Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Root Growth Dynamics
3.2. Growth, Physiology, Yield, and Head Quality
4. Materials and Methods
4.1. Site Description
4.2. Stand Establishment and Cultivar Treatments
4.3. Field Management
4.4. Root Measurements
4.5. Shoot Morphology, Leaf Physiology, and Yield
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dale, A.; Drennan, S. Transplanted maize (Zea mays) for grain production in Southern England. I. Effects of planting date, transplant age at transplanting and cultivar on grain yield. J. Agric. Sci. 1997, 128, 27–35. [Google Scholar] [CrossRef]
- Fanadzo, M.; Chiduza, C.; Mnkeni, P. Comparative response of direct seeded and transplanted maize (Zea mays L.) to nitrogen fertilization at Zanyokwe irrigation scheme, Eastern Cape, South Africa. Afr. J. Agric. Res. 2009, 4, 689–694. [Google Scholar]
- Leskovar, D.; Othman, Y. Low nitrogen and fertigation practices can improve artichoke transplant quality and yield. HortScience 2016, 51, 567–572. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y. Nitrogen management for improving root and shoot components of young ‘Arbequina’ olives. HortScience 2019, 54, 175–180. [Google Scholar] [CrossRef]
- Leskovar, D.; Cantliffe, D. Transplant production systems influence growth and yield of fresh-market tomatoes. J. Am. Soc. Hort. Sci. 1994, 119, 662–668. [Google Scholar] [CrossRef]
- Ketema, S.; Dessalegn, L.; Tesfaye, B. Effect of planting methods on maturity and yield of onion (Allium cepa var. cepa) in the central rift valley of Ethiopia. Ethiop. J. Agric. Sci. 2013, 24, 45–55. [Google Scholar]
- Palma, A.; Laurance, S. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Douglas, G.; Dodd, M.; Power, I. Potential of direct seeding for establishing native plants into pastoral land in New Zealand. N. Z. J. Ecol. 2007, 31, 143–153. [Google Scholar]
