Phytostabilization of Phosphate Mine Wastes Used as a Store-and-Release Cover to Control Acid Mine Drainage in a Semiarid Climate
Abstract
1. Introduction
2. Results and Discussion
2.1. Metal Accumulation in Phosphate Wastes
2.2. Screening of Native Plant Species in the Phosphate Wastes
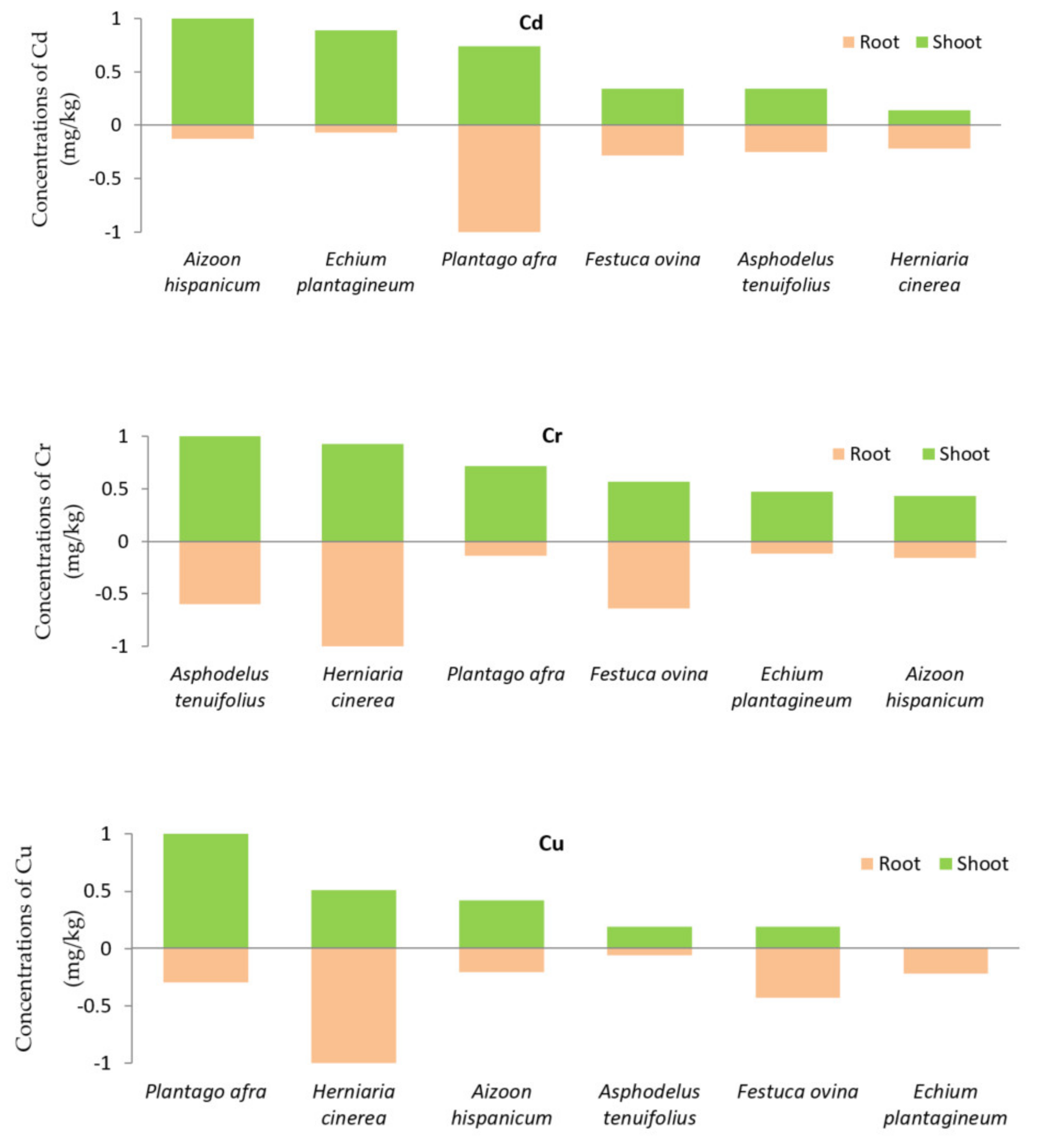
2.3. Heavy Metal Concentrations in Plant Tissues
2.4. Translocation Factor and Bioaccumulation Factor
2.5. Plant Species Candidate for Phytostabilization
3. Materials and Methods
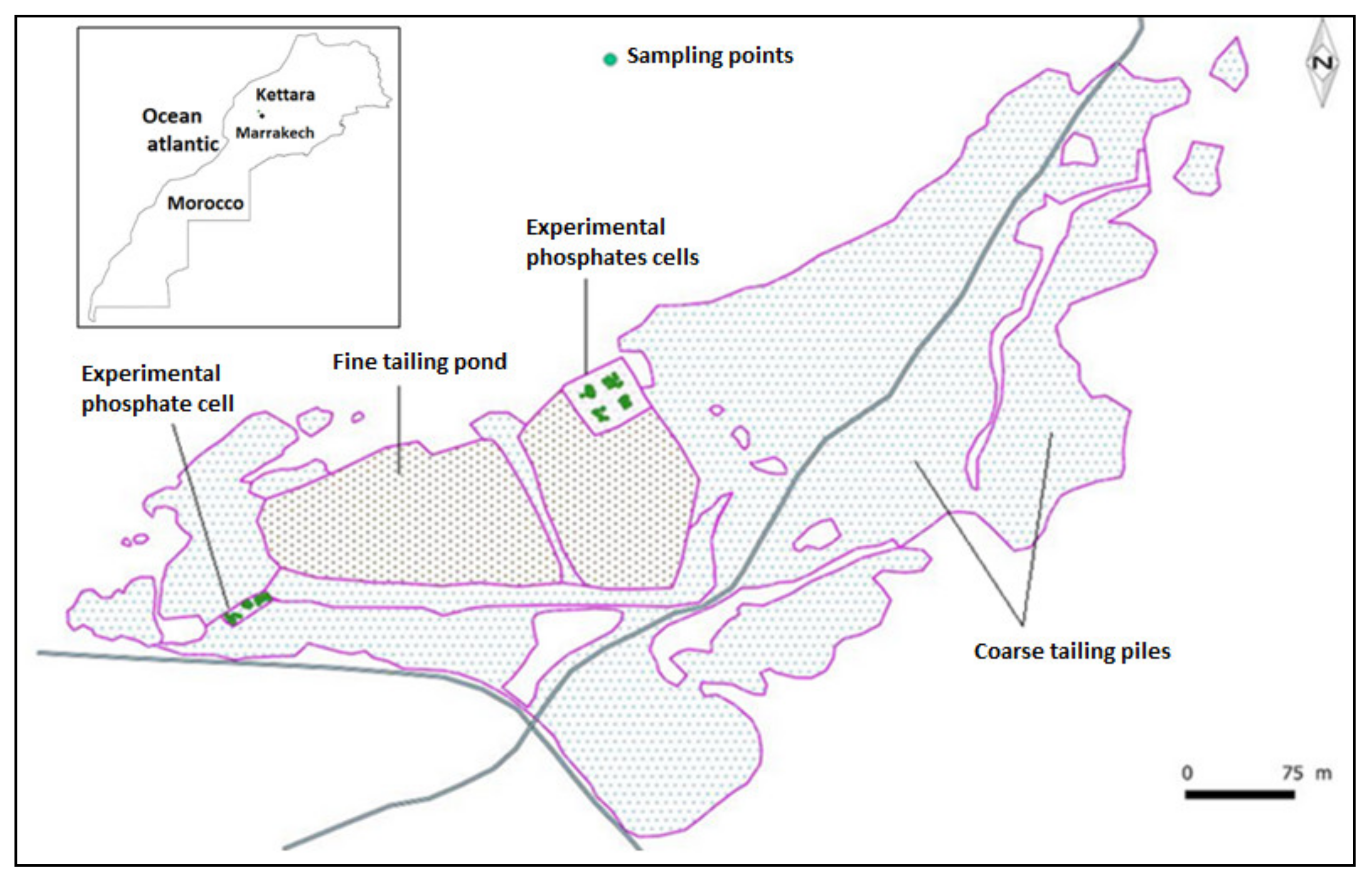
3.1. Study Area
3.2. Collection of Plant and Rhizospheric Substrata Samples
3.3. Plant and Soil Samples Analysis
3.4. Soil Pollution Index, Bioconcentration and Translocation Factors
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sonter, L.J.; Ali, S.H.; Watson, J.E. Mining and biodiversity: Key issues and research needs in conservation science. R. Soc. B 2018, 285, 20181926. [Google Scholar] [CrossRef]
- More, T.G.; Rajput, R.A.; Bandela, N.N. Impact of Heavy metals on DNA Contents in the whole body of fresh water Bivalve, Lamellidens marginalis. Pollut. Res. 2003, 22, 605–611. [Google Scholar]
- Wasi, S.; Tabrez, S.; Ahmad, M. Toxicological effects of major environmental pollutants: An overview. Environ. Monit. Assess. 2012, 185, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): 1. Environmental Characterization. Mine Water Environ. 2008, 27, 145–159. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Acid Mine Drainage at the Abandoned Kettara Mine (Morocco): 2. Mine Waste Geochemical Behavior. Mine Water Environ. 2008, 27, 160–170. [Google Scholar] [CrossRef]
- Khalil, A.; Hanich, L.; Bannari, A.; Zouhri, L.; Pourret, O.; Hakkou, R. Assessment of soil contamination around an abandoned mine in a semi-arid environment using geochemistry and geostatistics: Pre-work of geochemical process modeling with numerical models. J. Geochem. Explor. 2013, 125, 117–129. [Google Scholar] [CrossRef]
- Lghoul, M.; Kchikach, A.; Hakkou, R.; Zouhri, L.; Guerin, R.; Bendjoudi, H.; Teíxido, T.; Penã, J.A.; Enriqué, L.; Jaffal, M.; et al. Etude géophysique et hydrogéologique du site minier abandonné de Kettara (région de Marrakech, Maroc): Contribution au projet de réhabilitation. Hydrol. Sci. J. 2012, 57, 370–381. [Google Scholar] [CrossRef]
- Bossé, B.; Bussière, B.; Hakkou, R.; Maqsoud, A.; Benzaazoua, M. Assessment of Phosphate Limestone Wastes as a Component of a Store-and-Release Cover in a Semiarid Climate. Mine Water Environ. 2013, 32, 152–167. [Google Scholar] [CrossRef]
