Biological Properties of Essential Oils from Thymus algeriensis Boiss
Abstract
1. Introduction
- − Antioxidant test using two well-known reagents or assays DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid);
- − Antitumor test using two kinds of human cancer cells colon and hepatocellular carcinoma cells;
- − A microbiol test using several strains (bacteria, yeast, and fungi).
2. Results and Discussion
2.1. Chemical Composition
2.2. The Biological Assessment
2.2.1. DPPH and ABTS Radical Scavenging Activity test
2.2.2. Antimicrobial Susceptibility Assay
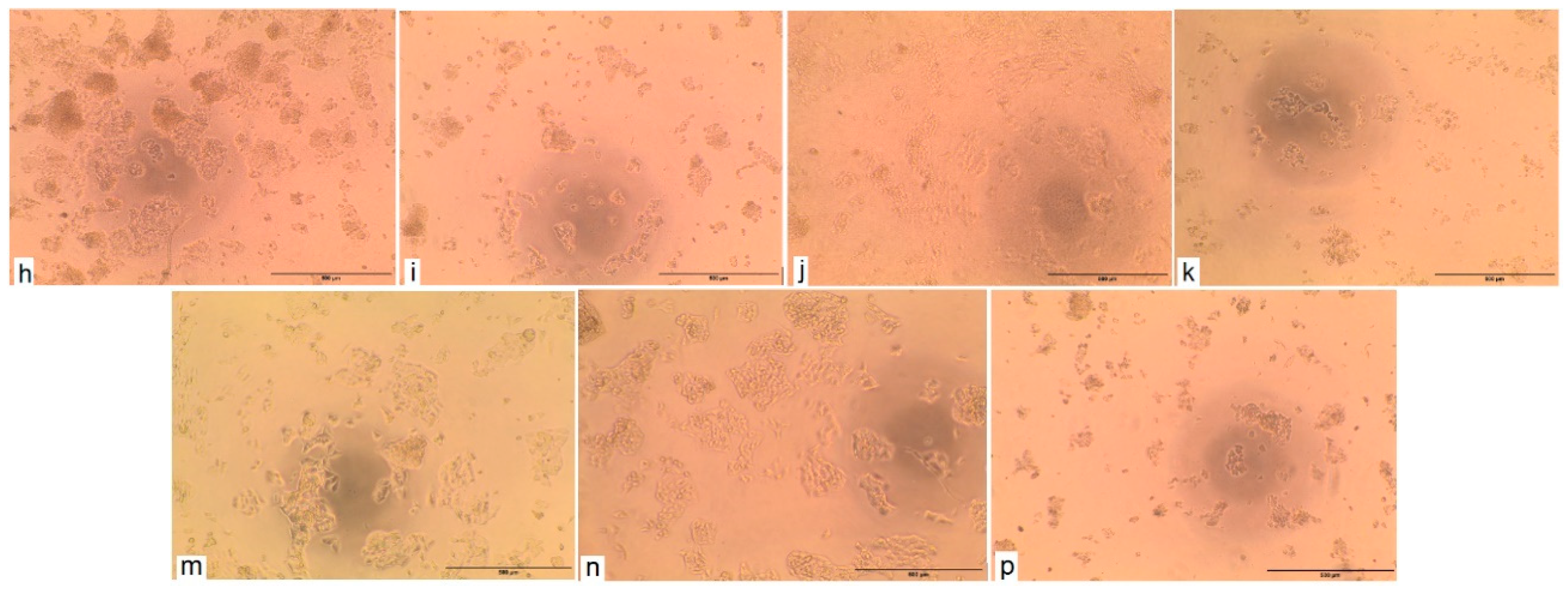
2.2.3. Cytotoxic Activity Test
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Extraction of the Essential oil (EOs)
3.3.1. GC-FID Analysis
3.3.2. GC-MS Analysis
3.3.3. Identification of Chemical Compounds
3.4. Biological Evaluation
3.4.1. Antioxidant Activity
DPPH (1,1-Diphenyl-2-Picrylhydrazyl) Assay
- − I%: Inhibition percentage (%).
- − Abs sample: Absorbance of methanolic-DPPH solution containing extracts after 30 min of reaction time.
- − Abs control: Absorbance of methanolic-DPPH solution without extracts.
