Two-Species Forests at the Treeline of Siberian Mountains: An Ecophysiological Perspective under Climate Change
Abstract
1. Introduction
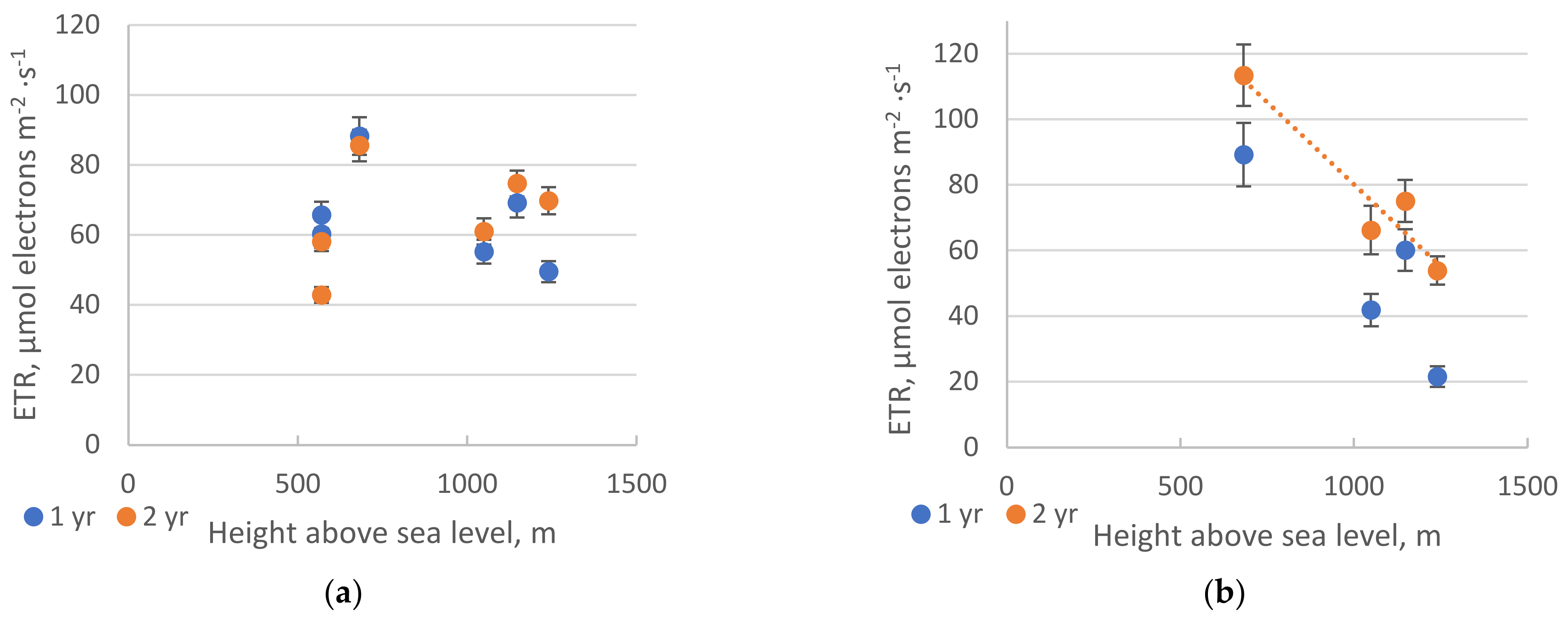