- Bossé, B.; Bussière, B.; Hakkou, R.; Maqsoud, A.; Benzaazoua, M. Field experimental cells to assess hydrogeological behaviour of store-and-release covers made with phosphate mine waste. Can. Geotech. J. 2015, 52, 1255–1269. [Google Scholar] [CrossRef]
- Bossé, B.; Bussière, B.; Maqsoud, A.; Hakkou, R.; Benzaazoua, M. Hydrogeological behavior of a store-and-release cover: A comparison between field column tests and numerical predictions with or without hysteresis effects. Mine Water Environ. 2016, 35, 221–234. [Google Scholar] [CrossRef]
- Knidiri, J.; Bussière, B.; Hakkou, R.; Bossé, B.; Maqsoud, A.; Benzaazoua, M. Hydrogeological behaviour of an inclined store-and-release cover experimental cell made with phosphate mine wastes. Can. Geotech. J. 2017, 54, 102–116. [Google Scholar] [CrossRef]
- Cooke, J.A.; Johnson, M.S. Ecological restoration of land with particular reference to the mining of metals and industrial minerals: A review of theory and practice. Environ. Rev. 2002, 10, 41–71. [Google Scholar] [CrossRef]
- Zine, H.; El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Smouni, A.; Fahr, M.; Bouab, N.; El Faiz, A.; Ouhammou, A. Screening for native plant species potential revegetation of phosphatic clay applied as a cover to abandoned Kettara mine tailings Marrakech, Morocco. SMETox J. 2018, 1, 83–88. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Galfati, I.; Bilal, E.; Sassi, A.B.; Abdallah, H.; Zaïer, A. Accumulation of heavy metals in native plants growing near the phosphate treatment industry, Tunisia. Carpathian J. Earth Environ. Sci. 2011, 6, 85–100. [Google Scholar]
- Li, M.S.; Luo, Y.P.; Su, Z.Y. Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ. Pollut. 2007, 147, 168–175. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B. Laboratory Evaluation of the Use of Alkaline Phosphate Wastes for the Control of Acidic Mine Drainage. Mine Water Environ. 2009, 28, 206–218. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M.; Ouyahya, A.; El Oulaidi, J. Flore Pratique du Maroc; Série Botanique; Travaux Institut Scientifique: Rabat, Morocco, 1999; Volume 2. [Google Scholar]
- Fennane, M.; Ibn Tattou, M.; El Oualidi, J. Flore Pratique du Maroc, Manuel de Détermination des Plantes Vasculaires; Série Botanique; Travaux de L’institut Scientifique: Rabat, Morocco, 2014; Volume 3, p. 793. [Google Scholar]