ABTS (2,2′-Azino-Bis (3-Ethylbenzothiazoline-6-Sulphonic Acid)) Test
3.4.2. Antimicrobial Susceptibility Assay
3.4.3. Cytotoxicity Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Mohammedi, H.; Mecherara-Idjeri, S.; Foudil-Cherif, Y.; Hassani, A. Chemical Composition and Antioxidant Activity of Essential Oils from Algerian Daucus Carota L. subsp. carota Aerial Parts. J. Essent. Oil Bear. Plants 2015, 18, 873–883. [Google Scholar] [CrossRef]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical and Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Boutemtam, L.; Boukhatem, M.N.; Messaoudi, M.; Begaa, S.; Benarfa, A.; Ferhat, M.A. Understanding the phenomena of extraction of essential oils by the microwave accelerated distillation process: Case of the Washington Navel variety. Eur. J. Biol. Res. 2020, 10, 167–181. [Google Scholar]
- Ouakouak, H.; Benchikha, N.; Hassani, A.; Ashour, M.L. Chemical composition and biological activity of Mentha citrata Ehrh., essential oils growing in southern Algeria. J. Food Sci. Technol. 2019, 56, 5346–5353. [Google Scholar] [CrossRef]
- Benarfa, A.; Gourine, N.; Harrat, M.; Yousfi, M. Composition and biovariability of Deverra scoparia volatile oil and its potential use as a source of bioactive phthalide components. Biochem. Syst. Ecol. 2020, 90, 104019. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Benarfa, A.; Gourine, N.; Mahfoudi, R.; Harrat, M.; Yousfi, M. Effect of seasonal and regional variations on Phenolic compounds of Deverra Scoparia (flowers/seeds) methanolic extract and the evaluation of its in-vitro antioxidant activity. Chem. Biodivers. 2019, 16, e1900420. [Google Scholar] [CrossRef]
- Dellai, A.; Souissi, H.; Borgi, W.; Bouraoui, A.; Chouchane, N. Antiinflammatory and antiulcerogenic activities of Pistacia lentiscus L. leaves extracts. Ind. Crops Prod. 2013, 49, 879–882. [Google Scholar] [CrossRef]
- Bertella, A.; Benlahcen, K.; Abouamama, S.; Pinto, D.C.G.A.; Maamar, K.; Kihal, M.; Silva, A.M.S. Artemisia herba-alba Asso. essential oil antibacterial activity and acute toxicity. Ind. Crops Prod. 2018, 116, 137–143. [Google Scholar] [CrossRef]
- Begaa, S.; Messaoudi, M. Toxicological Aspect of Some Selected Medicinal Plant Samples Collected from Djelfa, Algeria Region. Biol. Trace Elem. Res. 2019, 187, 301–306. [Google Scholar] [CrossRef]
- Chelalba, I.; Benchikha, N.; Begaa, S.; Messaoudi, M.; Debbeche, H.; Rebiai, A.; Youssef, F.S. Phytochemical composition and biological activity of Neurada procumbens L. growing in southern Algeria. J. Food Process. Preserv. 2020, 44, e14774. [Google Scholar] [CrossRef]
- Hamlat, N.; Benarfa, A.; Beladel, B.; Begaa, S.; Messaoudi, M.; Hassani, A. Assessment of the contents of essential and potentially toxic elements in Pistacia terebinthus L. and Pistacia lentiscus L. by INAA technique. J. Radioanal. Nucl. Chem. 2019, 322, 1127–1131. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Benabdelkader, T.; Chelghoum, C. Studies on the essential oil composition and antimicrobial activity of Thymus algeriensis Boiss. et Reut. Int. J. Aromather. 2006, 16, 95–100. [Google Scholar] [CrossRef]
- de Mesquita, L.S.S.; Luz, T.R.S.A.; de Mesquita, J.W.C.; Coutinho, D.F.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Exploring the anticancer properties of essential oils from family Lamiaceae. Food Rev. Int. 2019, 35, 105–131. [Google Scholar] [CrossRef]