- Leskovar, D.; Othman, Y. Pre-transplant conditioning to mitigate heat, drought and biotic stresses in artichoke. Acta Hortic. 2016, 1147, 145–154. [Google Scholar] [CrossRef]
- Hossain, M.; Salam, M.; Uddin, M.; Pervez, Z.; Sarkar, M. A comparative study of direct seeding versus transplanting method on the yield of Aus rice. Pak. J. Agron. 2002, 1, 86–88. [Google Scholar] [CrossRef]
- Macias-Leon, M.; Leskovar, D. Containerized onion transplants: A management system to enhance growth, yield, and quality. HortScience 2019, 54, 60–69. [Google Scholar] [CrossRef]
- Hongyan, L.; Weiqin, W.; Aibin, H.; Lixiao, N. Correlation of leaf and root senescence during ripening in dry seeded and transplanted rice. Rice Sci. 2018, 25, 279–285. [Google Scholar] [CrossRef]
- Othman, Y.; Leskovar, D. Nitrogen management influenced root length intensity of young olive trees. Sci. Hortic. 2019, 246, 726–733. [Google Scholar] [CrossRef]
- Leskovar, D.; Cantliffe, D.; Stoffella, P. x Root growth and root-shoot interaction in transplants and direct seeded pepper plants. Environ. Exp. Bot. 2019, 30, 349–354. [Google Scholar] [CrossRef]
- Leskovar, D.; Cantliffe, D. Comparison of plant establishment method, transplant, or direct seeding on growth and yield of bell pepper. J. Am. Soc. Hort. Sci. 1993, 118, 17–22. [Google Scholar] [CrossRef]
- Vemmos, S.; Petri, E.; Stournaras, V. Seasonal changes in photosynthetic activity and carbohydrate content in leaves and fruit of three fig cultivars (Ficus carica L.). Sci. Hort. 2013, 160, 198–207. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.; Yoon, T. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hort. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Ciancolini, A.; Alignan, M.; Pagnotta, M.; Miquel, J.; Vilarem, G.; Crinò, P. Morphological characterization, biomass and pharmaceutical compounds in Italian globe artichoke genotypes. Ind. Crop. Prod. 2013, 49, 326–333. [Google Scholar] [CrossRef]