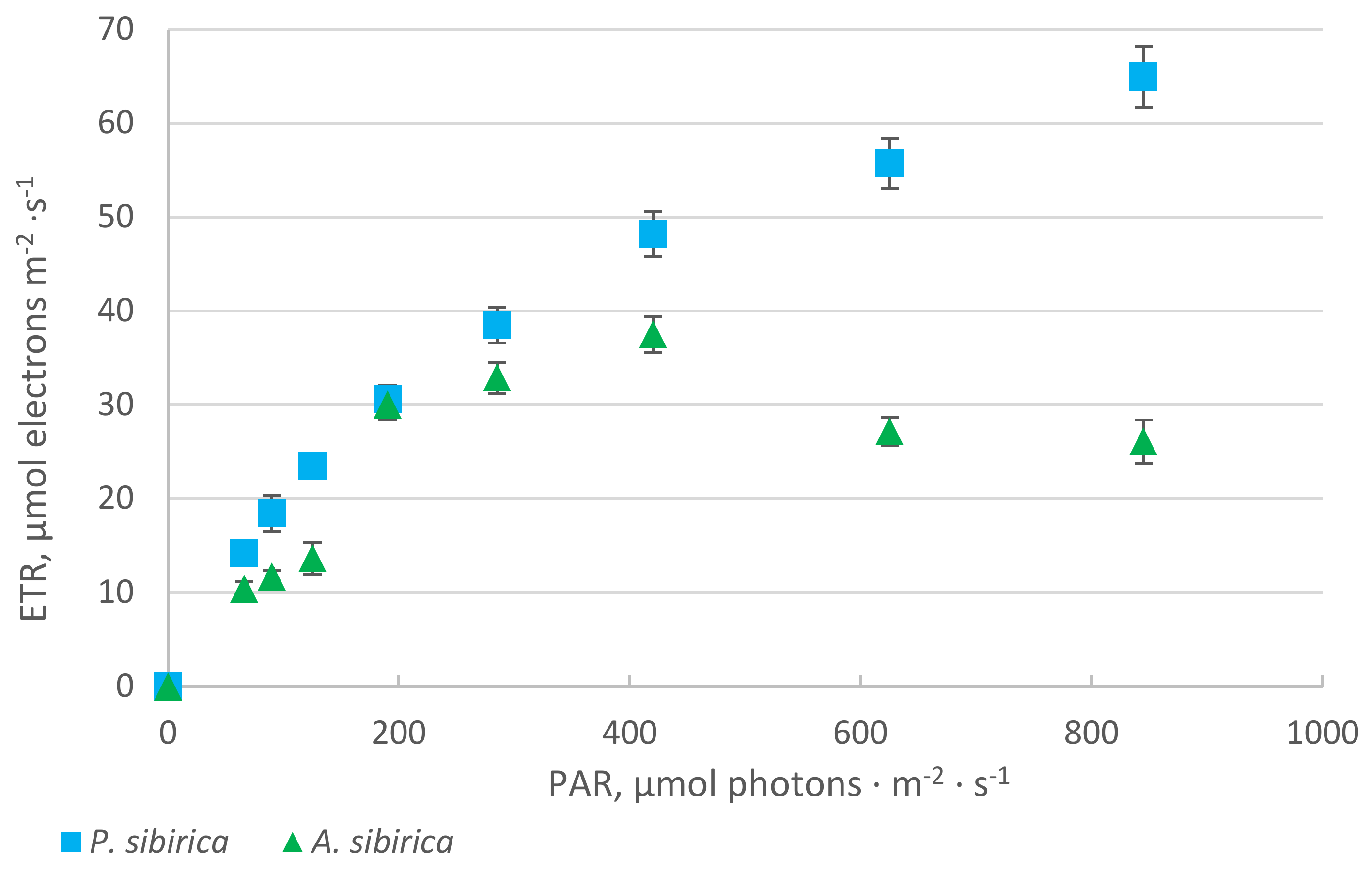
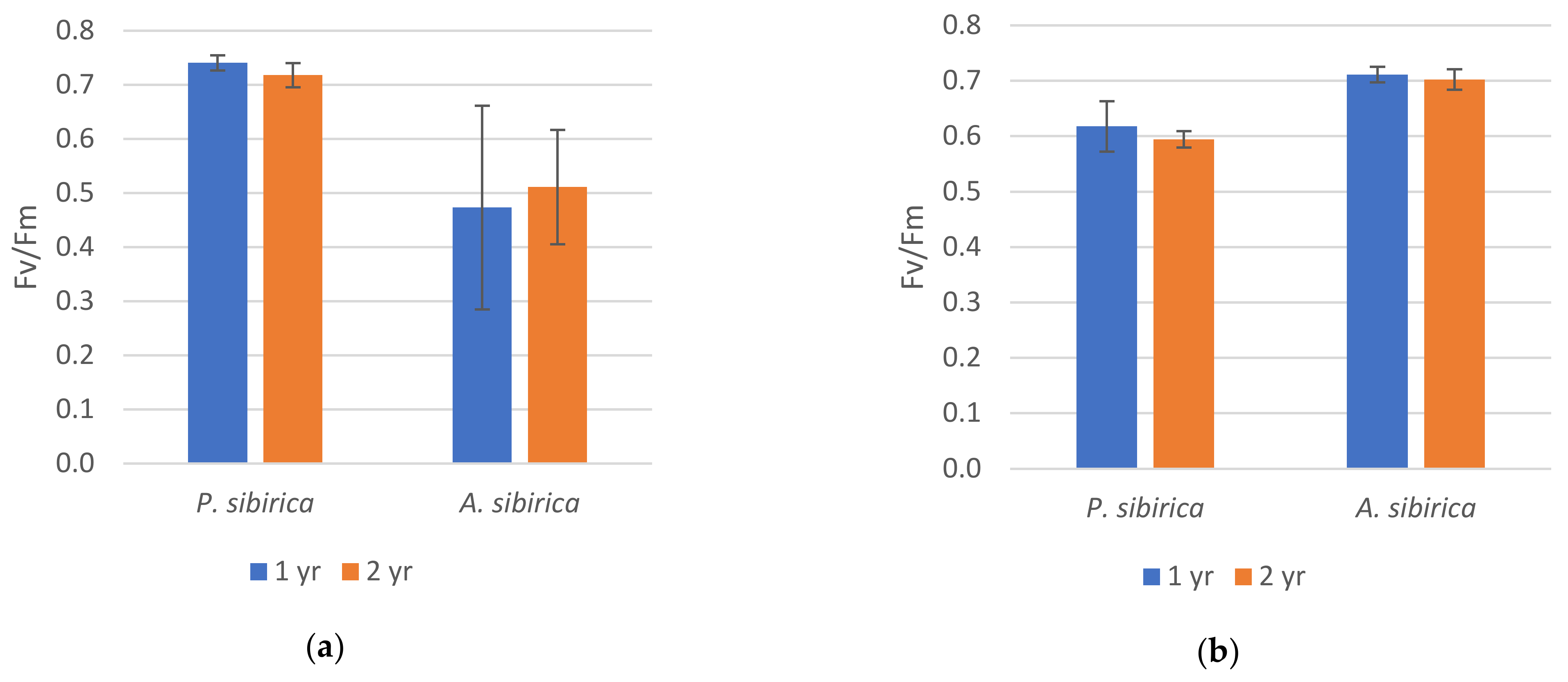
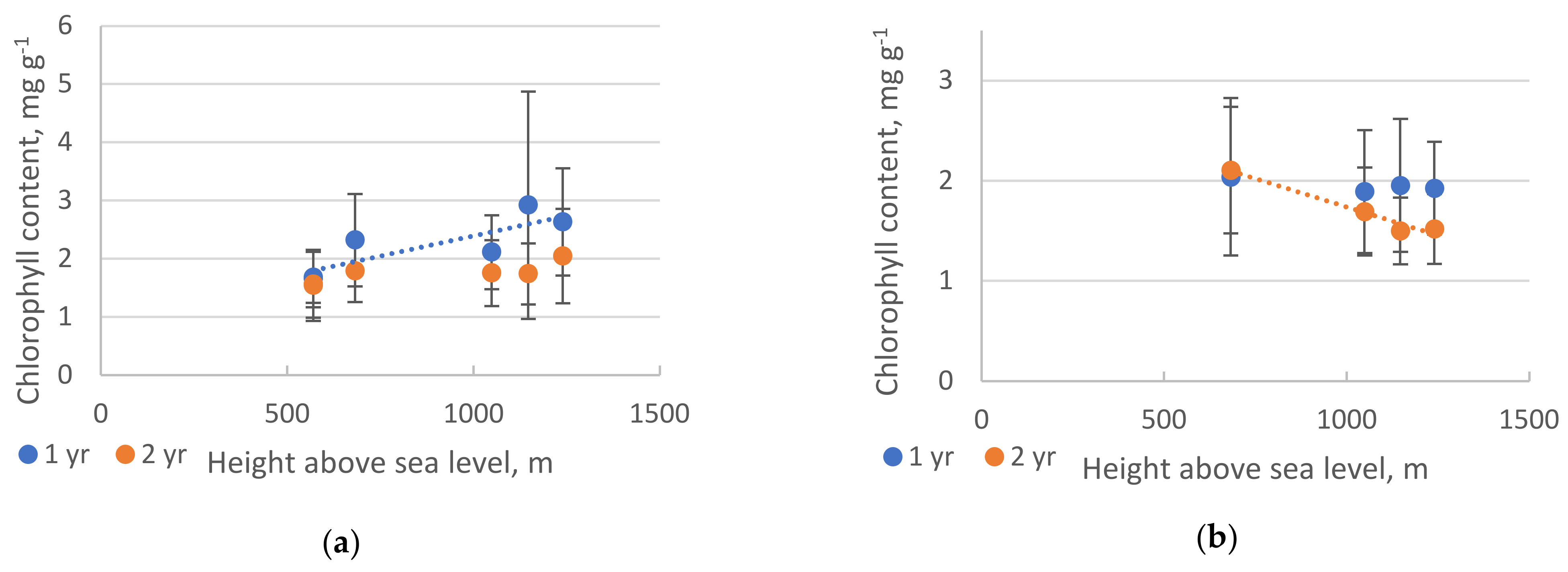
2. Results
2.1. Chlorophyll Fluorescence
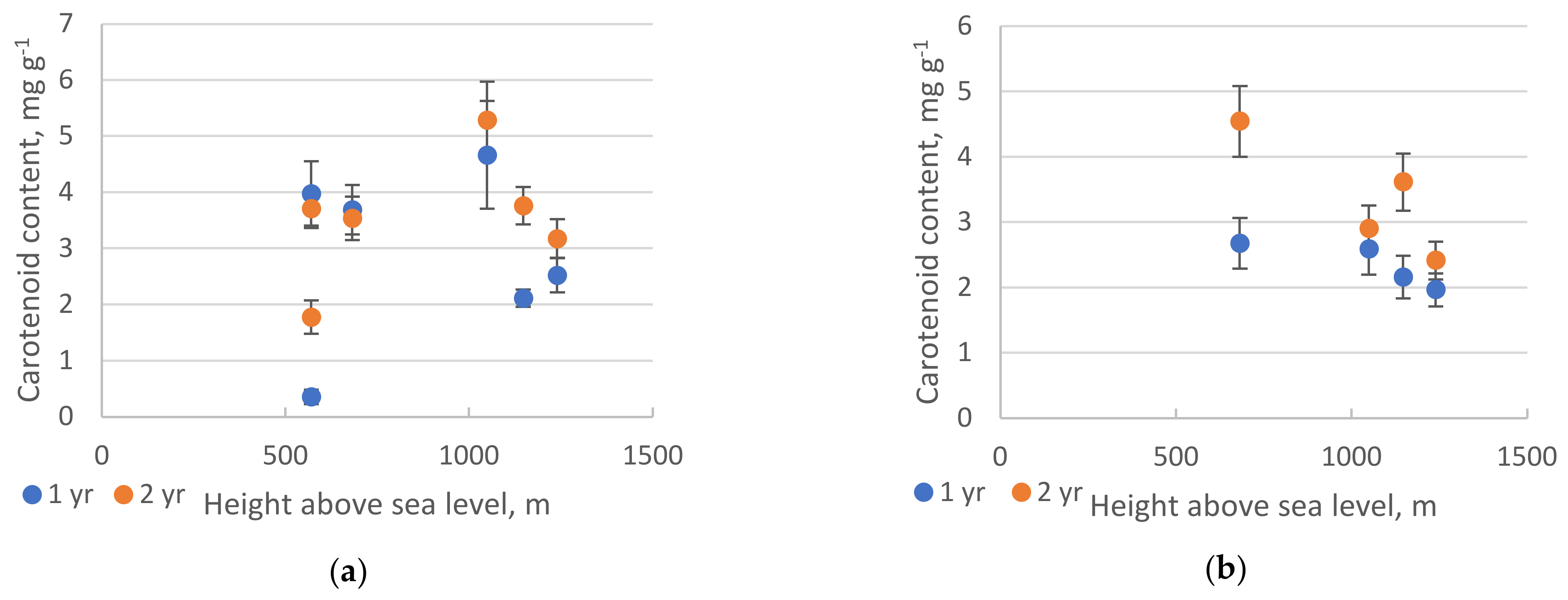