- Guesmi, F.; Ali, M.B.; Barkaoui, T.; Tahri, W.; Mejri, M.; Ben-Attia, M.; Bellamine, H.; Landoulsi, A. Effects of Thymus hirtus sp. algeriensis Boiss. et Reut.(Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids Health Dis. 2014, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calhelha, R.C.; Fernandes, Â.; Marković, T.; Marković, D.; Giweli, A.; Soković, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod. 2014, 52, 183–190. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar]
- Tran, T.A.; Ho, M.T.; Song, Y.W.; Cho, M.; Cho, S.K. Camphor induces proliferative and anti-senescence activities in human primary dermal fibroblasts and inhibits UV-induced wrinkle formation in mouse skin. Phyther. Res. 2015, 29, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, X.; Hu, K. Physically open BBB. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–217. [Google Scholar]
- Mokhtari, M.; Chabani, S.; Mouffouk, S.; Aberkane, M.-C.; Dibi, A.; Benkhaled, M.; Haba, H. Phytochemicals, Antihemolytic, Anti-inflammatory, Antioxidant, and Antibacterial Activities from Thymus Algeriensis. J. Herbs Spices Med. Plants 2021. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rasoul, M.A.A.; Abdelgaleil, S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar]
- Giacomino, A.; Abollino, O.; Malandrino, M.; Karthik, M.; Murugesan, V. Determination and assessment of the contents of essential and potentially toxic elements in Ayurvedic medicine formulations by inductively coupled plasma-optical emission spectrometry. Microchem. J. 2011, 99, 2–6. [Google Scholar] [CrossRef]
- Sas, I.I. SAS/STATÒ 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Ali, I.B.E.H.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. et Reut. Ind. Crops Prod. 2015, 77, 631–639. [Google Scholar]
- El Ajjouri, M.; Ghanmi, M.; Satrani, B.; Amarti, F.; Rahouti, M.; Aafi, A.; Ismaili, M.R.; Farah, A. Composition chimique et activité antifongique des huiles essentielles de Thymus algeriensis Boiss. & Reut. et Thymus ciliatus (Desf.) Benth. contre les champignons de pourriture du bois. Acta Bot. Gall. 2010, 157, 285–294. [Google Scholar]
- Hamdani, I.; Bouyanzer, A.; Hammouti, B.; Majidi, L.; Costa, J.; Paolini, J.; Chetouani, A. Chemical composition and antioxidant activity of essential oils of Thymus broussonetii Boiss. and Thymus algeriensis Boiss. from Morocco. Asian Pac. J. Trop. Dis. 2014, 4, 281–286. [Google Scholar]
- Zouari, N.; Fakhfakh, N.; Zouari, S.; Bougatef, A.; Karray, A.; Neffati, M.; Ayadi, M.A. Chemical composition, angiotensin I-converting enzyme inhibitory, antioxidant and antimicrobial activities of essential oil of Tunisian Thymus algeriensis Boiss. et Reut.(Lamiaceae). Food Bioprod. Process. 2011, 89, 257–265. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- de Vargas, F.S.; Almeida, P.D.O.; de Boleti, A.P.A.; Pereira, M.M.; de Souza, T.P.; de Vasconcellos, M.C.; Nunez, C.V.; Pohlit, A.M.; Lima, E.S. Antioxidant activity and peroxidase inhibition of Amazonian plants extracts traditionally used as anti-inflammatory. BMC Complement. Altern. Med. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Lue, B.-M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Rajurkar, N.S.; Hande, S.M. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146. [Google Scholar] [CrossRef] [PubMed]