- Yang, S.; Liang, S.; Yi, L.; Xu, B.; Cao, J.; Guo, Y.; Zhou, Y. Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front. Environ. Sci. Eng. 2014, 8, 394–404. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Kuczyńska, A.; Krajewski, P.; Sawikowska, A.; Surma, M.; Ogrodowicz, P.; Adamski, T.; Krystkowiak, K.; Górny, A.G.; Kempa, M.; et al. Quantitative trait loci for plant height in Maresi × CamB barley population and their associations with yield-related traits under different water regimes. J. Appl. Genet. 2017, 58, 23–35. [Google Scholar] [CrossRef]
- Krämer, U. Metal Hyperaccumulation in Plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Chaney, R.L. Toxic element accumulation in soils and crops: Protecting soil fertility and agricultural food-chains. In Inorganic Contaminants in the Vadose Zone; Springer: Berlin/Heidelberg, Germany, 1989; pp. 140–158. [Google Scholar]
- Shanker, A.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.; Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram ((L.) R.Wilczek. cv CO 4) roots. Plant Sci. 2004, 166, 1035–1043. [Google Scholar] [CrossRef]
- Bollard, E.G. Involvement of unusual elements in plant growth and nutrition. Encycl. Plant Physiol. New Ser. 1983, 15B, 695–744. [Google Scholar]
- Małecka, A.; Derba-Maceluch, M.; Kaczorowska, K.; Piechalak, A.; Tomaszewska, B. Reactive oxygen species production and antioxidative defense system in pea root tissues treated with lead ions: Mitochondrial and peroxisomal level. Acta Physiol. Plant 2009, 31, 1065–1075. [Google Scholar] [CrossRef]
- Påhlsson, A.-M.B. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water Air Soil Pollut. 1989, 47, 287–319. [Google Scholar] [CrossRef]
- Castagna, A.; Di Baccio, D.; Ranieri, A.M.; Sebastiani, L.; Tognetti, R. Effects of combined ozone and cadmium stresses on leaf traits in two poplar clones. Environ. Sci. Pollut. Res. 2014, 22, 2064–2075. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Rodriguez, E.; da Conceição Santos, M.; Azevedo, R.; Correia, C.; Moutinho-Pereira, J.; de Oliveira, J.M.; Dias, M.C. Photosynthesis light-independent reactions are sensitive biomarkers to monitor lead phytotoxicity in a Pb-tolerant Pisumsativum cultivar. Environ. Sci. Pollut. Res. 2015, 22, 574–585. [Google Scholar] [CrossRef]
- NCR (National Research Council). Mineral Tolerance of Animals; National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Baker, A.J.M. Accumulators and excluders-strategies in the response of plants to heavy metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, Q.; Wang, X. Identification of weed plants excluding the uptake of heavy metals. Environ. Int. 2005, 31, 