2.2. Pigments
3. Discussion
4. Materials and Methods
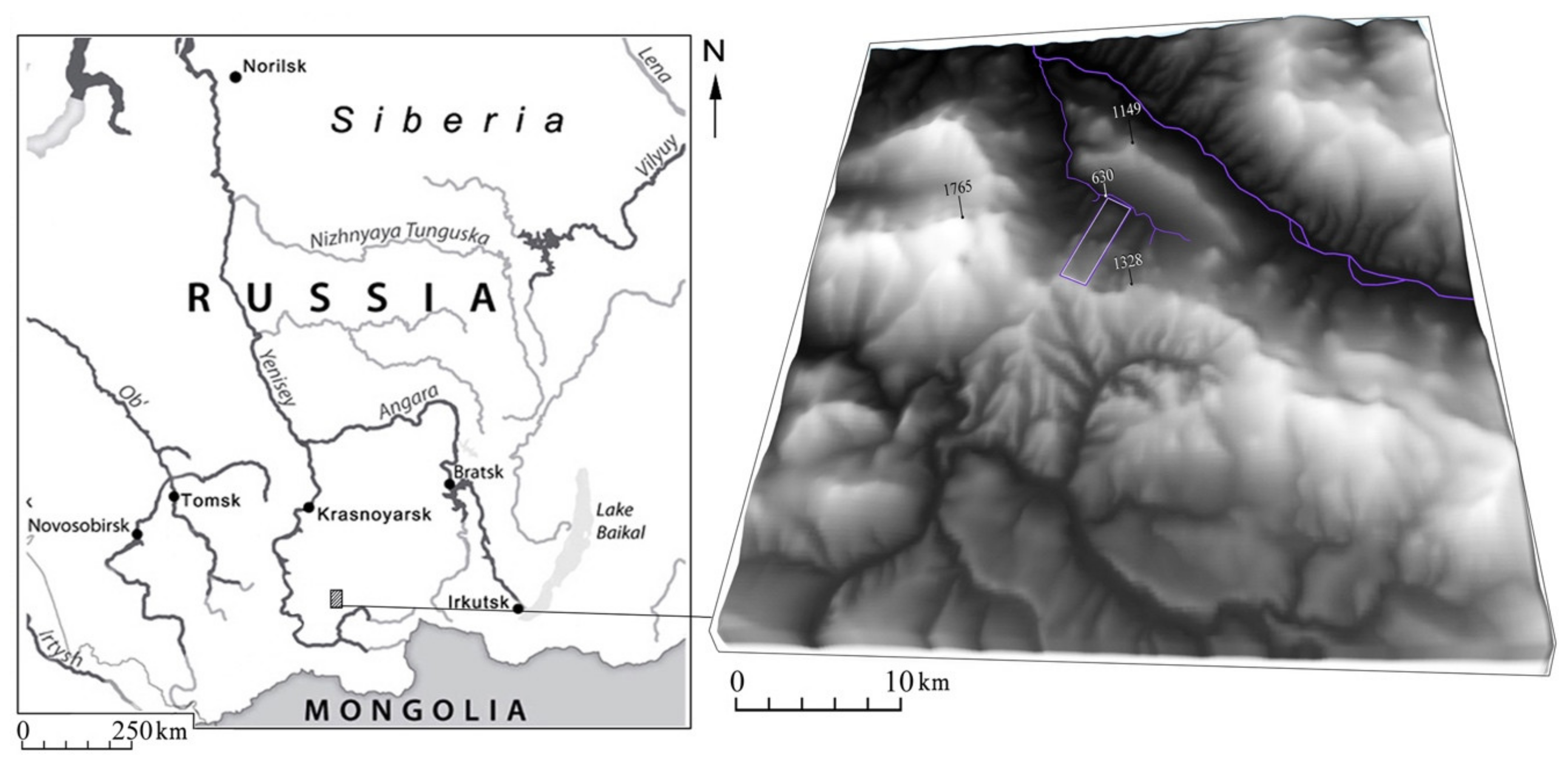
4.1. Area and Study Objects
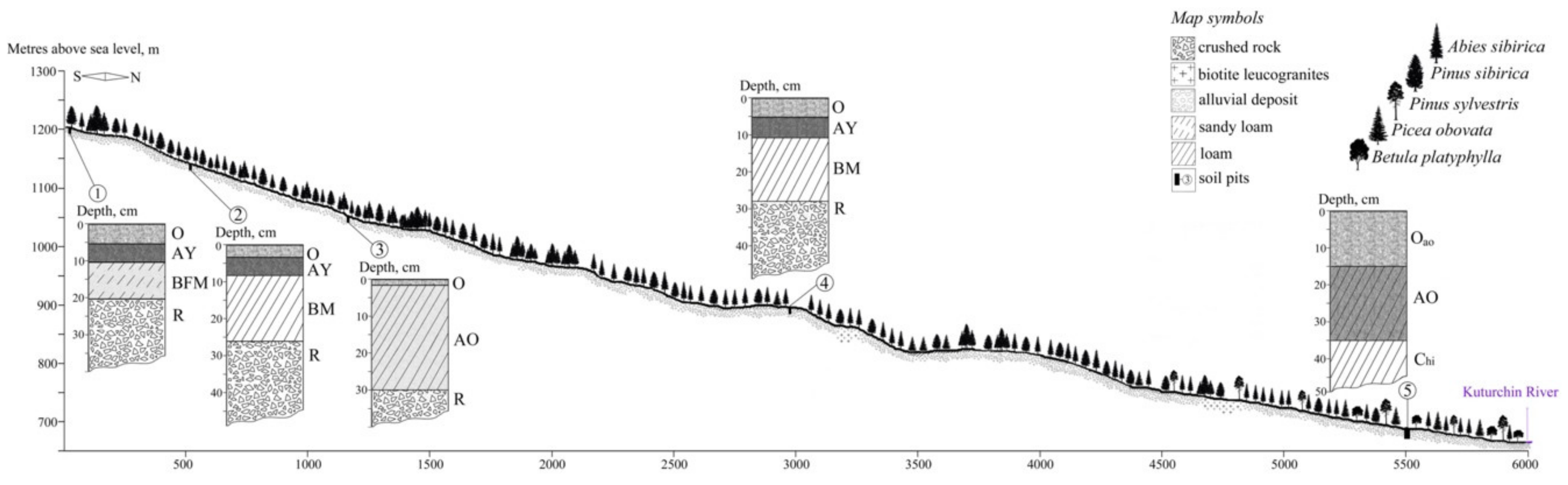
4.2. Soil Conditions
- 1200 m a.s.l, Haplic Folic Fractic Skeletic Ferralic CAMBISOLS Arenic Densic;
- 1147 m a.s.l, Leptic Folic Greyzemic Cambic Skeletic PHAEOZEMS Densic Abruptic Loamic;
- 1049 m a.s.l., Sceletic Fractic Umbric LEPTOSOLS Densic Humic Loamic;
- 890 m a.s.l., Leptic Folic Greyzemic Cambic Skeletic PHAEOZEMS Densic Abruptic Loamic (4);
- 682 m a.s.l., Gleyic Floatic Skeletic HISTOSOLS Loamic Petrogleyic.