- Moglia, A.; Lanteri, S.; Comino, C.; Acquadro, A.; Beekwilder, J. Stress-induced biosynthesis of dicaffeoylquinic acids in globe artichoke. J. Agric. Food Chem. 2008, 56, 8641–8649. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y. Organic and conventional farming differentially influenced soil respiration, physiology, growth and head quality of artichoke cultivars. J. Soil Sci. Plant Nutr. 2018, 18, 865–880. [Google Scholar] [CrossRef]
- Othman, Y.; Leskovar, D. Organic soil amendments influence soil health, yield, and phytochemicals of globe artichoke heads. Biol. Agric. Hortic. 2018, 34, 258–267. [Google Scholar] [CrossRef]
- Othman, Y.; Leskovar, D. Farming system and sources of organic matter: Influence on soil health, yield, and head quality of globe artichoke. Acta Hortic. 2020, 1284, 93–100. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.; Schnitzler, W.; Nitz, G.; Razin, A.; El-Oksh, I. The effect of gibberellic acid (GA3) on some phenolic substances in globe artichoke (Cynara cardunculus var. scolymus (L.) Fiori). Sci. Hortic. 2007, 111, 326–329. [Google Scholar] [CrossRef]
- Shinohara, T.; Agehara, S.; Yoo, K.; Leskovar, D. Irrigation and nitrogen management of artichoke: Yield, head quality, and phenolic content. HortScience 2011, 46, 377–386. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. Morphological and physiological traits of roots and their relationships with shoot growth in “super” rice. Field Crop. Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Leskovar, D.; Clark, J.; Sprague, R. Yield, quality, and water use efficiency of muskmelon are affected by irrigation and transplanting versus direct seeding. HortScience 2001, 36, 286–291. [Google Scholar] [CrossRef]