829–834. [Google Scholar] [CrossRef]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Heavy metals in native mediterranean grassland species growing at abandoned mine sites: Ecotoxicological assessment and phytoremediation of polluted soils. In Heavy Metal Contamination of Soils; Springer: Cham, Switzerland, 2015; pp. 159–178. [Google Scholar]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Alvarenga, P.; Gonçalves, A.; Fernandes, R.; De Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A. Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci. Total Environ. 2008, 406, 43–56. [Google Scholar] [CrossRef]
- Tordoff, G.; Baker, A.; Willis, A. Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere 2000, 41, 219–228. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytoremediation of mine tailings in temperate and arid environments. Rev. Environ. Sci. Bio/Technol. 2007, 7, 47–59. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization Potential of Jatropha Curcas L. in Polymetallic Acid Mine Tailings. Int. J. Phytoremed. 2011, 13, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- SRM (Service Régional des Mines de Marrakech). Le Gisement de Sulfures Massifs de Kettara; SRM: Marrakech, Morocco, 1983. [Google Scholar]
- ONEM (Observatoire Nationale de l’Environnement du Maroc). Monographie locale de l’environnement de la ville de Marrakech. In Etude Réalisée Pour le Compte de la Wilaya de Marrakech; ONEM: Marrakech, Morocco, 1997. [Google Scholar]
- Marguí, E.; Queralt, I.; Carvalho, M.; Hidalgo, M. Comparison of EDXRF and ICP-OES after microwave digestion for element determination in plant specimens from an abandoned mining area. Anal. Chim. Acta 2005, 549, 197–204. [Google Scholar] [CrossRef]
- Temminghoff, E.J.; Hoba, V.J. Digestion with HNO3-H2O2-HF. In Plant Analysis Procedures, 2nd ed.; Kluwer: Dordrecht, The Netherlands, 2004; pp. 16–19. [Google Scholar]
- Moreno, J.L.; Garcia, C.; Hernandez, T.; Pascual, J.A. Transference of heavy metals from a calcareous soil amended with sewage-sludge compost to barley plants. Bioresour. Technol. 1996, 55, 251–258. [Google Scholar] [CrossRef]
- Emoyan, O.; Ogban, F.; Akarah, E. Evaluation of Heavy Metals Loading of River Ijana in Ekpan–Warri, Nigeria. J. Appl. Sci. Environ. Manag. 2009, 10. [Google Scholar] [CrossRef]
- Nishida, H.; Miyai, M.; Tada, F.; Suzuki, S. Computation of the index of pollution caused by heavy metals in river sediment. Environ. Pollut. Ser. B Chem. Phys. 1982, 4, 241–248. [Google Scholar] [CrossRef]
