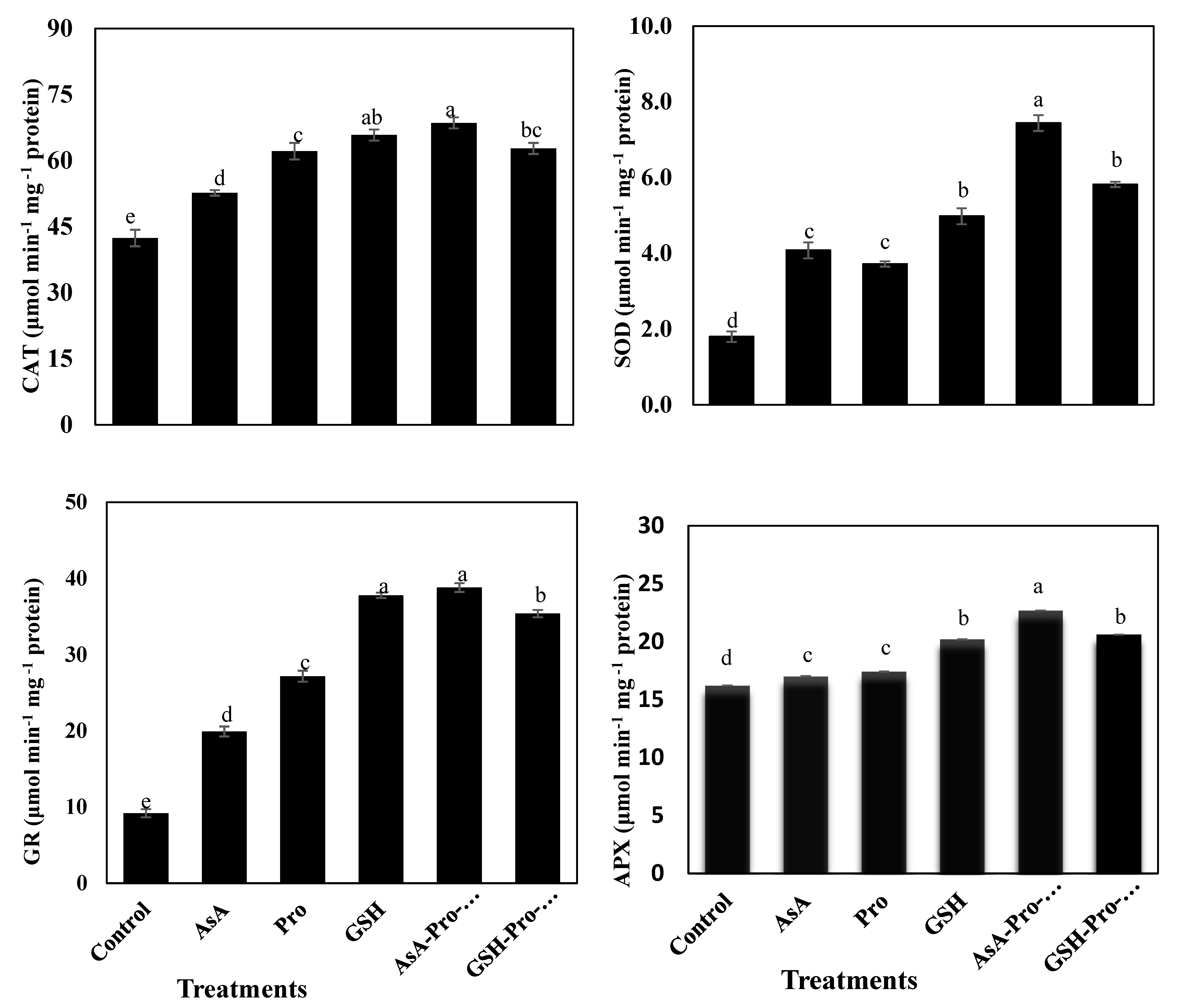
2.5. Enzymatic Antioxidants Assays
2.6. Non-Enzymatic Antioxidants
2.7. Statistical Analysis
3. Results
3.1. Effect of Antioxidants on Growth Characteristics
3.2. Effect of Antioxidants on Physiological Attributes
3.3. Enzymatic and Non-Enzymatic Antioxidants
3.4. Effect of Antioxidants on Yield and Yield Attributes
4. Discussion
4.1. Improvement of Salinity Tolerance by Integrating Three Powerful Antioxidants into One Sequential Treatment
4.2. Sequential AsA-Pro-GSH Improves Photosynthetic Efficiency and Relative Chlorophyll Content
4.3. Application of AsA-Pro-GSH Increases Enzymatic Antioxidant Levels for Better Defense System
4.4. Application of AsA-Pro-GSH Alleviates Membrane Damage and Enhances Water Status of Plants
4.5. Application of AsA-Pro-GSH Enhances Stomatal Conductance
4.6. Integration of AsA-Pro-GSH Promotes the Overall Growth and Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zörb, C.; Geilfus, C.-M.M.; Dietz, K.-J.J.; Zorb, C.; Geilfus, C.-M.M.; Dietz, K.-J.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, M.A.; Chaudhary, H.J.; Ullah, A. Technology Combating soil salinity with combining saline agriculture and phytomanagement with salt- accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1085–1115. [Google Scholar] [CrossRef]
- Desoky, E.M.; El-maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. Amst. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Hemida, K.; Rady, M.M. Natural bee-honey based biostimulants confer salt tolerance in onion via modulation of the antioxidant defence system. J. Hortic. Sci. Biotechnol. 2019, 94, 632–642. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Marcelis, L.F.M.; Hooijdonk, V. Effect of salinity on growth, water use and nutrient use in radish (Raphanus sativus L.). Plant Soil 1999, 215, 57–64. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; Abd El-mageed, T.A.A.; Howladar, S.M.; Shaaban, A. Foliage Applied Selenium Improves Photosynthetic Efficiency, Antioxidant Potential and Wheat Productivity under Drought Stress. Int. J. Agric. Biol. 2020, 24, 1293–1300. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant. Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Rady, M.M.; Taha, R.S.; Semida, W.M.; Alharby, H.F. Modulation of salt stress effects on vicia faba l. plants grown on a reclaimed-saline soil by salicylic acid application. Rom. Agric. Res. 2017, 34, 175–185. [Google Scholar]
- Parvaiz, A.; Azooz, M.M.; Khan, M.I.R.; Asgher, M.; Iqbal, N.; Nafees, A.K.; Ahmad, P.; Azooz, M.M.; Prasad, M.N. V Ecophysiology and Responses of Plants Under Salt Stress; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 9781461447, ISBN 9781461447474. [Google Scholar]
- Semida, W.M.; Taha, R.S.; Abdelhamid, M.T.; Rady, M.M. Foliar-applied α-tocopherol enhances salt-tolerance in Vicia faba L. plants grown under saline conditions. S. Afr. J. Bot. 2014, 95, 24–31. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Howladar, S.M.; Mohamed, G.F.; Rady, M.M. Response of Solanum melongena L. seedlings grown under saline calcareous soil conditions to a new organo-mineral fertilizer. J. Anim. Plant Sci. 2015, 25, 485–493. [Google Scholar]