4.3. Measurements of Pigment Content
4.4. Measurement of Needle Fluorescence
4.5. Statistical Treatment of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Parameter | Factor (Source of Variation) | SS 1 | df | MS | F | p-Value | F Critical |
|---|---|---|---|---|---|---|---|
| ETR in the needles of P. sibirica | Needle age | 115.64 | 1 | 115.64 | 0.95 | 0.34 | 4.35 |
| Altitude | 4231.58 | 4 | 1057.90 | 8.72 | <0.0001 * | 2.87 | |
| Needle age × a altitude | 1538.25 | 4 | 384.56 | 3.17 | 0.04 | 2.87 | |
| Error | 2426.65 | 20 | 121.33 | ||||
| ETR in the needles of A. sibirica | Needle age | 3436.83 | 1 | 3436.83 | 3.28 | 0.09 | 4.49 |
| Altitude | 13145.64 | 3 | 4381.88 | 4.18 | 0.02 * | 3.24 | |
| Needle age × altitude | 226.82 | 3 | 75.61 | 0.07 | 0.97 | 3.24 | |
| Error | 16790.29 | 16 | 1049.39 | ||||
| ETR in the needles of P. sibirica and A. sibirica | Brightness of flash | 2436.49 | 1 | 2436.49 | 1073.30 | <0.0001 * | 4.02 |
| Species | 16383.13 | 8 | 2047.89 | 902.12 | <0.0001 * | 2.12 | |
| Brightness of flash × species | 2819.23 | 8 | 352.40 | 155.24 | <0.0001 | 2.12 | |
| Error | 122.58 | 54 | 2.27 | ||||
| Fv/Fm | Needle age | 0.00 | 1 | 0.00 | 0.01 | 0.91 | 5.32 |
| Species | 0.17 | 1 | 0.17 | 14.30 | 0.01 * | 5.32 | |
| Needle age × species | 0.00 | 1 | 0.00 | 0.23 | 0.64 | 5.32 | |
| Error | 0.09 | 8 | 0.01 |
| Parameter | Factor (Source of Variation) | SS 1 | df | MS | F | p-Value | F Critical |
|---|---|---|---|---|---|---|---|
| Chlorophyll in 1-year-old P. sibirica needles | Altitude | 5.64 | 3 | 1.88 | 1.32 | 0.28 | 2.77 |
| Error | 79.88 | 56 | 1.43 | ||||
| Chlorophyll in 1-year-old A. sibirica needles | Altitude | 0.18 | 3 | 0.06 | 0.14 | 0.94 | 2.77 |
| Error | 23.89 | 56 | 0.43 | ||||
| Chlorophyll in 2-year-old P. sibirica needles | Altitude | 0.93 | 3 | 0.31 | 0.81 | 0.50 | 2.77 |
| Error | 23.89 | 56 | 0.39 | ||||
| Chlorophyll in 2-year-old A. sibirica needles | Altitude | 3.57 | 3 | 1.19 | 5.61 | <0.0001 * | 2.77 |
| Error | 11.86 | 56 | 0.21 | ||||
| Carotenoids in 1-year-old P. sibirica needles | Altitude | 13.91 | 4 | 3.48 | 159.48 | <0.0001 * | 3.48 |
| Error | 0.22 | 10 | 0.02 | ||||
| Carotenoids l in 1-year-old A. sibirica needles | Altitude | 1.04 | 3 | 0.35 | 24.75 | <0.0001 * | 4.07 |
| Error | 0.11 | 8 | 0.01 | ||||
| Carotenoids in 2-year-old P. sibirica needles | Altitude | 19.83 | 4 | 4.96 | 207.90 | <0.0001 * | 3.48 |
| Error | 0.24 | 10 | 0.02 | ||||
| Carotenoids in 2-year-old A. sibirica needles | Altitude | 7.95 | 3 | 2.65 | 251.88 | <0.0001 * | 4.07 |
| Error | 0.08 | 8 | 0.01 | ||||
| Carotenoids in 1-year-old needles | P. sibirica vs. A. sibirica | 1829214.06 | 2 | 914607.03 | 45.66 | <0.0001 * | 5.14 |
| Error | 120176.14 | 6 | 20029.36 | ||||
| Carotenoids in 2-year-old needles | P. sibirica vs. A. sibirica | 1825545.31 | 2 | 912772.66 | 45.57 | <0.0001 * | 5.14 |
| Error | 120175.83 | 6 | 20029.31 |
References
- Takahashi, K. Virtual issue: Alpine and subalpine plant communities: Importance of plant growth, reproduction and community assemblage processes for changing environments. J. Plant Res. 2018, 131, 891–894. [Google Scholar] [CrossRef]
- Shi, C.; Shen, M.; Wu, X.; Cheng, X.; Li, X.; Fan, T.; Wu, G. Growth response of alpine treeline forests to a warmer and drier climate on the southeastern Tibetan Plateau. Agric. For. Meteorol. 2019, 264, 73–79. [Google Scholar] [CrossRef]