- Mishra, T.; Pandey, V.C. Phytoremediation of red mud deposits through natural succession. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–424. [Google Scholar]

| Parameters | As | Cd | Co | Cr | Cu | Ni | Pb | Zn | PIs | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mean | 10.63 ± 1.91 | 10.67 ± 4.03 | 0.72 ± 0.16 | 138.04 ± 47.72 | 119.86 ± 59.89 | 25.07 ± 9.35 | 27.28 ± 15.42 | 104.70 ± 14.34 | 0.94 | 7.53 |
| Critical values a | 20.00 | 3.00 | 50.00 | 100.00 | 100.00 | 100.00 | 100.00 | 300.00 | |||
| Mobilizable EDTA | Mean | 0.11 ± 0.06 | 0.22 ± 0.07 | < 0.05 | - | 2.35 ± 0.64 | - | 0.24 ± 0.13 | 2.57 ± 1.05 | ||
| Mean percent | 0.011 ± 0.006 | 0.02 ± 0.01 | < 0.07 | - | 0.02 ± 0.01 | - | 0.011 ± 0.07 | 0.03 ± 0.01 | |||
| Mobile CaCl2 | Mean | < 0.05 | < 0.02 | < 0.05 | - | < 0.02 | - | < 0.06 | < 0.01 | ||
| Mean percent | < 0.005 | < 0.002 | < 0.07 | - | < 0.002 | - | < 0.003 | < 0.0001 | |||
| Family | Species No | Species | Life-Form | Shoot System Size (cm) | Root System Size (cm) |
|---|---|---|---|---|---|
| Aizoaceae | 1 | Aizoon hispanicum | Annual | 9.67 ± 4.04 a | 5.83 ± 2.25 a |
| Asphodelaceae | 2 | Asphodelus tenuifolius | Annual/perennial | 20.00 ± 5.00 a | 9.17 ± 2.47 a |
| Asteraceae | 3 | Chrysanthemum coronarium | Annual | 36.67 ± 15.28 ab | 14.83 ± 3.25 a |
| 4 | Reichardia tingitana | Annual | 27.33 ± 6.43 ab | 8.33 ± 3.21 a | |
| 5 | Scolymus hispanicus | Biennial/perennial | 12.50 ± 1.32 ab | 9.67 ± 1.53 a | |
| Boraginaceae | 6 | Echium plantagineum | Annual | 24.67 ± 17.79 ab | 14.33 ± 3.79 a |
| Polygonaceae | 7 | Emex spinosus | Annual | 22.50 ± 5.22 ab | 7.33 ± 2.08 a |
| Apiaceae | 8 | Eryngium ilicifolium | Annual | 4.33 ± 0.58 a | 10.43 ± 0.93 a |
| Brassicaceae | 9 | Hirschfeldia incana | Annual | 69.67 ± 21.50 b | 19 ± 11.27 a |
| Poaceae | 10 | Festuca ovina | Annual | 25.67 ± 5.51 ab | 12.33 ± 4.93 a |
| Caryophyllaceae | 11 | Herniaria cinerea | Annual | 11 ± 12.17 a | 6.77 ± 4.61 a |
| Plantaginaceae | 12 | Plantago afra | Biennial/perennial | 13.67 ± 2.93 a | 6.33 ± 1.76 a |
| Plumbaginaceae | 13 | Spergularia rubra | Annual | 14.50 ± 5.07 a | 5.33 ± 3.33 a |
| Species | As | Cd | Co | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Aizoon hispanicum | 2.03 ± 1.22 a | 3.65 ± 1.43 a | 0.96 ± 0.54 a | 8.12 ± 2.65 ab | 23.77 ± 7.03 abc | 3.64 ± 1.56 ab | 22.55 ± 3.02 b | 162.86 ± 64.97 bc |
| Asphodelus tenuifolius | 1.30 ± 0.07 a | 7.10 ± 3.20 a | 1.30 ± 0.07 a | 30.01 ± 17.88 ab | 6.53 ± 3.81 a | 9.48 ± 4.04 ab | 12.51 ± 2.56 ab | 50.90 ± 12.98 a |