- Semida, W.M.; Abd El-mageed, T.A.; Howladar, S.M.; Rady, M.M. Foliar-applied ɑ -tocopherol enhances salt-tolerance in onion plants by improving antioxidant defence system. Aust. J. Crop Sci. 2016, 10, 1030–1039. [Google Scholar] [CrossRef]
- Gill, S.G.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Kumar, D.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Paull, J.; Rengasamy, P.; McDonald, G.K. Comparing genotypic variation in faba bean (Vicia faba L.) in response to salinity in hydroponic and field experiments. Field Crop. Res. 2012, 127, 99–108. [Google Scholar] [CrossRef]
- Smirnoff, N. (Ed.) Ascorbate, tocopherol and carotenoids: Metabolism, pathway engineering and functions. In Antioxidants and Reactive Oxygen Species in Plants; Blackwell Publishing: Oxford, UK, 2005; pp. 53–86. ISBN 9781405125291. [Google Scholar]
- Semida, W.M.; Hemida, K.A.; Rady, M.M. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol. Environ. Saf. 2018, 154, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Burritt, D.J.; Fujita, M.; Munne-Bosch, S.; Diaz-Vivancos, P. Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 9783319740560. [Google Scholar]
- Horemans, N.; Foyer, H.C.; Potters, G.; Asard, H. Ascorbate function and associated transport systems in plants. Plant Physiol. Biochem. 2000, 38, 531–540. [Google Scholar] [CrossRef]
- Potters, G.; De Gara, L.; Asard, H.; Horemans, N. Ascorbate and glutathione: Guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 2002, 40, 537–548. [Google Scholar] [CrossRef]
- Noctor, G. Metabolic signalling in defence and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ouyang, B.; Yang, C.; Zhang, X.; Liu, H.; Zhang, Y.; Zhang, J. Reducing AsA leads to leaf lesion and defence response in knock-down of the AsA biosynthetic enzyme GDP-D-mannose pyrophosphorylase gene in tomato plant. PLoS ONE 2013, 8, e61987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zheng, F.; Wei, S.; Zhang, S.; Li, G. Evolution of disease defense genes and their regulators in plants. Int. J. Mol. Sci. 2019, 20, 335. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 4, 1011–1019. [Google Scholar] [CrossRef]
- Xiang, C.; Werner, B.L.; Christensen, E.L.M.; Oliver, D.J. The biological functions of glutathione revisited in arabidopsis transgenic plants with altered glutathione levels. Plant Physiol. 2001, 126, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Hajdinák, P.; Czobor, Á.; Lőrincz, T.; Szarka, A. The problem of glutathione determination: A comparative study on the measurement of glutathione from plant cells. Period. Polytech. Chem. Eng. 2019, 63, 1–10. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Szalai, G.; Kellos, T.; Galiba, G.; Kocsy, G. Glutathione as an antioxidant and regulatory molecule in plants under abiotic stress conditions. J. Plant Growth Regul. 2009, 28, 66–80. [Google Scholar] [CrossRef]