| Thymus algeriensis | Aerial Parts Parts (Stems, Leaves, and Flowers) | Leaves | |||||||
|---|---|---|---|---|---|---|---|---|---|
| before Flowering | during Flowering | after Flowering | during the Flowering | ||||||
| N° | Compounds a | RI b | RI c | HD% | HD% | HD% | MAD% | HD% | SD% |
| 1 | Tricyclene | 921 | 918 | 0.475 | 0.617 | 0.866 | 0.343 | 0.52 | 0.27 |
| 2 | α-Thujene | 924 | 921 | 0.183 | 0.210 | 0.084 | 0.132 | 0.1 | 0.05 |
| 3 | α-Pinene | 932 | 928 | 4.562 | 5.007 | 2.808 | 3.027 | 3.74 | 1.88 |
| 4 | Camphene | 946 | 941 | 10.732 | 12.784 | 12.858 | 8.729 | 14.88 | 7.53 |
| 5 | Thuja-2,4(10)-diene | 953 | 950 | - | - | - | - | 0.09 | 0.08 |
| 6 | verbenene | 961 | 962 | 0.384 | - | 0.022 | 0.363 | - | - |
| 7 | Sabinene | 969 | 963 | - | - | 0.226 | 0.95 | 0.22 | 0.15 |
| 8 | β-Pinene | 974 | 965 | 1.118 | 1.218 | 0.73 | 0.752 | 0.83 | 0.48 |
| 9 | β-Myrcene (Myrcene) | 988 | 977 | 1.146 | 1.014 | 0.216 | 0.029 | 0.42 | 0.18 |
| 10 | α-Terpinene | 1014 | 1012 | 0.271 | - | 0.303 | 0.081 | 0.11 | 0.06 |
| 11 | p-Cymene (p-Cymol) | 1020 | 1017 | 1.177 | - | - | 0.153 | 0.15 | 0.15 |
| 12 | Limonene | 1024 | 1018 | - | - | - | 0.909 | 0.66 | 0.86 |
| 13 | 1,8-Cineole | 1026 | 1020 | 5.165 | 5.940 | 11.209 | 7.009 | 7.88 | 7.72 |
| 14 | (Z)-β-ocimene | 1032 | 1026 | 0.141 | 0.019 | 0.093 | - | - | |
| 15 | (E)-β-ocimene | 1044 | 1039 | 2.151 | 1.728 | 0.185 | 1.669 | 0.53 | 0.31 |
| 16 | γ-Terpinene | 1054 | 1051 | 0.151 | 0.111 | 0.256 | 0.149 | 0.19 | 0.09 |
| 17 | cis-Sabinene hydrate | 1065 | 1061 | 0.78 | 0.656 | 0.827 | 0.824 | 0.35 | 0.97 |
| 18 | cis-Linaloloxide (furanoid) | 1067 | 1068 | 0.061 | 0.134 | 0.107 | 0.048 | 0.06 | 0.05 |
| 19 | Camphenilone | 1078 | 1081 | 0.132 | 0.235 | 1.103 | 0.199 | 0.3 | 0.44 |
| 20 | α–Tterpinolene (Tterpinolene) | 1086 | 1086 | 0.566 | 0.205 | 0.259 | 0.253 | 0.17 | 0.12 |
| 21 | trans-Sabinene hydrate | 1098 | 1099 | 2.402 | 0.866 | 0.929 | 2.007 | 0.83 | 0.88 |
| 22 | Linalool | 1095 | 1105 | 0.193 | 1.030 | 0.18 | 0.033 | - | - |
| 23 | Isopropyl-5-methyl-(2E)-hexenal<2-> | 1104 | 1108 | - | - | - | - | 0.08 | 0.21 |
| 24 | Cis-p-menth-2-en-1-ol | 1118 | 1124 | 0.41 | 0.499 | 0.369 | |||
| 25 | α—Campholenal | 1122 | 1128 | 0.069 | 0.051 | 0.091 | 0.31 | 0.33 | |
| 26 | Mentha-2,8-dien-1-ol<cis-p-> | 1133 | 1138 | - | - | - | - | 0.05 | 0.13 |
| 27 | Nopinone | 1135 | 1140 | - | - | - | 0.086 | - | - |
| 28 | trans-Pinocarveol | 1135 | 1142 | - | - | 0.239 | 0.15 | 0.19 | |
| 29 | Camphor | 1141 | 1145 | 17.452 | 22.602 | 34.31 | 20.738 | 32.56 | 24.25 |
| 30 | Camphene hydrate | 1145 | 1146 | - | - | 0.091 | 0.046 | - | - |
| 31 | Borneol | 1165 | 1165 | 13.907 | 11.164 | 14.479 | 16.741 | 17.13 | 22.2 |
| 32 | Terpinene-4-ol | 1174 | 1174 | 0.56 | 0.704 | 1.113 | 0.761 | 1.05 | 0.8 |
| 33 | p-Cymen-8-ol | 1179 | 1181 | 0.049 | 0.104 | 0.207 | 0.047 | - | - |
| 34 | α–Terpineol | 1186 | 1186 | 0.583 | 0.489 | 0.342 | 0.631 | 0.33 | 0.49 |
| 35 | Myrtenol | 1195 | 1192 | 0.224 | 0.214 | 0.341 | 0.249 | 0.17 | 0.3 |
| 36 | Verbenone | 1204 | 1202 | 0.046 | 0.053 | 0.016 | 0.032 | 0.09 | 0.2 |
| 37 | trans-Carveol | 1215 | 1213 | 0.127 | 0.139 | 0.213 | 0.132 | - | 0.13 |
| 38 | Isobornyl formate | 1235 | 1223 | 0.18 | 0.137 | 0.59 | 0.195 | 0.15 | 0.39 |