- McKee, J. Physiological aspects of transplanting vegetables and other crops. I. Factors which influence re-establishment. Hort. Abstr. 1981, 51, 265–272. [Google Scholar]
- Grossnickle, C. Importance of root growth in overcoming planting stress. New For. 2005, 30, 273–294. [Google Scholar] [CrossRef]
- Song, C.; Sheng-guan, C.; Xin, C.; Guo-ping, Z. Genotypic differences in growth and physiological responses to transplanting and direct seeding cultivation in rice. Rice Sci. 2009, 16, 143–150. [Google Scholar] [CrossRef]
- Kerbiriou, P.; Stomph, T.; Bueren, E.; Struik, P. Influence of transplant size on the above- and below-ground performance of four contrasting field-grown lettuce cultivars. Front. Plant Sci. 2013, 4, 379. [Google Scholar] [CrossRef] [PubMed]
- Khozaei, M.; Haghighi, A.; Parsa, S.; Sepaskhah, A.; Razzaghi, F.; Yousefabadi, V.; Emam, Y. Evaluation of direct seeding and transplanting in sugar beet for water productivity, yield and quality under different irrigation regimes and planting densities. Agric. Water Manag. 2020, 238, 106230. [Google Scholar] [CrossRef]
- Javanmardi, J.; Moradiani, M. Tomato transplant production method affects plant development and field performance. Int. J. Veg. Sci. 2017, 23, 31–41. [Google Scholar] [CrossRef]
- Leskovar, D.; Othman, Y. Morpho-physiological characteristics and yield of early and Mid-season globe artichoke. Acta Hortic. 2016, 1147, 155–158. [Google Scholar] [CrossRef]