- Gallé, A.; Czekus, Z.; Bela, K.; Horvath, E.; Ördög, A.; Csiszar, J.; Poor, P. Plant glutathione transferases and light. Front. Plant Sci. 2019, 9, 1944. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Frendo, P.; Baldacci-Cresp, F.; Benyamina, M.; Puppo, A. Glutathione and plant response to the biotic environment. Free Radic. Biol. Med. 2013, 65, 724–730. [Google Scholar] [CrossRef]
- Tausz, M.; Grill, D. The role of glutathione in stress adaptation of plants. Phyton (B. Aires) 2000, 40, 111–118. [Google Scholar]
- Moustafa, E.S.A.; El-Sobky, E.-S.E.A.; Farag, H.I.A.; Yasin, M.A.T.; Attia, A.; Rady, M.O.A.; Awad, M.F.; Mansour, E. Sowing Date and Genotype Influence on Yield and Quality of Dual-Purpose Barley in a Salt-Affected Arid Region. Agronomy 2021, 11, 717. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Alagu, G.M.; Sridharan, R.; Panneerselvam, R. NaCl as a physiological modulator of proline metabolism and antioxidant potential in Phyllanthus amarus. Comptes Rendus Biol. 2007, 330, 806–813. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Abd El-mageed, T.A.; Abdelkhalik, A.; Abd El-mageed, S.A.; Semida, W.M. Co-composted poultry litter biochar enhanced soil quality and eggplant productivity under different irrigation regimes. J. Soil Sci. Plant Nutr. 2021. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt stress encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem Artichoke Plantlets. PLoS ONE 2013, 8, e62085. [Google Scholar] [CrossRef]
- Abdul Jaleel, C.; Gopi, R.; Sankar, B.; Manivannan, P.; Kishorekumar, A.; Sridharan, R.; Panneerselvam, R. Studies on germination, seedling vigour, lipid peroxidation, and proline metabolism in Catharanthus roseus seedlings under salt stress. South Afr. J. Bot. 2007, 73, 190–195. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Rady, M.O.A.; Semida, W.M.; Shaaban, A.; Mekdad, A.A.A. Exogenous Micronutrients Modulate Morpho-physiological Attributes, Yield, and Sugar Quality in Two Salt-Stressed Sugar Beet Cultivars. J. Soil Sci. Plant Nutr. 2021. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-mageed, T.A.; Abdelkhalik, A.; Hemida, K.A.; Abdurrahman, H.A.; Howladar, S.M.; Leilah, A.A.A.; Rady, M.O.A. Selenium modulates antioxidant activity, osmoprotectants, and photosynthetic efficiency of onion under saline. Agronomy 2021, 11, 855. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.A.; Marey, R.A.; El-mageed, T.A.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. Amst. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Bulut, F.; Akinci, S. The effect of salinity on growth and nutrient composition in broad bean (Vicia faba L.) seedlings. Fresenius Environ. Bull. 2010, 19, 2901–2910. [Google Scholar]