| Chrysanthemum coronarium | 2.37 ± 0.68 a | 5.36 ± 2.93 a | 0.69 ± 0.18 a | 46.95 ± 15.29 c | 9.94 ± 4.11 ab | 3.86 ± 0.66 ab | 14.22 ± 1.55 ab | 60.59 ± 7.71 a |
| Echium plantagineum | 1.85 ± 1.55 a | nd | 1.3 ± 0.74 a | 6.21 ± 1.42 ab | 24.28 ± 8.34 abc | 5.21 ± 0.21 ab | nd | 43.91 ± 25.7 a |
| Emex spinosa | 1 ± 0.44 a | 6.95 ± 1.78 a | 0.52 ± 0.02 a | 4.37 ± 0.28 ab | 30.63 ± 9 abc | 3.98 ± 1.59 ab | 2.83 ± 0.75 a | 70.62 ± 5.82 ab |
| Eryngium ilicifolium | 2.80 ± 0.99 a | 2.04 ± 1.61 a | 0.56 ± 0.05 a | 6.22 ± 1.47 ab | 4.34 ± 2.30 a | 3.89 ± 1.54 ab | 3.19 ± 0.58 a | 54.55 ± 22.25 a |
| Festuca ovina | 2.02 ± 0.61 a | 8.17 ± 3.41 a | 2.02 ± 0.61 a | 31.67 ± 10.93 c | 48.23 ± 24.63 c | 15.11 ± 6.54 c | 5.40 ± 0.25 a | 74.32 ± 10.77 ab |
| Herniaria cinerea | 15.98 ± 5.74 b | 6.18 ± 2.14 a | <0.91 b | 49.77 ± 27.13 c | 111.22 ± 34.36 d | 45.93 ± 5.10 d | 55.96 ± 19.92 c | 127.92 ± 7.47 abc |
| Hirschfeldia incana | <0.69 a | 4.59 ± 1.95 a | <0.54 a | 3.08 ± 0.56 a | 5.1 ± 1.27 a | 3.14 ± 2.01 a | 0.88 ± 0.49 a | 44.19 ± 9.52 a |
| Plantago afra | 1.94 ± 0.16 a | 28.72 ± 19.15 b | <2.25 a | 7 ± 1.67 ab | 32.97 ± 17.66 abc | 10.89 ± 4.55 bc | 5.33 ± 2.28 a | 182.88 ± 60.18 c |
| Reichardia tingitana | 0.84 ± 0.29 a | nd | <0.63 a | 4.62 ± 1.08 ab | nd | 3.95 ± 0.32 ab | 0.85 ± 0.21 a | 120.97 ± 82.59 abc |
| Scolymus hispanicus | 0.73 ± 0.3 a | 4.73 ± 1.4 a | <0.51 a | 6.02 ± 3.78 ab | 27.91 ± 12.08 abc | 2.27 ± 0.01 a | 2.57 ± 0.63 a | 64.9 ± 31.5 ab |
| Spergularia rubra | 1.21 ± 0.54 a | 6.01 ± 0.35 a | <0.7 a | 7.87 ± 4.54 ab | 9.59 ± 5.36 ab | 5.53 ± 2.45 ab | <0.80 a | 151.50 ± 89.51 abc |
| Toxicity levels 1 | <2–80 | 6–10 | 0.4–several | 0.2–1 | 20–30 | 10–50 | 0.6–28 | 100–300 |
| The critical hyperaccumulating levels 2 | >1000 | >100 | >1000 | >1000 | >1000 | >1000 | >1000 | >10,000 |
| Species | As | Cd | Co | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| Aizoon hispanicum | 1.58 ± 0.74 ab | 7.59 ± 3.62 a | <0.51 a | 6.34 ± 3.46 abc | 25.68 ± 4.52 a | 3.24 ± 0.33 ab | 12.89 ± 3.94 abc | 83.57 ± 12.82 ab |
| Asphodelus tenuifolius | 0.64 ± 0.1 a | 2.61 ± 0.07 a | <0.57 a | 14.70 ± 6.74 c | 11.45 ± 8.02 a | 6.69 ± 1.52 ab | 4.72 ±0.32 a | 22.73 ± 5.79 ab |
| Chrysanthemumcoronarium | <0.54 a | 3.40 ± 1.15 a | <0.52 a | 4.19 ± 1.90 a | 10.83 ± 1.47 a | 3.97 ± 1.01 bc | 4.91 ± 2.02 a | 50.20 ± 8.01 c |
| Echium plantagineum | 2.52 ± 0.91 b | 6.75 ± 1.85 a | <0.68 a | 6.94 ± 3.89 abc | 0.24 ± 0.07 a | 4.09 ± 1.81 a | 17.71 ± 4.74 bcd | 93.42 ± 25.63 ab |