- Gezer, C.; Yucecan, S.; Rattan, S. Artichoke compound cynarin differentially affects the survival, growth, and stress response of normal, immortalized, and cancerous human cells. Turk. J. Biol. 2015, 39, 299–305. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.; Salami, S.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Jarrell, W.; Beverly, R. The dilution effect in plant nutrition studies. In Advances in Agronomy; Academic Press: New York, NY, USA, 1981; pp. 197–224. [Google Scholar] [CrossRef]
- Sharma, S.; Leskovar, D.; Crosby, K.; Volder, A. Root growth dynamics and fruit yield of melon (Cucumis melo L.) genotypes at two locations with sandy loam and clay soils. Soil Tillage Res. 2017, 168, 50–62. [Google Scholar] [CrossRef]
- Rasmussen, I.; Thorup-Kristensen, K. Does earlier sowing of winter wheat improve root growth and Nuptake? Field Crop. Res. 2016, 196, 10–21. [Google Scholar] [CrossRef]
- Othman, Y.; Van Leeuwen, D.; Heerema, R.; St. Hilaire, R. Midday stem water potential values needed to maintain photosynthesis and gas exchange established for pecans. J. Amer. Soc. Hort. Sci. 2014, 139, 537–546. [Google Scholar] [CrossRef]
- Tadros, M.; Al-Mefleh, N.; Othman, Y.; Al-Assaf, A. Water harvesting techniques for improving soil water content, and morpho-physiology of pistachio trees under rainfed conditions. Agric. Water Manag. 2021, 243, 106464. [Google Scholar] [CrossRef]
- A’saf, T.; Al-Ajlouni, M.; Ayad, J.; Othman, Y.; St. Hilaire, R. Performance of six different soilless green roof substrates for the Mediterranean region. Sci. Total Environ. 2020, 730, 139182. [Google Scholar] [CrossRef] [PubMed]
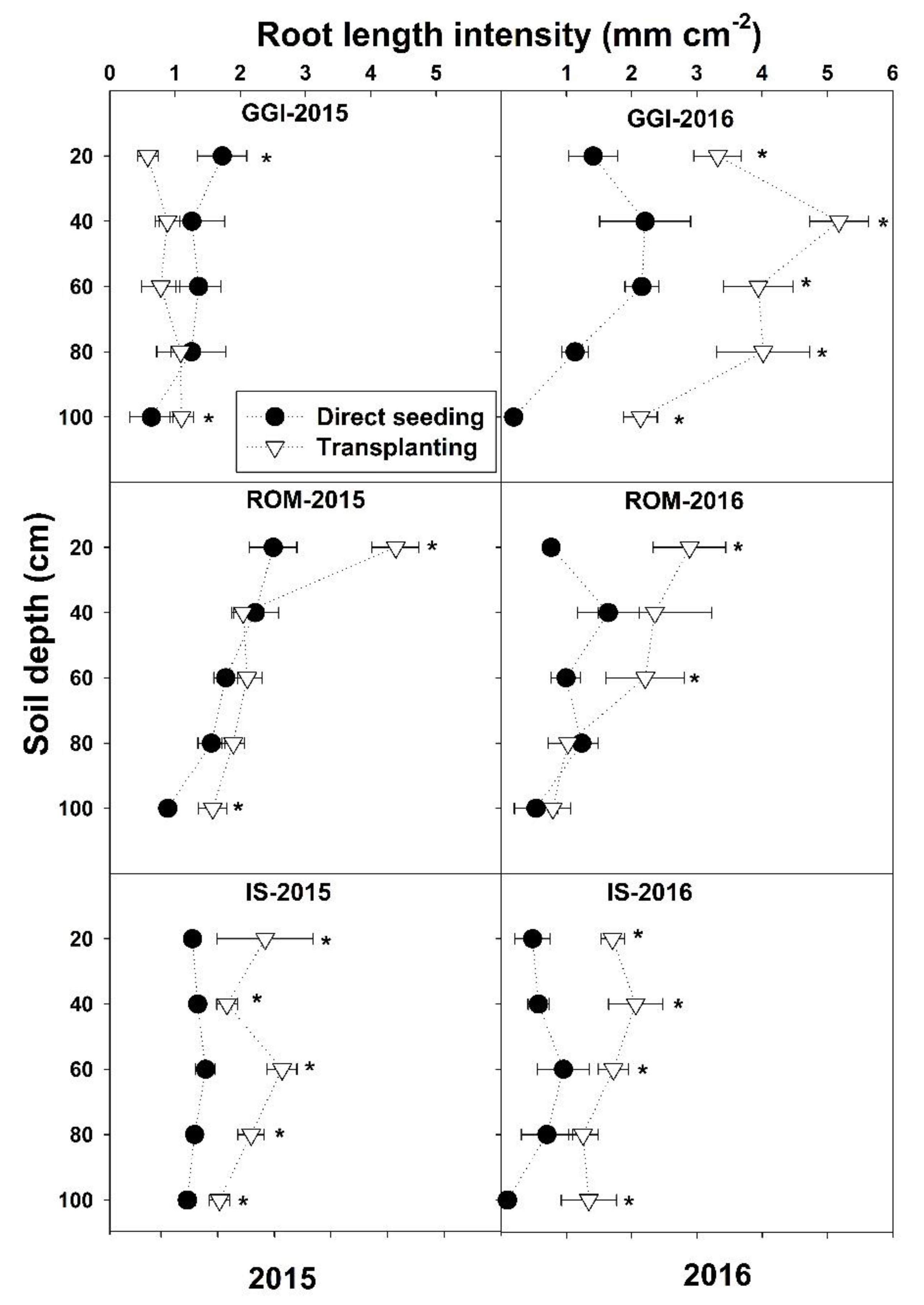
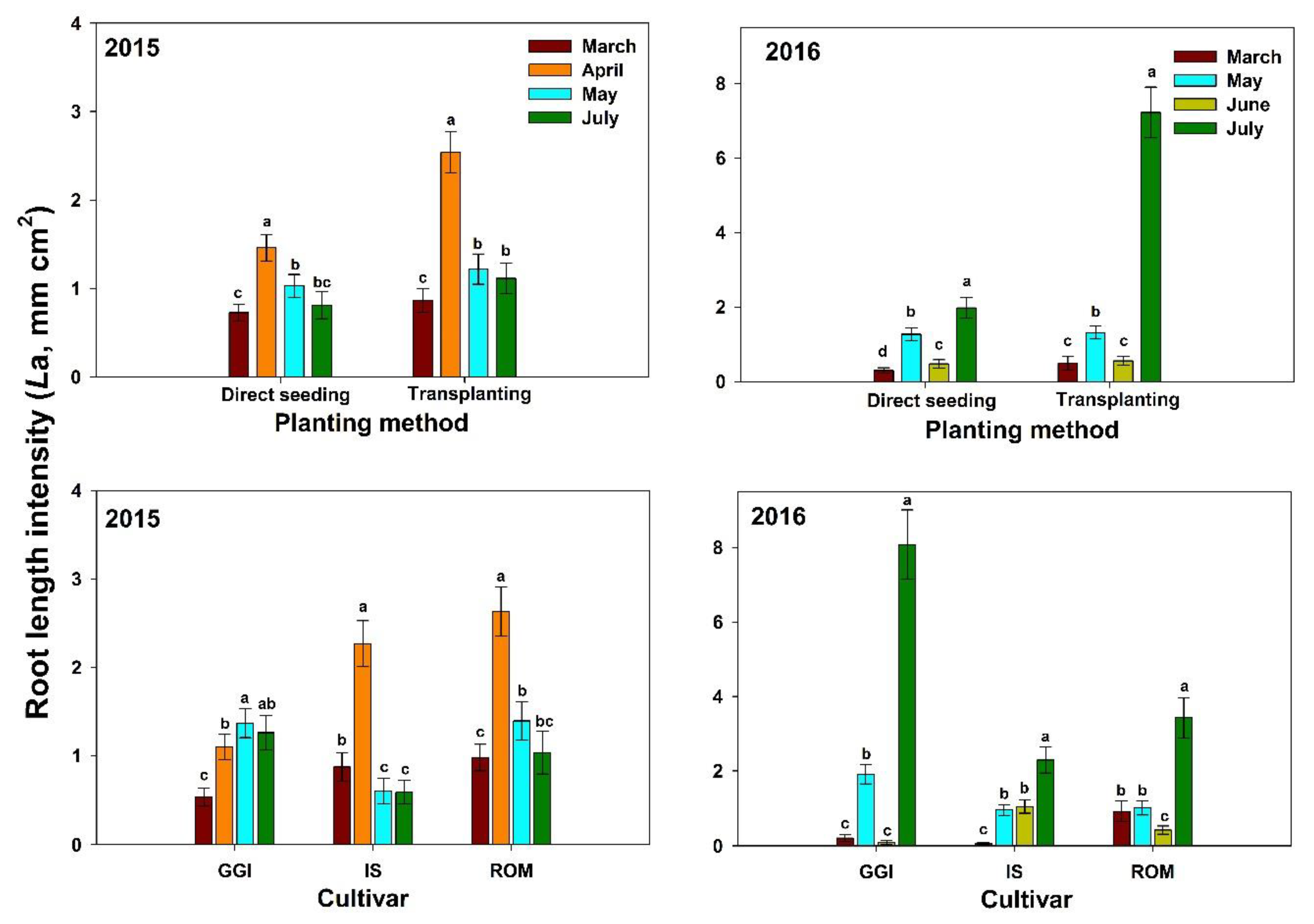
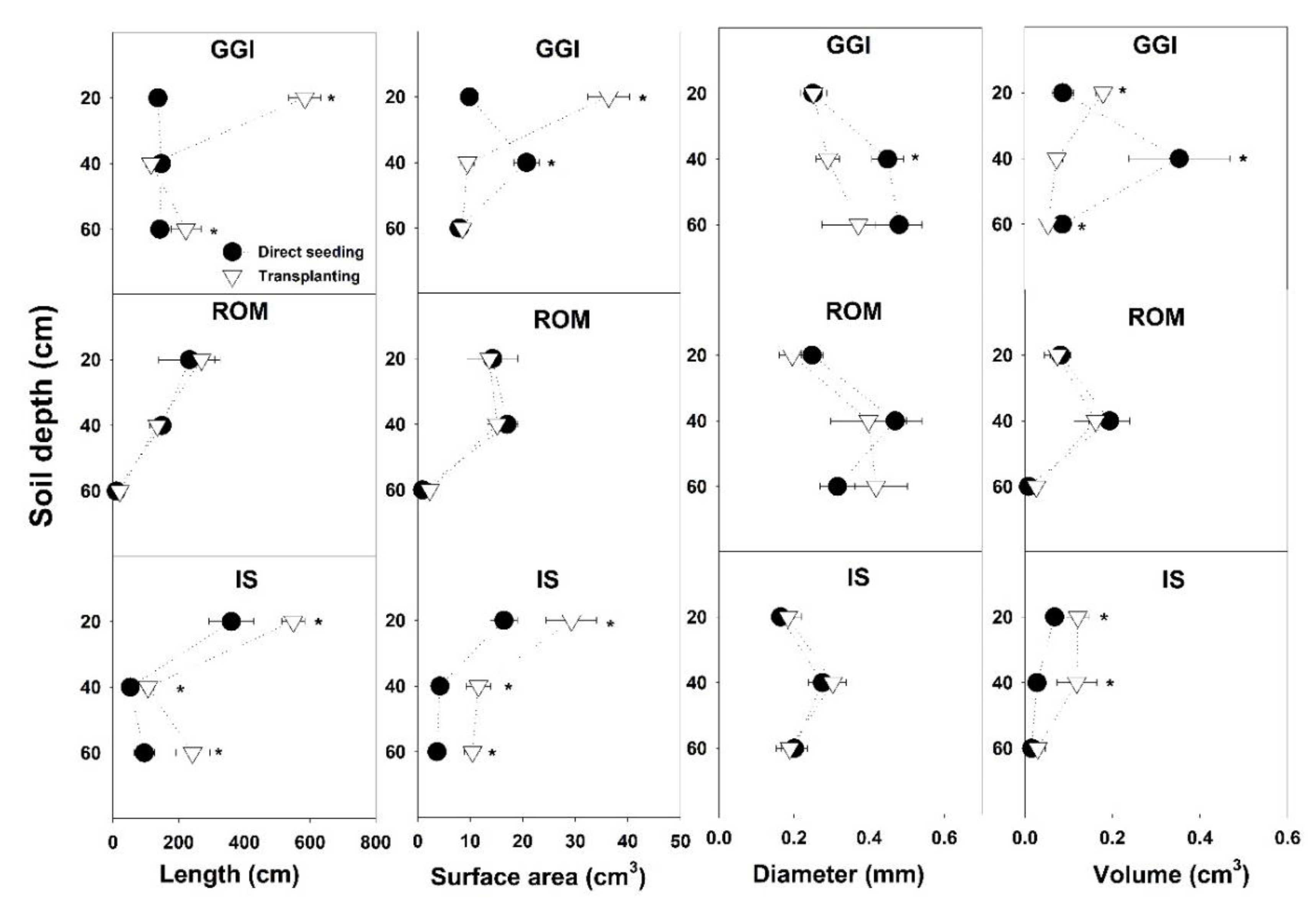
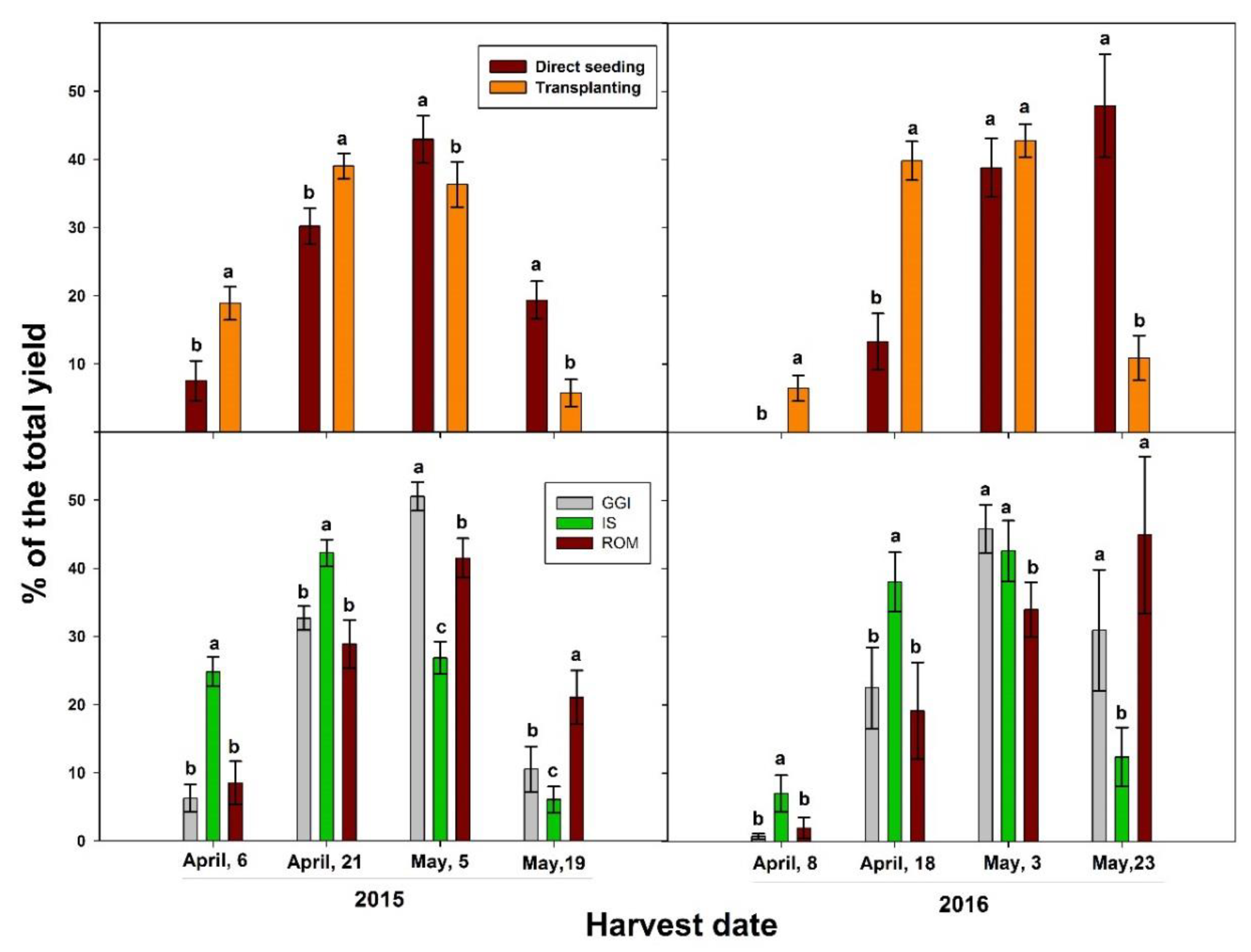

| Main Effect | Root Length Intensity (La, mm cm−2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | Overall Mean | |||||||||
| March | April | May | July | Mean | March | May | June | July | Mean | ||