- Manchanda, G.; Garg, Æ.N. Salinity and its effects on the functional biology of legumes. Acta Physiol Plant 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Maas, E.; Hoffman, G. Crop salt tolerance: Evaluation of existing data. ASCE 1977, 103, 187–198. [Google Scholar]
- Grieve, C.M.; Grattan, S.R.; Maas, E.V. Plant salt tolerance. In Agricultural Salinity Assessment and Management; ASCE: Reston, VA, USA, 2012; pp. 405–460. ISBN 9780784411698. [Google Scholar]
- Abdelhamid, M.T.; Shokr, M.B.; Bekheta, M. Effects of induced salinity on four Vicia faba cultivars. In Proceedings of the Fourtenth International Water Technology Conference, Cairo, Egypt, 21–23 March 2010; pp. 421–427. [Google Scholar]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, S.; Shu, S.; Sun, J.; Tezuka, T. Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 2011, 10, 18381–18390. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Akhter Banu, M.N.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Al Mahmud, J.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Akram, S.; Siddique, B.M.N.; Al Bari, M.A.; Mostafa, M.G.; Hossain, M.A.; Lam-Son, T.P. Exogenous glutathione modulates salinity tolerance of soybean [Glycine max (L.) Merrill] at reproductive stage. J. Plant Growth Regul. 2017, 36, 877–888. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Black, C.A.; Evans, D.D.; Ensminger, L.E.; White, L.L.; Clark, E. Methods of Soil Analysis Part 2: Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Whitney, D.A. Recommended Chemical Soil Test Procedures for the North Central Region. North Cent. Reg. Publ. 1988, 221, 32–34. [Google Scholar]
- Semida, W.M.; El-Mageed, T.A.A.; Mohamed, S.E.; El-Sawah, N.A. Combined effect of deficit irrigation and foliar-applied salicylic acid on physiological responses, yield, and water-use efficiency of onion plants in saline calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 1227–1239. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Clark, A.J.J.; Landolt, W.; Bucher, J.B.B.; Strasser, R.J.J. Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ. Pollut. 2000, 109, 501–507. [Google Scholar] [CrossRef]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. Amst. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Hayat, S.; Ali, B.; Aiman Hasan, S.; Ahmad, A. Brassinosteroid enhanced the level of antioxidants under cadmium stress in Brassica juncea. Environ. Exp. Bot. 2007, 60, 33–41. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, P. Ultraviolet-9- and ozone-lnduced biochemical changes in antioxidant enzymes of arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, ISBN 0076-6879. [Google Scholar]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldeen, R.P.; Teare, I.D. Rapid determination of free proline for water—Stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Sauvesty, A.; Page, F.; Huot, J. A simple method for extracting plant phenolic compounds. Can. J. For. Res. 1992, 22, 654–659. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- Horemans, N.; Foyer, C.H.; Asard, H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000, 5, 263–267. [Google Scholar] [CrossRef]