| 39 | Neral (Z-Citral) | 1235 | 1239 | 0.075 | 0.079 | 0.136 | 0.239 | - | - |
| 40 | Carvone | 1239 | 1241 | - | - | - | - | - | 0.08 |
| 41 | Piperitone | 1249 | 1249 | 1.89 | - | 0.245 | 0.085 | - | - |
| 42 | Geraniol | 1249 | 1251 | - | 1.361 | - | 1.913 | 0.32 | 0.16 |
| 43 | Geranial(E-Citral) | 1264 | 1265 | 0.279 | 0.258 | 0.074 | 0.33 | - | 0.03 |
| 44 | Perilla aldehyde | 1269 | 1268 | - | - | - | - | - | 13.21 |
| 45 | Bornyl acetate | 1284 | 1281 | 3.859 | 3.860 | 4.278 | 5.707 | 5.21 | 7.92 |
| 46 | α–Copaene | 1374 | 1372 | - | - | 0.029 | 0.056 | 0.04 | 0.04 |
| 47 | β-Bourbonene | 1387 | 1371 | - | - | - | 0.093 | 1.3 | 0.83 |
| 48 | Geranyl acetate | 1379 | 1370 | 4.263 | 2.649 | 0.206 | 3.781 | - | - |
| 49 | β-Elemene | 1389 | 1377 | 0.408 | 0.253 | 0.07 | 0.268 | 0.12 | 0.12 |
| 50 | α–Gurjunene | 1409 | 1392 | 0.554 | 0.279 | 0.088 | 0.232 | 0.21 | 0.26 |
| 51 | (E)-β–Caryophyllene | 1417 | 1401 | 0.123 | 0.103 | 0.022 | 0.068 | - | 0.05 |
| 52 | α–Humulene | 1452 | 1447 | - | - | - | 0.025 | - | - |
| 53 | AROMADENDRENE | 1439 | 1449 | 0.717 | 0.564 | 0.176 | - | - | |
| 54 | Alloaromadendrene | 1458 | 1455 | - | - | - | 0.416 | 0.39 | 0.49 |
| 55 | Germancrene D | 1480 | 1478 | - | - | - | - | 0.08 | 0.08 |
| 56 | α–Amorphene | 1483 | 1473 | 0.521 | 0.300 | 0.05 | 0.288 | - | - |
| 57 | Valencene | 1496 | 1484 | 0.114 | 0.146 | 0.046 | 0.041 | 0.11 | |
| 58 | Bicyclogermacrene | 1500 | 1491 | 1.583 | 1.042 | 0.219 | 0.762 | 0.53 | 0.6 |
| 59 | α-Muurolene | 1500 | 1495 | - | 0.101 | 0.012 | 0.018 | - | - |
| 60 | α-Bulnesene | 1509 | 1499 | 0.407 | 0.334 | 0.162 | 0.277 | 0.25 | 0.35 |
| 61 | γ-Cadinene | 1513 | 1509 | 0.158 | 0.182 | 0.038 | 0.107 | 0.12 | 0.09 |
| 62 | Cis-dihydroagarofuran | 1519 | 1515 | 0.59 | 0.351 | 0.113 | 0.681 | - | - |
| 63 | δ-Cadinene | 1522 | 1519 | 0.489 | 0.724 | 0.095 | 0.497 | 0.56 | 0.22 |
| 64 | α-Agarofuran | 1548 | 1544 | 0.386 | 0.274 | 0.209 | 0.061 | - | - |
| 65 | Bourbonanone<1-nor-> | 1561 | 1558 | - | - | - | - | 0.22 | 0.04 |
| 66 | Palustrol | 1567 | 1562 | 0.118 | 0.126 | 0.1 | 0.103 | - | - |
| 67 | Germancrene D-4-ol | 1574 | 1570 | 1.179 | 1.195 | 1.189 | 1.209 | - | - |
| 68 | Spathulenol | 1577 | 1572 | 0.858 | 0.844 | 0.231 | - | - | |
| 69 | Caryophyllene oxide | 1582 | 1578 | 0.161 | 0.276 | 0.264 | 0.035 | - | - |
| 70 | Capaen-4-α-ol<α-> | 1590 | 1588 | - | - | - | - | 0.9 | 0.58 |
| 71 | Viridiflorol | 1592 | 1590 | - | - | - | - | 0.27 | 0.16 |
| 72 | Ledol | 1602 | 1597 | 0.242 | 0.306 | 0.26 | 0.264 | - | - |
| 73 | Isolongifolan-7-α-ol | 1618 | 1615 | - | - | - | - | 0.19 | 0.1 |
| 74 | t-Cadinol (α-epi- Cadinol) | 1638 | 1635 | 0.331 | 0.864 | 0.102 | 0.169 | - | - |
| 75 | α-epi- Muurolol (tau-Muurolol) | 1640 | 1642 | 0.383 | 0.530 | 0.259 | 0.363 | - | - |
| 76 | Muurolol<α-> | 1644 | 1645 | - | - | - | - | 0.31 | - |
| 77 | α-Cadinol | 1652 | 1653 | 4.139 | 2.166 | 1.634 | 0.224 | - | - |
| 78 | Eudesmpl<α-> | 1652 | 1657 | - | - | - | - | 0.12 | 0.07 |
| 79 | Alloimachalol | 1661 | 1659 | - | - | - | - | 0.43 | 0.09 |
| 80 | 7-epi-α-Eudesmol | 1662 | 1661 | - | 2.630 | - | 4.129 | 2.14 | 0.89 |
| 81 | Cedranol<5-neo-> | 1684 | 1682 | - | - | - | 6.034 | 1.16 | 0.59 |
| 82 | Acorenone | 1692 | 1686 | 8.032 | 5.844 | 1.248 | - | - | - |