| Planting method (P) | |||||||||||
| Seed | 0.72 a | 1.46 b | 1.03 a | 0.81 a | 1.01 b | 0.29 a | 1.27 a | 0.48 a | 1.97 b | 1.01 b | 1.01 b |
| Transplant | 0.87 a | 2.54 a | 1.22 a | 1.12 a | 1.43 a | 0.49 a | 1.32 a | 0.55 a | 7.22 a | 2.40 a | 1.92 a |
| Cultivar (C) | |||||||||||
| GGI | 0.53 b | 1.01 b | 1.40 a | 1.26 a | 1.07 b | 0.21 b | 1.91 a | 0.08 c | 8.06 a | 2.56 a | 1.82 a |
| ROM | 0.71 a | 2.63 a | 1.39 a | 1.04 ab | 1.51 a | 0.92 a | 1.01 b | 0.42 b | 3.43 b | 1.44 b | 1.48 b |
| IS | 0.56 a | 2.27 a | 0.60 b | 0.59 b | 1.08 b | 0.06 c | 0.95 b | 1.05 a | 2.31 c | 1.09 b | 1.01 c |
| Depth (D, cm) | |||||||||||
| 20 | 1.06 a | 2.52 a | 1.77 a | 1.86 a | 1.80 a | 1.06 a | 1.43 ab | 0.66 a | 3.91 cd | 1.77 b | 1.78 a |
| 40 | 1.05 a | 1.95 ab | 0.78 b | 0.93 b | 1.18 b | 0.39 b | 1.92 a | 0.69 a | 6.34 a | 2.33 a | 1.76 a |
| 60 | 1.01 a | 2.17 a | 1.08 b | 0.90 b | 1.29 b | 0.33 bc | 1.50 ab | 0.51 a | 5.66 ab | 2.00 ab | 1.65 a |
| 80 | 0.68 a | 1.95 ab | 1.14 b | 0.69 b | 1.12 b | 0.14 bc | 1.17 b | 0.43 a | 4.51 bc | 1.56 b | 1.34 b |
| 100 | 0.19 b | 1.41 b | 0.82 b | 0.43 b | 0.71 c | 0.04 c | 0.43 c | 0.30 a | 2.62 d | 0.85 c | 0.78 c |
| ANOVA | |||||||||||
| P | NS | *** | NS | NS | *** | † | NS | NS | *** | *** | *** |
| C | ** | *** | ** | * | ** | *** | ** | *** | *** | *** | *** |
| P×C | ** | *** | NS | ** | *** | *** | * | ** | *** | ** | NS |
| D | *** | ** | ** | ** | *** | *** | * | NS | *** | ** | *** |
| P×D | NS | NS | NS | NS | NS | *** | NS | NS | NS | NS | NS |
| C×D | ** | *** | *** | NS | *** | *** | NS | NS | * | † | ** |
| P×C×D | NS | NS | NS | * | * | *** | NS | NS | NS | NS | ** |
| Main Effect | Length (cm) | Surface Area (cm2) | Average Diameter (mm) | Volume (cm3) |
|---|---|---|---|---|
| Planting method (P) | ||||