- Venkatesh, J.; Park, S. Role of L-ascorbate in alleviating abiotic stresses in crop plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, W.; Guo, J.; Chang, X.; Su, P.; Zhang, L. Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J. Exp. Bot. 2005, 56, 3041–3049. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.-B. Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front. Plant Sci. 2015, 6, 792. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Gaafar, R.M.; Seyam, M.M. Ascorbate—Glutathione cycle confers salt tolerance in Egyptian lentil cultivars. Physiol. Mol. Biol. Plants 2018, 24, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ming, D.; Cui, J.X.; Chen, X.J.; Wen, Z.L.; Zhang, J.W.; Liu, H.Y. Exogenous GSH protects tomatoes against salt stress by modulating photosystem II efficiency, absorbed light allocation and H2O2 -scavenging system in chloroplasts. J. Integr. Agric. 2018, 17, 2257–2272. [Google Scholar] [CrossRef]
- Rady, M.O.A.; Semida, W.M.; Howladar, S.M.; Abd El-Mageed, T.A. Raised beds modulate physiological responses, yield and water use efficiency of wheat (Triticum aestivum L) under deficit irrigation. Agric. Water Manag. 2021, 245, 106629. [Google Scholar] [CrossRef]
- Smirnoff, N.; Sciences, B.; Laboratories, H.; Road, W. The function and metabolism of ascorbic acid in plants. Bot. Brief. 1996, 78, 661–669. [Google Scholar]
- Mehler, A.H. Studies on reactions of illuminated I. Mechanism of the reduction of oxygen and other hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef]
- Borisova, M.M.M.; Kozuleva, M.A.; Rudenko, N.N.; Naydov, I.A.; Klenina, I.B.; Ivanov, B.N. Photosynthetic electron fl ow to oxygen and diffusion of hydrogen peroxide through the chloroplast envelope via aquaporins. Biochim. Biophys. Acta 2012, 1817, 1314–1321. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nio; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.M.; Hemida, K.A. Sequenced application of ascorbate-proline-glutathione improves salt tolerance in maize seedlings. Ecotoxicol. Environ. Saf. 2016, 133, 252–259. [Google Scholar] [CrossRef]
- Bastam, N.; Baninasab, B.; Ghobadi, C. Interactive effects of ascorbic acid and salinity stress on the growth and photosynthetic capacity of pistachio (Pistacia vera L.) seedlings. J. Hortic. Sci. Biotechnol. 2013, 88, 610–616. [Google Scholar] [CrossRef]
- Gadallah, M.A.A. Effects of proline and glycinebetaine on Vicia faba responses to salt stress. Biol. Plant. 1999, 42, 249–257. [Google Scholar] [CrossRef]
- Aly-Salama, K.H.; Al-Mutawa, M.M. Glutathione-triggered mitigation in salt-induced alterations in plasmalemma of onion epidermal cells. Interanational J. Agric. Biol. 2009, 11, 639–642. [Google Scholar]