- Wei, C.; Karger, D.N.; Wilson, A.M. Spatial detection of alpine treeline ecotones in the Western United States. Remote Sens. Environ. 2020, 240, 111672. [Google Scholar] [CrossRef]
- Heilmeier, H. Functional traits explaining plant responses to past and future climate changes. Flora 2019, 254, 1–11. [Google Scholar] [CrossRef]
- Li, K.; Liao, M.; Ni, J.; Liu, X.; Wang, Y. Treeline composition and biodiversity change on the southeastern Tibetan Plateau during the past millennium, inferred from a high-resolution alpine pollen record. Quat. Sci. Rev. 2019, 206, 44–55. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, L.; Guo, Y.; Luo, T. Causes for treeline stability under climate warming: Evidence from seed and seedling transplant experiments in southeast Tibet. For. Ecol. Manag. 2018, 408, 45–53. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Hagedorn, F.; Knorre, A.A.; Fedotova, E.V.; Vaganov, E.A.; Naurzbaev, M.M.; Rigling, A. 20th century tree-line advance and vegetation changes along an altitudinal transect in the Putorana Mountains, northern Siberia. Boreas 2012, 41, 56–67. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Liu, S.; Gu, F.; Wang, X.; Li, Z.; Fan, Z. Divergent growth between spruce and fir at alpine treelines on the east edge of the Tibetan Plateau in response to recent climate warming. Agric. For. Meteorol. 2019, 276, 107631. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.; Wang, X.; Gu, F.; Liu, S. The responses of dominant tree species to climate warming at the treeline on the eastern edge of the Tibetan Plateau. For. Ecol. Manag. 2018, 425, 21–26. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Dvinskaya, M.L.; Im, S.T.; Ranson, K.D. Woody vegetation of forest-tundra ecotone in West Sayan and climatic trends. Ekologia 2008, 1, 10–15. (In Russian) [Google Scholar]
- Petrov, I.A.; Kharuk, V.I.; Dvinskaya, M.L.; Im, S.T. Reaction of Coniferous Trees of Kuznetsky Alatau Alpine Tundra Ecotone on Climate Change. Sib. Ekol. Zhurnal 2015, 22, 518–527. (In Russian) [Google Scholar]
- Pakharkova, N.V.; Heilmeier, H.; Gette, I.G.; Andreeva, E.B.; Grachev, A.M.; Gaevskiy, N.A.; Grigoriev, Y.S. Quantitative characteristics of the phases of winter dormancy of conifer species at a site in Central Siberia. Rev. Bras. Bot. 2016, 39, 1005–1014. [Google Scholar] [CrossRef]
- Pakharkova, N.V.; Borisova, I.V.; Sharafutdinov, R.A.; Gavrikov, V.L. Photosynthetic Pigments in Siberian Pine and Fir under Climate Warming and Shift of the Timberline. Forests 2020, 11, 63. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Zivcak, M. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Damm, A.; Guanter, L.; Paul-Limoges, E.; van der Tol, C.; Hueni, A.; Buchmann, N.; Eugster, W.; Ammann, C.; Schaepman, M.E. Far-red sun-induced chlorophyll fluorescence shows ecosystem-specific relationships to gross primary production: An assessment based on observational and modeling approaches. Remote Sens. Environ. 2015, 166, 91–105. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Qiu, N.; Zhou, F.; Gu, Z.; Jia, S.; Wang, X. Photosynthetic functions and chlorophyll fast fluorescence characteristics of five Pinus species. Chin. J. Appl. Ecol. 2012, 23, 1181–1187. [Google Scholar]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Flexas, J.; Galmés, J.; Niinemets, Ü.; Gil-Pelegrín, E. Light acclimation of photosynthesis in two closely related firs (Abies pinsapo Boiss. and Abies alba Mill.): The role of leaf anatomy and mesophyll conductance to CO2. Tree Physiol. 2016, 36, 300–310. [Google Scholar] [CrossRef]