| Seed | 136 b | 10.6 b | 0.32 a | 0.10 a |
| Transplant | 231 a | 15.2 a | 0.29 a | 0.09 a |
| Cultivar (C) | ||||
| GGI | 197 a | 15.5 a | 0.35 a | 0.14 a |
| ROM | 119 b | 10.6 b | 0.34 a | 0.09 b |
| IS | 235 a | 12.6 b | 0.22 b | 0.06 b |
| Depth (D) | ||||
| 20 | 330 a | 20.0 a | 0.22 b | 0.10 b |
| 40 | 107 b | 13.0 b | 0.36 a | 0.15 a |
| 60 | 114 b | 5.6 c | 0.33 a | 0.04 c |
| ANOVA | ||||
| P | *** | ** | NS | NS |
| C | *** | *** | ** | * |
| P×C | ** | ** | NS | * |
| D | *** | *** | *** | *** |
| P×D | *** | *** | NS | NS |
| C×D | *** | *** | NS | * |
| P×C×D | ** | *** | NS | ** |
| Year | Main Effect | Width (cm) | Height (cm) | Leaf Area Index | SPAD | ||||
|---|---|---|---|---|---|---|---|---|---|
| Vegetative | Harvesting | Vegetative | Harvesting | Vegetative | Harvesting | Vegetative | Harvesting | ||
| 2015 | Planting method (P) | ||||||||
| Seed | 80.3 b | 162 b | 12.0 a | 71.9 b | 1.60 b | 3.33 b | 38.4 b | 57.0 a | |
| Transplant | 105 a | 191 a | 15.3 a | 93.1 a | 2.10 a | 3.80 a | 44.0 a | 58.7 a | |
| Cultivar (C) | |||||||||
| GGI | 88.5 b | 180 a | 15.2 a | 86.4 a | 1.77 a | 3.19 b | 42.3 a | 61.0 a | |
| ROM | 97.2 a | 178 a | 14.6 a | 82.5 a | 1.94 a | 3.73 a | 39.8 a | 56.2 a | |
| IS | 92.1 ab | 172 a | 11.0 a | 78.6 a | 1.84 a | 3.77 a | 41.5 a | 56.5 a | |
| ANOVA | |||||||||
| P | * | ** | NS | ** | * | * | ** | NS | |
| C | * | NS | NS | NS | NS | * | NS | NS | |
| P×C | NS | NS | NS | NS | NS | NS | NS | NS | |
| 2016 | Planting method | ||||||||
| Seed | 67.2 b | 160 a | 18.3 b | 73.5 a | 1.4 a | 5.25 b | 56.7 a | 60.0 a | |
| Transplant | 89.1 a | 167 a | 21.8 a | 77.8 a | 1.5 a | 5.86 a | 57.2 a | 61.4 a | |
| Cultivar | |||||||||
| GGI | 73.3 a | 167 a | 23.8 a | 85.9 a | 1.3 a | 5.10 b | 59.4 a | 64.5 a | |