- Purohit, S.; Kumar, G.P.; Lalorya, M.; Lalorya, M.M. Involvement of superoxide radical in signal transduction regulating stomatal movements. BMC Plant Biol 1994, 19, 30–37. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Am. Soc. Plant Biol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Cheng, M.; Ko, K.; Chang, W.; Kuo, W.; Chen, G.; Lin, T. Increased glutathione contributes to stress tolerance and global translational changes in Arabidopsis. Plant J. 2015, 83, 926–939. [Google Scholar] [CrossRef]
- Klein, A.; Itai, C. Is proline involved in stomata regulation of Commelina communis plants recovering from salinity stress? Physiol. Plant. 1989, 75, 399–404. [Google Scholar] [CrossRef]
- Rajagopal, V. The influence of exogenous proline on the stomatal resistance in Vicia faba. Physiol. Plant. 1981, 52, 292–296. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, R.R.; Zhang, C.Q.; Tang, K.X.; Sun, M.F.; Yan, G.H.; Liu, Q.Q. Manipulation of the rice L-galactose pathway: Evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance. PLoS ONE 2015, 10, e125870. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene knockout study reveals that cytosolic ascorbate peroxidase 2(OsAPX2) plays a critical role in growth and reproduction in rice under drought, salt and cold stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef]
- Cordoba, F.; Gonzfilez-Reyes, J.A. Ascorbate and plant cell growth. J. Bioenerg. Biomembr. 1994, 26, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Esaka, M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol. Plant. 1999, 105, 321–329. [Google Scholar] [CrossRef]
- Sakr, M.T.; El-Sarkassy, N.M.; Fuller, M.P. Osmoregulators proline and glycine betaine counteract salinity stress in canola. Agron. Sustain. Dev. 2012, 32, 747–754. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]

| Layer (cm) | Particle Size Distribution | Bulk density g cm−3 | Ksat cm h−1 | Soil Moisture Content AT | pH | ECe dS m−1 | CaCO3, % | OM % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand % | Silt % | Clay % | Texture Class | F.C % | W.P % | A.W % | |||||||
| 0–20 | 65.07 | 16.08 | 18.85 | S.L. | 1.44 | 2.21 | 23.00 | 10.02 | 12.98 | 7.63 | 4.3 | 7.60 | 0.96 |
| 20–40 | 71.62 | 12.09 | 16.29 | S.L. | 1.47 | 2.01 | 20.72 | 9.15 | 11.57 | 7.60 | 4.5 | 6.4 | 0.83 |
| 40–60 | 73.61 | 12.15 | 14.24 | S.L. | 1.56 | 1.89 | 18.71 | 8.05 | 10.66 | 7.43 | 4.8 | 6.20 | 0.51 |
| Treatments | Shoot Length (cm) | No. of Leaves Plant−1 | No. of branches Plant−1 | Shoot FW (g) | Shoot DW (g) | Leaves area (dm2) |
|---|---|---|---|---|---|---|
| SI | ||||||
| Control | 87.0 ± 2.9 b | 50.5 ± 1.0 c | 2.25± 0.25 b | 161.9 ± 3.7 c | 19.5 ± 0.9 c | 28.9 ± 1.40 d |
| AsA | 108.0 ± 2.5 a | 57.0 ± 2.7 bc | 3.50± 0.65 ab | 210.6 ± 2.1 b | 27.1 ± 0.3 b | 42.5 ± 0.84 c |
| Pro | 105.2 ± 2. 8 a | 58.3 ± 1.2 bc | 3.75± 0.25 a | 216.2 ± 1.6 b | 26.0 ± 1.4 b | 43.5 ± 1.60 bc |
| GSH | 111.2 ± 3.6 a | 64.8 ± 0.9 ab | 4.00± 0.41 a | 230.2 ± 2.0 ab | 30.3 ± 1.1 ab | 48.6 ± 1.43 b |
| AsA-Pro-GSH | 105.0 ± 3.8 a | 67.5 ± 1.0 a | 4.25± 0.48 a | 245.7 ± 2.2 a | 33.3 ± 0.2 a | 54.4 ± 0.67 a |