| 83 | Acorenone B | 1697 | 1695 | 0.389 | 0.425 | 0.256 | 0.404 | - | - |
| 84 | Nootkatol | 1714 | 1712 | 0.232 | 0.251 | 0.164 | 0.194 | - | - |
| 85 | Eudesn-11-en-4-α,6-α-diol | 1808 | 1806 | - | 0.314 | 0.083 | 0.274 | - | - |
| Total Identified | 97.877 | 95.992 | 97.266 | 97.687 | 98.97 | 99.050 | |||
| Yield of Essential Oil (v/w) % | 1.20 | 1.30 | 0.84 | 1.07 | 1.53 | 1.08 | |||
| monoterpene hydrocarbons | 26.90 | 25.59 | 21.37 | 20.91 | 23.93 | 14.32 | |||
| oxygenated monoterpenes | 40.56 | 43.34 | 63.93 | 49.57 | 60.34 | 70.66 | |||
| sesquiterpene hydrocarbons | 5.07 | 4.03 | 1.01 | 3.15 | 3.60 | 3.24 | |||
| oxygenated sesquiterpene | 17.04 | 16.40 | 5.88 | 14.38 | 5.52 | 2.48 | |||
| other compounds | 8.30 | 6.65 | 5.07 | 9.68 | 5.58 | 8.35 | |||
| Specification | x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 |
|---|---|---|---|---|---|---|---|---|
| Average | 1315.61 | 1313.09 | 1.15 | 1.13 | 1.16 | 1.15 | 1.16 | 1.17 |
| Median | 1264.00 | 1265.00 | 0.18 | 0.21 | 0.10 | 0.20 | 0.11 | 0.09 |
| Standard deviation | 249.87 | 249.18 | 2.87 | 3.15 | 4.40 | 3.18 | 4.33 | 3.98 |
| Kurtosis | −1.36 | −1.33 | 17.41 | 28.22 | 40.87 | 23.24 | 35.86 | 22.71 |
| Skewness | 0.09 | 0.09 | 4.00 | 4.94 | 6.01 | 4.58 | 5.68 | 4.67 |
| Range | 887.00 | 888.00 | 17.45 | 22.60 | 34.31 | 20.74 | 32.56 | 24.25 |
| Minimum | 921.00 | 918.00 | - | - | - | - | - | - |
| Maximum | 1808.00 | 1806.00 | 17.45 | 22.60 | 34.31 | 20.74 | 32.56 | 24.25 |
| Coefficients of variation (%) | 18.99 | 18.98 | 248.99 | 279.32 | 379.68 | 277.13 | 372.15 | 341.50 |
| x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 | |
|---|---|---|---|---|---|---|---|---|
| x1 | 1.00 * | |||||||
| x2 | 1.00 * | 1.00 * | ||||||
| x3 | −0.15 | −0.15 | 1.00 * | |||||
| x4 | −0.16 | −0.16 | 0.96 * | 1.00 * | ||||
| x5 | −0.19 | −0.19 | 0.89 * | 0.96 * | 1.00 * | |||
| x6 | −0.15 | −0.15 | 0.89 * | 0.91 * | 0.92 * | 1.00 * | ||
| x7 | −0.19 | −0.19 | 0.90 * | 0.96 * | 0.99 * | 0.95 * | 1.00 * | |
| x8 | −0.18 | −0.18 | 0.82 * | 0.83 * | 0.87 * | 0.89 * | 0.89 * | 1.00 * |
| Experimental Factors | Camphene | 1,8-Cineole | Camphor | Borneol | Bornyl Acetate | ||
|---|---|---|---|---|---|---|---|
| Our data Aerial parts | Before flowering | HD | 10.732 b | 5.165 c | 17.452 c | 13.907 b | 3.859 b |
| During flowering | HD | 12.784 a | 5.940 b | 22.602 b | 11.164 c | 3.860 b | |
| After flowering | HD | 12.858 a | 11.209 a | 34.310 a | 14.479 a | 4.278 a | |
| HSD0.05 | 0.670 | 0.412 | 1.363 | 0.731 | 0.220 | ||
| Our data leaves | Leaves during the flowering | MAD | 8.729 b | 7.009 b | 20.738 c | 16.741 b | 5.707 b |
| HD | 14.880 a | 7.880 a | 32.561 a | 17.130 b | 5.210 c | ||
| SD | 7.531 c | 7.720 a | 24.25 b | 22.200 a | 7.92 a | ||
| HSD0.05 | 0.410 | 0.408 | 1.423 | 1.035 | 0.354 | ||
| Algeria [26] | HD% | 2.3 | 6.5 | 10.1 | 1.6 | 0.7 | |
| Tunisia [16] | HD% | 1.83 | 19.96 | 19.2 | 7.64 | 11.67 | |
| Tunisia [27] | HD% | 5.9 | 7.4 | 14.8 | 1.2 | 1.3 | |
| Morocco [28] | HD% | 0.07 | 7.64 | 27.7 | 2.53 | 0 | |
| Morocco [29] | HD% | 11.8 | 4.9 | 10 | 18.3 | 1.2 | |
| Specification | C (mg/mL) | |||||
|---|---|---|---|---|---|---|
| 0.4 | 0.8 | 1 | 10 | 50 | 150 | |
| Average | 13.90 | 20.78 | 29.36 | 54.46 | 66.72 | 87.14 |
| Median | 13.87 | 20.93 | 29.14 | 54.66 | 67.52 | 87.34 |