| Emexspinosa | <0.56 a | 8.75 ± 2.80 a | <0.53 a | 3.05 ± 0.90 a | 9.29 ± 3.87 a | 2.19 ± 0.53 ab | 1.45 ± 0.41 abc | 44.95 ± 9.02 ab |
| Eryngium ilicifolium | 1.39 ± 0.62 ab | 5.11 ± 3.71 a | <0.54 a | 9 ± 1.68 abc | 9.74 ± 0.06 a | 4.41 ± 0.23 a | 1.62 ± 0.73 a | 33.21 ± 13.19 ab |
| Festuca ovina | <0.55 a | 2.56 ± 0.89 a | <0.58 a | 8.33 ± 3.66 abc | 11.30 ± 5.12 a | 3.92 ± 0.14 ab | 4.79 ± 2.88 cd | 97.31 ± 50.53 ab |
| Herniaria cinerea | 1.58 ± 0.54 ab | nd | <0.52 a | 13.74 ± 7.63 bc | 30.62 ± 13.54 a | 4.29 ± 1.67 a | 13.90 ± 7.49 cd | 99.84 ± 27.58 ab |
| Hirschfeldia incana | 1.27 ± 0.94 ab | 2.44 ± 0.69 a | <0.57 a | 9.54 ± 2.67 abc | <0.23 a | 2.72 ± 1.1 ab | 11.68 ± 6.2 abc | 100.4 ± 47.11 ab |
| Plantago afra | 2.49 ± 1.39 b | 5.61 ± 3.52 a | <0.54 a | 10.53 ± 0.63 abc | 60.56 ± 32.752 b | 4.24 ± 1.27 a | 7.27 ± 0.99 abc | 81.14 ± 13.46 ab |
| Reichardia tingitana | <0.55 a | nd | <0.55 a | 4.84 ± 1.39 a | 31.24 ± 20.34 a | 4.24 ± 0.64 ab | 3.01 ±2.05 d | 147.13 ± 107.06 ab |
| Scolymus hispanicus | 1.20 ± 0.98 ab | 5.20 ± 0.11 a | <0.51 a | 5.31 ± 3.26 ab | nd | 1.98 ± 0.30 ab | nd | 82.91 ± 7.9 bc |
| Spergularia rubra | 2.34 ± 1.21 ab | 7.23 ± 1.71 a | 0.52 ± 0.01 a | 8.80 ± 0.19 abc | 16.43 ± 6.14 a | 6.21 ± 0.94 c | 2.22 ± 1.26 abc | 211.97 ± 90.41 ab |
| Toxicity levels 1 | <2–80 | 6–10 | 0.4–several | 0.2–1 | 20–30 | 10–50 | 0.6–28 | 100–300 |
| The critical hyperaccumulating levels 2 | >1000 | >100 | >1000 | >1000 | >1000 | >1000 | >1000 | >10,000 |
| Species | BCF | TF | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Co | Cr | Cu | Ni | Pb | Zn | As | Cd | Co | Cr | Cu | Ni | Pb | Zn | |
| Aizoon hispanicum | 0.19 a | 0.34 a | 1.34 a | 0.06 ab | 0.20 abc | 0.15 ab | 0.83 b | 1.56 bc | 0.80 a | 2.36 a | 0.64 cdef | 0.74 ab | 1.11 ab | 0.95 bcd | 0.56 a | 0.56 a |
| Asphodelus tenuifolius | 0.12 a | 0.67 a | 1.81 a | 0.22 abc | 0.05 ab | 0.38 abc | 0.46 ab | 0.49 a | 0.49 a | 0.42 a | 0.43 bcd | 0.60 ab | 1.88 ab | 0.77 abcd | 0.39 a | 0.45 a |
| Chrysanthemum coronarium | 0.22 a | 0.66 a | 0.96 a | 0.34 c | 0.08 abc | 0.15 ab | 0.52 ab | 0.58 ab | 0.24 a | 0.54 a | 0.78 defg | 0.09 a | 1.23 ab | 1.07 bcd | 0.34 a | 0.83 a |
| Echium plantagineum | 0.17 a | 0.12 a | 1.81 a | 0.04 ab | 0.20 abc | 0.21 ab | 0.07 a | 0.42 a | 2.06 a | 14.07 a | 0.55 bcde | 1.09 ab | 0.01 a | 0.78 abcd | 14.07 b | 2.28 b |
| Emex spinosa | 0.09 a | 0.65 a | 0.72 a | 0.03 ab | 0.26 abc | 0.16 ab | 0.10 a | 0.67 ab | 0.65 a | 1.26 a | 1.02 g | 0.70 ab | 0.35 ab | 0.59 abcd | 0.56 a | 0.65 a |