| GSH-Pro-ASA | 102.5 ± 1.2 a | 62.3 ± 1.7 ab | 3.50± 0.29 ab | 222.5 ± 2.4 ab | 28.1 ± 0.8 b | 45.3 ± 2.48 bc |
| SII | ||||||
| Control | 94.5 ± 1.9 b | 50.0 ± 1.3 d | 2.50±0.29 b | 168.9 ± 2.5 d | 20.6 ± 1.8 c | 32.7 ± 2.18 d |
| AsA | 111.0 ± 3.4 a | 58.1 ± 1.2 c | 3.86±0.43 a | 210.7 ± 2.4 c | 26.9 ± 0.45 ab | 42.2 ± 2.01 bc |
| Pro | 102.5 ± 3.3 ab | 58.8 ± 1.5 bc | 3.80±0.27 a | 214.1 ± 3.7 bc | 27.0 ± 0.90 a | 42.4 ± 0.26 c |
| GSH | 110.2 ± 4.8 a | 62.5 ± 0.9 a | 4.00±0.01 a | 228.1 ± 3.6 ab | 29.4 ± 0.99 a | 49.0 ± 2.23 ab |
| AsA-Pro-GSH | 106.2 ± 2.4 a | 65.3 ± 1.0 a | 4.6±0.12 a | 243.4 ± 4.3 a | 34.3 ± 0.68 a | 51.8 ± 2.52 a |
| GSH-Pro-ASA | 101.0 ± 2.5 ab | 59.5 ± 0.5 a | 3.83±0.28 a | 225.7 ± 9.5 bc | 27.8.0 ± 1.8 a | 45.1 ± 2.02 bc |
| Treatments | SPAD Value | Fv/Fm | Fv/F0 | PI | gs (mmol m−2 S−1) |
|---|---|---|---|---|---|
| SI | |||||
| Control | 31.98 ± 4.2 c | 0.822 ± 0.007 b | 4.60 ± 0.16 b | 3.04 ± 0.27 c | 122.9 ± 2.1 c |
| AsA | 40.46 ± 1.4 b | 0.827 ± 0.004 ab | 4.82 ± 0.12 ab | 4.59 ± 0.15 b | 172.0 ± 2.5 a |
| Pro | 40.14 ± 2.2 b | 0.836 ± 0.004 a | 5.13 ± 0.13 a | 4.81 ± 0.19 ab | 152.1 ± 1.9 b |
| GSH | 47.88 ± 1.4 a | 0.839 ± 0.004 a | 5.25 ± 0.13 a | 5.46 ± 0.19 a | 167.8 ± 1.7 a |
| AsA-Pro-GSH | 48.78 ± 0.5 a | 0.840 ± 0.004 a | 5.23 ± 0.16 a | 5.40 ± 0.27 a | 171.4 ± 1.2 a |
| GSH-Pro-ASA | 43.38 ± 1.5 ab | 0.839 ± 0.005 a | 5.24 ± 0.18 a | 4.88 ±0.31 ab | 163.1 ± 1.8 a |
| SII | |||||
| Control | 29.90 ± 1.6 c | 0.807 ± 0.004 b | 3.34 ± 0.43 b | 3.14 ± 0.48 c | 131.9 ± 2.2 d |
| AsA | 43.46 ± 2.0 b | 0.825 ± 0.004 a | 4.89 ± 0.16 ab | 4.15 ± 0.28 b | 174.5 ± 2.8 bc |
| Pro | 44.80 ± 1.6 ab | 0.834 ± 0.007 a | 4.42 ± 0.12 a | 4.56 ± 0.39 ab | 163.9 ± 0.3 c |
| GSH | 44.32 ± 1.6 ab | 0.828 ± 0.007 a | 5.07 ± 0.21 a | 4.90 ± 0.66 a | 186.0 ± 2.6 ab |
| AsA-Pro-GSH | 49.24 ± 0.87 a | 0.840 ± 0.002 a | 5.24 ± 0.18 a | 5.31 ± 0.34 a | 190.3 ± 0.8 a |
| GSH-Pro-ASA | 42.94 ± 2.6 b | 0.825 ± 0.006 a | 4.76 ±0.20 a | 4.65 ± 0.42 ab | 178.0 ± 1.6 ab |
| Treatments | RWC % | MSI % | WUE (Kg m3) |
|---|---|---|---|
| SI | |||
| Control | 77.0 ± 0.57 c | 64.9 ± 0.82 c | 0.56±0.01 d |
| AsA | 85.1 ± 0.76 b | 69.7 ± 2.5 bc | 0.75±0.00 c |
| Pro | 87.4 ± 1.5 ab | 69.3 ± 2.6 bc | 0.75±0.02 c |
| GSH | 86.8 ± 1.5 b | 67.1 ± 1.1 bc | 0.87±0.02 b |
| AsA-Pro-GSH | 91.9 ± 0.57 a | 77.3 ± 1.6 a | 0.95±0.01 a |
| GSH-Pro-ASA | 88.9 ± 1.8 ab | 72.7 ± 3.7 ab | 0.78±0.01 c |
| SII | |||
| Control | 75.3 ± 0.77 c | 63.1 ± 3.1 c | 0.55±0.02 d |
| AsA | 83.9 ± 2.1 b | 72.0 ± 1.7 ab | 0.78±0.02 bc |
| Pro | 84.2 ± 2.6 b | 68.0 ± 2.6 bc | 0.75±0.00 c |
| GSH | 87.1 ± 2.7 ab | 73.9 ± 0.94 ab | 0.85±0.03 ab |
| AsA-Pro-GSH | 92.0 ± 1.8 a | 77.7 ± 1.8 a | 0.91±0.02 a |
| GSH-Pro-ASA | 86.2 ±1.4 ab | 71.0 ±1.5 ab | 0.81±0.03 bc |
| Treatments | No. of Pods Plant−1 | Pods Weight Plant−1 (g) | Pods Yield Hectare−1 (ton) |
|---|---|---|---|
| SI | |||
| Control | 10.8 ± 0.48 d | 109.5 ± 1.8 c | 8.8 ± 1.03 c |