| ROM | 90.7 a | 174 a | 20.1 ab | 75.3 ab | 1.6 a | 6.62 a | 56.4 ab | 61.1 ab | |
| IS | 70.5 a | 150 b | 16.3 b | 65.7 b | 1.4 a | 4.95 b | 55.0 b | 56.6 b | |
| ANOVA | |||||||||
| P | * | NS | * | NS | NS | ** | NS | NS | |
| C | NS | *** | ** | * | NS | ** | * | * | |
| P×C | NS | NS | NS | NS | NS | NS | NS | NS | |
| Year | Main Effect | Marketable Yield (ton ha−1) | Chlorogenic Acid (µg g−1) | Cynarin (µg g−1) |
|---|---|---|---|---|
| 2015 | Planting method (P) | |||
| Seed | 19.9 b | 125 a | 5.63 a | |
| Transplant | 21.1 a | 138 a | 6.26 a | |
| Cultivar (C) | ||||
| GGI | 24.3 a | 95 b | 5.65 a | |
| ROM | 21.9 b | 140 ab | 6.65 a | |
| IS | 15.2 c | 159 a | 5.53 a | |
| ANOVA | ||||
| P | * | NS | NS | |
| C | * | * | NS | |
| P×C | NS | NS | NS | |
| 2016 | Planting method | |||
| Seed | 13.7 b | 312 a | 6.70 a | |
| Transplant | 18.3 a | 144 b | 6.23 a | |
| Cultivar | ||||
| GGI | 17.5 a | 241 ab | 6.76 a | |
| ROM | 15.1 b | 284 a | 6.21 a | |
| IS | 15.5 ab | 175 b | 6.52 a | |
| ANOVA | ||||
| P | *** | *** | NS | |
| C | * | * | NS | |
| P×C | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leskovar, D.I.; Othman, Y.A. Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke. Plants 2021, 10, 899. https://doi.org/10.3390/plants10050899
Leskovar DI, Othman YA. Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke. Plants. 2021; 10(5):899. https://doi.org/10.3390/plants10050899
Chicago/Turabian StyleLeskovar, Daniel I., and Yahia A. Othman. 2021. "Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke" Plants 10, no. 5: 899. https://doi.org/10.3390/plants10050899
APA StyleLeskovar, D. I., & Othman, Y. A. (2021). Direct Seeding and Transplanting Influence Root Dynamics, Morpho-Physiology, Yield, and Head Quality of Globe Artichoke. Plants, 10(5), 899. https://doi.org/10.3390/plants10050899