| Eryngium ilicifolium | 0.26 a | 0.19 a | 0.78 a | 0.05 ab | 0.04 a | 0.16 ab | 0.12 a | 0.52 a | 0.49 a | 6.60 ab | 0.96 fg | 1.49 b | 2.67 b | 1.26 d | 0.49 a | 0.62 a |
| Festuca ovina | 0.19 a | 0.77 a | 2.80 a | 0.23 bc | 0.40 c | 0.60 c | 0.20 a | 0.71 ab | 0.28 a | 0.34 a | 0.30 abc | 0.28 ab | 0.26 ab | 0.30 ab | 0.88 a | 1.29 ab |
| Herniaria cinerea | 1.50 b | 0.58 a | 16.91 b | 0.36 c | 0.93 d | 1.83 d | 2.05 c | 1.22 abc | 0.11 a | 0.21 a | 0.05 a | 0.28 ab | 0.30 ab | 0.09 a | 0.24 a | 0.78 a |
| Hirschfeldia incana | 0.06 a | 0.43 a | 0.74 a | 0.02 a | 0.04 ab | 0.13 ab | 0.03 a | 0.42 a | 2.08 a | 0.56 a | 1.08 g | 3.21 c | 0.05 a | 0.96 bcd | 16.99 b | 2.40 b |
| Plantago afra | 0.18 a | 2.69 b | 3.13 a | 0.05 ab | 0.28 abc | 0.43 bc | 0.20 a | 1.75 c | 1.33 a | 0.31 a | 0.25 ab | 1.57 b | 2.63 b | 0.41 abc | 1.42 a | 0.47 a |
| Reichardia tingitana | 0.08 a | 0.81 a | 0.87 a | 0.03 ab | 0.38 bc | 0.16 ab | 0.03 a | 1.16 abc | 0.67 a | 0.84 a | 0.88 efg | 1.04 ab | 0.76 ab | 1.07 bcd | 3.83 a | 1.20 ab |
| Scolymus hispanicus | 0.07 a | 0.44 a | 0.71 a | 0.04 ab | 0.23 abc | 0.09 a | 0.09 a | 0.62 ab | 1.95 a | 1.13 a | 1.00 fg | 0.88 ab | 0.44 ab | 0.87 bcd | 1.86 a | 1.41 ab |
| Spergularia rubra | 0.11 a | 0.57 a | 0.71 a | 0.06 ab | 0.08 abc | 0.22 ab | 0.03 a | 1.45 abc | 0.80 a | 2.36 a | 0.64 cdef | 0.74 ab | 1.11 ab | 0.95 bcd | 0.56 a | 0.56 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Phytostabilization of Phosphate Mine Wastes Used as a Store-and-Release Cover to Control Acid Mine Drainage in a Semiarid Climate. Plants 2021, 10, 900. https://doi.org/10.3390/plants10050900
El Berkaoui M, El Adnani M, Hakkou R, Ouhammou A, Bendaou N, Smouni A. Phytostabilization of Phosphate Mine Wastes Used as a Store-and-Release Cover to Control Acid Mine Drainage in a Semiarid Climate. Plants. 2021; 10(5):900. https://doi.org/10.3390/plants10050900
Chicago/Turabian StyleEl Berkaoui, Meryem, Mariam El Adnani, Rachid Hakkou, Ahmed Ouhammou, Najib Bendaou, and Abdelaziz Smouni. 2021. "Phytostabilization of Phosphate Mine Wastes Used as a Store-and-Release Cover to Control Acid Mine Drainage in a Semiarid Climate" Plants 10, no. 5: 900. https://doi.org/10.3390/plants10050900
APA StyleEl Berkaoui, M., El Adnani, M., Hakkou, R., Ouhammou, A., Bendaou, N., & Smouni, A. (2021). Phytostabilization of Phosphate Mine Wastes Used as a Store-and-Release Cover to Control Acid Mine Drainage in a Semiarid Climate. Plants, 10(5), 900. https://doi.org/10.3390/plants10050900