| AsA | 13.3 ± 0.85 bc | 125.5 ± 4.6 b | 10.5 ± 0.19 b |
| Pro | 12.3 ± 0.25 cd | 123.0 ± 4.2 b | 10.6 ± 0.25 b |
| GSH | 15.5 ± 1.79 ab | 141.7 ± 2.6 a | 11.8 ± 0.63 a |
| AsA-Pro-GSH | 16.8 ± 1.08 a | 143.0 ± 2.9 a | 12.2 ± 0.83 a |
| GSH-Pro-ASA | 13.8 ±1.38 bc | 135.1 ± 4.69 ab | 11.2 ± 0.89 ab |
| SII | |||
| Control | 10.50 ± 1.2 b | 109.5 ± 1.8 c | 8.9 ± 0.15 c |
| AsA | 12.75 ± 1.1 ab | 125.5 ± 4.6 b | 10.5 ± 0.38 b |
| Pro | 13.25 ± 1. 8 ab | 123.0 ± 4.2 b | 10.3 ± 0.35 b |
| GSH | 16.00 ± 0.7 a | 141.7 ± 2.6 a | 11.9 ± 0.22 a |
| AsA-Pro-GSH | 15.50 ± 1.2 a | 143.0 ± 2.9 a | 12.0 ± 0.24 a |
| GSH-Pro-ASA | 13.75 ± 0.9 ab | 135.1 ± 4.6 ab | 11.4 ± 0.39 a |
| Treatments | Biological Yield Hectare−1 (ton) | Straw Yield Hectare−1 (ton) | Seed Yield Hectare−1 (ton) | 100-Seed Weight Average | HI (%) |
|---|---|---|---|---|---|
| SI | |||||
| Control | 7.9 ± 0.45 b | 5.6 ± 0.24 b | 2.3 ± 0.06 c | 90.2 ± 0.21 c | 29.4 ± 0.3 e |
| AsA | 9.3 ± 0.43 ab | 6.2 ± 0.21 ab | 3.1 ± 0.01 b | 93.9 ± 0.27 ab | 33.2 ± 0.6 cd |
| Pro | 9.7 ± 0.49 ab | 6.7 ± 0.23 ab | 3.1 ± 0.09 b | 92.4 ± 0.07 b | 31.7 ± 0.6 d |
| GSH | 10.0 ± 0.77 ab | 6.4 ±0.18 b | 3.6 ± 0.07 a | 95.0 ± 0.51 a | 36.4 ±1.1 a |
| AsA-Pro-GSH | 11.2 ± 0.63 a | 7.23 ± 0.23 a | 3.9 ± 0.06 a | 95.2 ± 0.35 a | 35.1 ±0.5 ab |
| GSH-Pro-ASA | 9.2 ± 0.36 ab | 6.1 ± 0.31 ab | 3.2 ± 0.06 b | 93.3 ± 0.21 b | 34.6 ±0.8 bc |
| SII | |||||
| Control | 7.6 ± 0.07 d | 5.4 ± 0.07 c | 2.21± 0.07 c | 91.3 ± 0.18 b | 29.3 ± 1.8 b |
| AsA | 9.4± 0.03 c | 6.3 ± 0.07 bc | 3.2 ± 0.07 ab | 92.2 ± 0.94 b | 33.6 ± 0.8 ab |
| Pro | 9.0 ± 0.13 c | 6.0 ± 0.14 bc | 3.0 ± 0.01 b | 92.4 ± 0.94 b | 33.7 ± 0.60 ab |
| GSH | 10.0 ± 0.09 b | 6.6 ± 0.05 ab | 3.4 ± 0.12 ab | 94.6 ± 1.24 ab | 35.5 ± 1.6 ab |
| AsA-Pro-GSH | 11.1 ± 0.07 a | 7.5 ± 0.13 a | 3.7 ± 0.09 a | 97.5 ± 0.55 a | 32.3 ± 0.65 ab |
| GSH-Pro-ASA | 9.6 ± 0.24 c | 6.2 ± 0.03 bc | 3.4 ± 0.12 ab | 94.2 ± 0.78 ab | 36.4 ± 0.34 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semida, W.M.; Abd El-Mageed, T.A.; Abdalla, R.M.; Hemida, K.A.; Howladar, S.M.; Leilah, A.A.A.; Rady, M.O.A. Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L. Plants 2021, 10, 914. https://doi.org/10.3390/plants10050914
Semida WM, Abd El-Mageed TA, Abdalla RM, Hemida KA, Howladar SM, Leilah AAA, Rady MOA. Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L. Plants. 2021; 10(5):914. https://doi.org/10.3390/plants10050914
Chicago/Turabian StyleSemida, Wael M., Taia A. Abd El-Mageed, Reham M. Abdalla, Khaulood A. Hemida, Saad. M. Howladar, Ahmed A. A. Leilah, and Mohamed O. A. Rady. 2021. "Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L." Plants 10, no. 5: 914. https://doi.org/10.3390/plants10050914
APA StyleSemida, W. M., Abd El-Mageed, T. A., Abdalla, R. M., Hemida, K. A., Howladar, S. M., Leilah, A. A. A., & Rady, M. O. A. (2021). Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L. Plants, 10(5), 914. https://doi.org/10.3390/plants10050914