| Standard deviation | 0.37 | 0.51 | 0.46 | 0.44 | 1.41 | 0.37 |
| Skewness | 0.37 | −1.21 | 1.66 | −1.63 | −1.73 | −1.72 |
| Range | 0.73 | 0.99 | 0.84 | 0.80 | 2.46 | 0.66 |
| Minimum | 13.55 | 20.21 | 29.05 | 53.96 | 65.09 | 86.71 |
| Maximum | 14.28 | 21.20 | 29.89 | 54.76 | 67.55 | 87.37 |
| Coefficient of variation V (%) | 2.63 | 2.46 | 1.57 | 0.80 | 2.12 | 0.43 |
| Concentration (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Specification | 5 | 10 | 25 | 50 | 100 | 200 | 500 | IC50 (µg/mL) | |
| I(%) | BHT | 0.5 c ± 0.001 | 1.0 b ± 0.003 | 3.4 c ± 0.002 | 7.8 c ± 0.008 | 18.5 c ± 0.002 | 51.3 c ± 0.004 | 67 b ± 0.007 | 180.0 a |
| Vitamin E | 15.4 a ± 0.016 | 31.0 a ± 0.002 | 79.5 a ± 0.008 | 89.9 a ± 0.0014 | 93.1 a ± 0.0014 | 91.1 a ± 0.008 | 100.0 a | 15.0 c | |
| BHA | 13.9 b ± 0.28 | 32.0 a ± 2.98 | 53.0 b ± 0.92 | 78.1 b ± 1.42 | 83.7 b ± 1.49 | 84.4 b ± 0.28 | 10.00 a | 22.5 b | |
| HSD0.05 | 0.8 | 1.66 | 4.21 | 4.9 | 4.7 | 5.2 | 5.5 | 10.4 | |
| Specification | Concentration (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | 200 | 500 | IC50 | |
| Average | 9.93 | 21.33 | 45.30 | 58.60 | 65.10 | 75.60 | 89.00 | 72.50 |
| Standard error | 2.37 | 5.10 | 11.15 | 12.82 | 11.73 | 6.15 | 5.50 | 26.90 |
| Median | 13.86 | 30.69 | 52.98 | 78.02 | 83.09 | 84.33 | 100.00 | 22.50 |
| Standard deviation | 7.11 | 15.31 | 33.46 | 38.45 | 35.19 | 18.45 | 16.50 | 80.69 |
| Kurtosis | −1.71 | −1.71 | −1.72 | −1.72 | −1.71 | −1.71 | −1.71 | −1.71 |
| Skewness | −0.82 | −0.82 | −0.43 | −0.79 | −0.80 | −0.76 | −0.86 | 0.85 |
| Range | 14.91 | 33.98 | 76.11 | 82.11 | 74.60 | 39.81 | 33.01 | 165.00 |
| Minimum | 0.50 | 1.00 | 3.40 | 7.79 | 18.50 | 51.30 | 66.99 | 15.00 |
| Maximum | 15.41 | 34.98 | 79.51 | 89.90 | 93.10 | 91.11 | 100.00 | 180.00 |
| Coefficient of variation V. | 71.54 | 71.77 | 73.86 | 65.61 | 54.06 | 24.41 | 18.54 | 111.30 |
| C (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| Specification | 16.44 | 32.87 | 49.31 | 65.74 | 82.18 | IC50 |
| Average | 20.61 | 41.58 | 58.41 | 60.25 | 65.86 | 41.09 |
| Median | 20.87 | 41.55 | 57.66 | 60.20 | 66.66 | 41.09 |
| Standard deviation | 1.09 | 0.97 | 1.30 | 1.38 | 1.56 | 0.00 |
| Skewness | −1.01 | 0.14 | 1.73 | 0.16 | −1.70 | 0.00 |
| Range | 2.14 | 1.93 | 2.25 | 2.75 | 2.80 | 0.00 |
| Minimum | 19.41 | 40.63 | 57.66 | 58.90 | 64.06 | 41.09 |
| Maximum | 21.55 | 42.56 | 59.91 | 61.65 | 66.86 | 41.09 |
| Coefficient of variation V. | 5.31 | 2.32 | 2.22 | 2.28 | 2.37 | 0.00 |
| Specification | Concentration (µg/mL) | |||||
|---|---|---|---|---|---|---|
| 4.60 | 9.20 | 13.80 | 18.40 | 23.00 | 27.60 | |
| Average | 0.48 | 12.94 | 20.86 | 26.73 | 35.49 | 52.27 |
| Median | 0.49 | 12.88 | 20.68 | 26.77 | 35.02 | 52.13 |
| Standard deviation | 0.03 | 0.85 | 0.85 | 1.08 | 0.96 | 1.45 |
| Skewness | −1.46 | 0.32 | 0.91 | −0.17 | 1.68 | 0.43 |
| Range | 0.05 | 1.70 | 1.68 | 2.16 | 1.73 | 2.88 |
| Minimum | 0.45 | 12.12 | 20.11 | 25.63 | 34.86 | 50.90 |
| Maximum | 0.50 | 13.82 | 21.79 | 27.79 | 36.59 | 53.78 |
| Coefficient of variation V. | 5.51 | 6.58 | 4.10 | 4.04 | 2.69 | 2.76 |
| Specification | Concentration (µg/mL) | |||
|---|---|---|---|---|
| 2.71 | 5.42 | 10.83 | 60.00 | |
| Average | 25.08 | 44.21 | 62.31 | 93.69 |
| Median | 24.99 | 44.03 | 62.62 | 93.04 |
| Standard deviation | 0.74 | 0.88 | 1.00 | 1.21 |