- Formighieri, C. Light Saturation of Photosynthesis. In Solar-To-Fuel Conversion in Algae and Cyanobacteria; Springer: Cham, Switzerland, 2015; pp. 55–58. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. N. Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Kunugi, M.; Satoh, S.; Ihara, K.; Shibata, K.; Yamagishi, Y.; Kogame, K.; Obokata, J.; Takabayashi, A.; Tanaka, A. Evolution of green plants accompanied changes in light-harvesting systems. Plant Cell Physiol. 2016, 57, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.-P.; Niyogi, K.K. Non-photochemical quenching. A response to excess of light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Bascuñán-Godoy, L.; García-Plazaola, J.I.; Bravo, L.A.; Corcuera, L.J. Leaf functional and micro-morphological photoprotective attributes in two ecotypes of Colobanthus quitensis from the Andes and Maritime Antarctic. Pol. Biol. 2010, 83, 885–897. [Google Scholar] [CrossRef]
- Wintermans, J.E.G.; De Mots, A. Spectrophotometric Characteristics of Chlorophyll a and b and Their Phaeophytins in Ethanol. Biochim. Biophys. Acta 1965, 109, 448–453. [Google Scholar] [CrossRef]
- Mohammed, G.H.; Binder, W.D.; Gillies, S.L. Chlorophyll fluorescence: A review of its practical forestry applications and instrumentation. Scand. J. For. Res. 1995, 10, 383–410. [Google Scholar] [CrossRef]
- Huner, N.P.A.; Ivanov, A.G.; Sane, P.V.; Pocock, T.; Król, M.; Balseris, A.; Rosso, D.; Savitch, L.V.; Hurry, V.M.; Öquist, G. Photoprotection of Photosystem II: Reaction center quenching versus antenna quenching. In Photoprotection, Photoinhibition, Gene Regulation, and Environment. In Advances in Photosynthesis and Respiration; Demmig-Adams, B., Iii, W.W.A., Mattoo, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 155–173. ISBN 978-1-4020-3564-7. [Google Scholar]
- L’Hirondelle, S.J.; Simpson, D.G.; Binder, W.D. Chlorophyll fluorescence, root growth potential, and stomatal conductance as estimates of field performance potential in conifer seedlings. New For. 2007, 34, 235–251. [Google Scholar] [CrossRef]
- Zavafer, A.; Labeeuw, L.; Mancilla, C. Global Trends of Usage of Chlorophyll Fluorescence and Projections for the Next Decade. Plant Phenomics 2020. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Buendia, E.C., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakharkova, N.; Kazantseva, A.; Sharafutdinov, R.; Borisova, I.; Gavrikov, V. Two-Species Forests at the Treeline of Siberian Mountains: An Ecophysiological Perspective under Climate Change. Plants 2021, 10, 763. https://doi.org/10.3390/plants10040763
Pakharkova N, Kazantseva A, Sharafutdinov R, Borisova I, Gavrikov V. Two-Species Forests at the Treeline of Siberian Mountains: An Ecophysiological Perspective under Climate Change. Plants. 2021; 10(4):763. https://doi.org/10.3390/plants10040763
Chicago/Turabian StylePakharkova, Nina, Anna Kazantseva, Ruslan Sharafutdinov, Irina Borisova, and Vladimir Gavrikov. 2021. "Two-Species Forests at the Treeline of Siberian Mountains: An Ecophysiological Perspective under Climate Change" Plants 10, no. 4: 763. https://doi.org/10.3390/plants10040763
APA StylePakharkova, N., Kazantseva, A., Sharafutdinov, R., Borisova, I., & Gavrikov, V. (2021). Two-Species Forests at the Treeline of Siberian Mountains: An Ecophysiological Perspective under Climate Change. Plants, 10(4), 763. https://doi.org/10.3390/plants10040763