| Skewness | 0.54 | 0.88 | −1.26 | 1.72 |
| Range | 1.47 | 1.74 | 1.93 | 2.15 |
| Minimum | 24.39 | 43.43 | 61.19 | 92.94 |
| Maximum | 25.86 | 45.17 | 63.12 | 95.09 |
| Coefficient of variation V | 2.95 | 2.00 | 1.61 | 1.30 |
| Specification | Concentration (µg/mL) | |||
|---|---|---|---|---|
| 2.58 | 9.54 | 19.08 | 28.62 | |
| Average | 30.77 | 52.31 | 61.54 | 90.77 |
| Median | 30.73 | 52.29 | 60.77 | 90.55 |
| Standard deviation | 0.78 | 1.19 | 1.47 | 2.00 |
| Skewness | 0.23 | 0.08 | 1.71 | 0.49 |
| Range | 1.56 | 2.38 | 2.63 | 3.98 |
| Minimum | 30.01 | 51.13 | 60.61 | 88.89 |
| Maximum | 31.57 | 53.51 | 63.24 | 92.87 |
| Coefficient of variation V | 2.54 | 2.28 | 2.40 | 2.20 |
| Specification | Concentration (µg/mL) | |||
|---|---|---|---|---|
| 6.22 | 22.97 | 45.95 | 68.92 | |
| Average | 11.97 | 32.12 | 59.85 | 81.06 |
| Median | 12.12 | 32.01 | 60.01 | 82.19 |
| Standard deviation | 0.35 | 0.85 | 1.83 | 2.08 |
| Skewness | −1.57 | 0.57 | −0.39 | −1.72 |
| Range | 0.65 | 1.69 | 3.64 | 3.67 |
| Minimum | 11.57 | 31.33 | 57.95 | 78.66 |
| Maximum | 12.22 | 33.02 | 61.59 | 82.33 |
| Coefficient of variation V | 2.92 | 2.65 | 3.05 | 2.57 |
| The Bacteria Strain | Diameter of Inhibition Zones (mm) | |
|---|---|---|
| Essential Oil (40 µL/Disk) | Amoxicillin (25 µg/Disk) | |
| Micrococcus luteus (Ml) | 18.0 a ± 0.6 | 0.0 |
| Staphylococcus aureus CIP 7625 | 18.0 a ± 0.7 | 10.0 b ± 0.5 |
| Escherichia coli ATCC 10536 | 13.0 b ± 0.9 | 27.0 a ± 0.7 |
| HSD0.05 | 1.0 | 1.5 |
| The Yeasts and Fungi Strain | Diameter of Inhibition Zones (mm) | |
|---|---|---|
| Essential Oil (40 µL/Disk) | Itraconazole (25 µg/Disk) | |
| Saccharomyces cerevisiae ATCC 4226 | 17.0 b ± 0.5 | 0.0 d |
| Candida albicans IPA200 | 13.0 c ± 0.4 | 24.0 a ± 0.9 |
| Candida tropicalis (Ct) | 2.04 d ± 0.8 | 17.0 b ± 0.7 |
| Candida glabrata (Cg) | 18.0 a ± 0.6 | 13.0 c ± 0.4 |
| HSD0.05 | 1.1 | 1.3 |
| Sample Code (Cell Line) | Concentration (µg/mL) | LC50 (μg/mL) | LC90 (g/mL) | Doxorubicin LC50 (μg/mL) | DMSO at 100 ppm | |||
|---|---|---|---|---|---|---|---|---|
| 100 | 50 | 25 | 12.5 | |||||
| HCT116 | 100 a | 71.37 a | 22.36 a | 4.77 a | 39.8 | 59.6 | 37.6 | 1% |
| HePG2 | 44.2 b | 0.0 b | 0.0 b | 0.0 b | >100 | >100 | 21.6 | 1% |
| HSD0.05 | 5.5 | 4.0 | 1.3 | 0.3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouakouak, H.; Benarfa, A.; Messaoudi, M.; Begaa, S.; Sawicka, B.; Benchikha, N.; Simal-Gandara, J. Biological Properties of Essential Oils from Thymus algeriensis Boiss. Plants 2021, 10, 786. https://doi.org/10.3390/plants10040786
Ouakouak H, Benarfa A, Messaoudi M, Begaa S, Sawicka B, Benchikha N, Simal-Gandara J. Biological Properties of Essential Oils from Thymus algeriensis Boiss. Plants. 2021; 10(4):786. https://doi.org/10.3390/plants10040786
Chicago/Turabian StyleOuakouak, Hamza, Adel Benarfa, Mohammed Messaoudi, Samir Begaa, Barbara Sawicka, Naima Benchikha, and Jesus Simal-Gandara. 2021. "Biological Properties of Essential Oils from Thymus algeriensis Boiss" Plants 10, no. 4: 786. https://doi.org/10.3390/plants10040786
APA StyleOuakouak, H., Benarfa, A., Messaoudi, M., Begaa, S., Sawicka, B., Benchikha, N., & Simal-Gandara, J. (2021). Biological Properties of Essential Oils from Thymus algeriensis Boiss. Plants, 10(4), 786. https://doi.org/10.3390/plants10040786









