Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide
Abstract
1. Introduction
2. Results
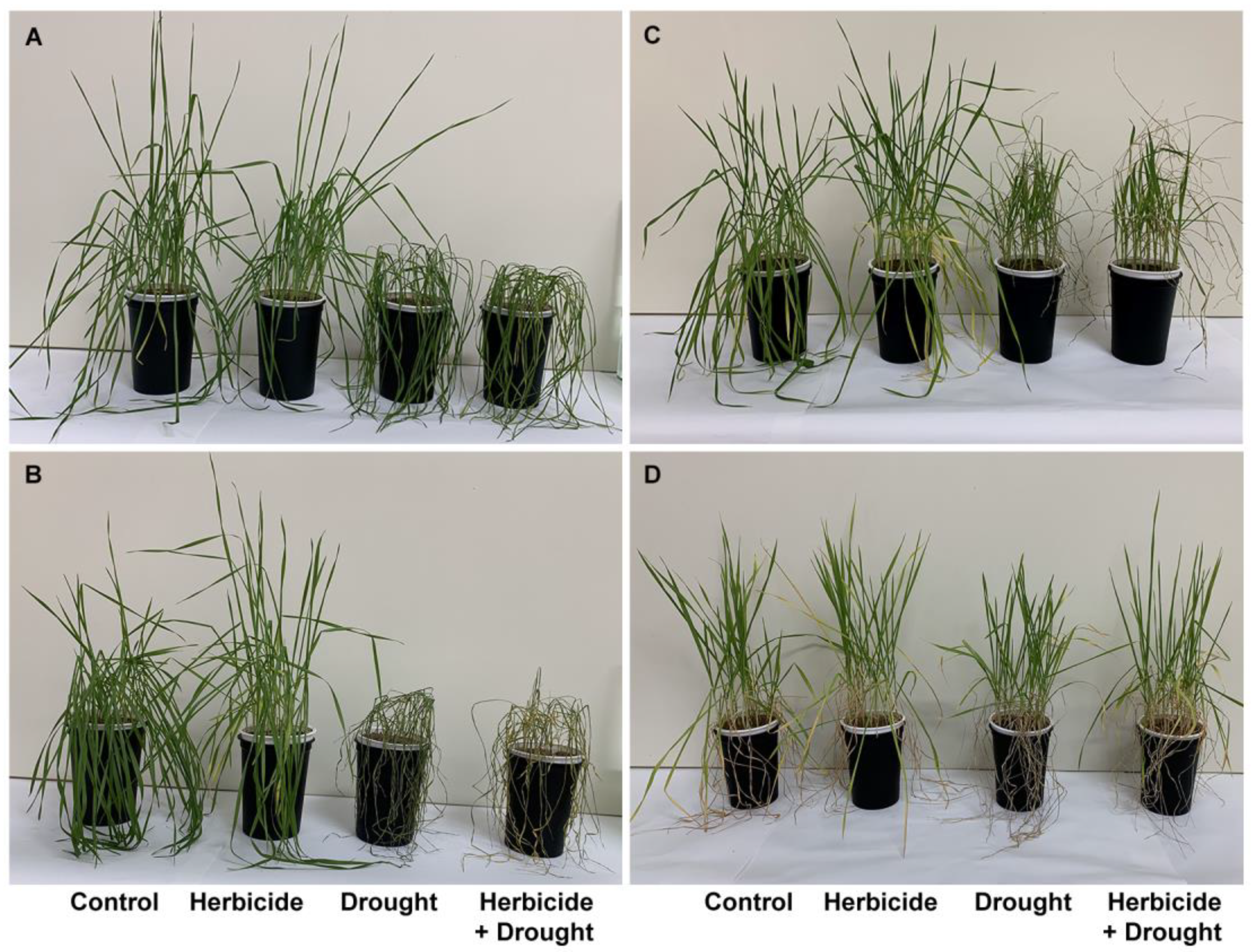
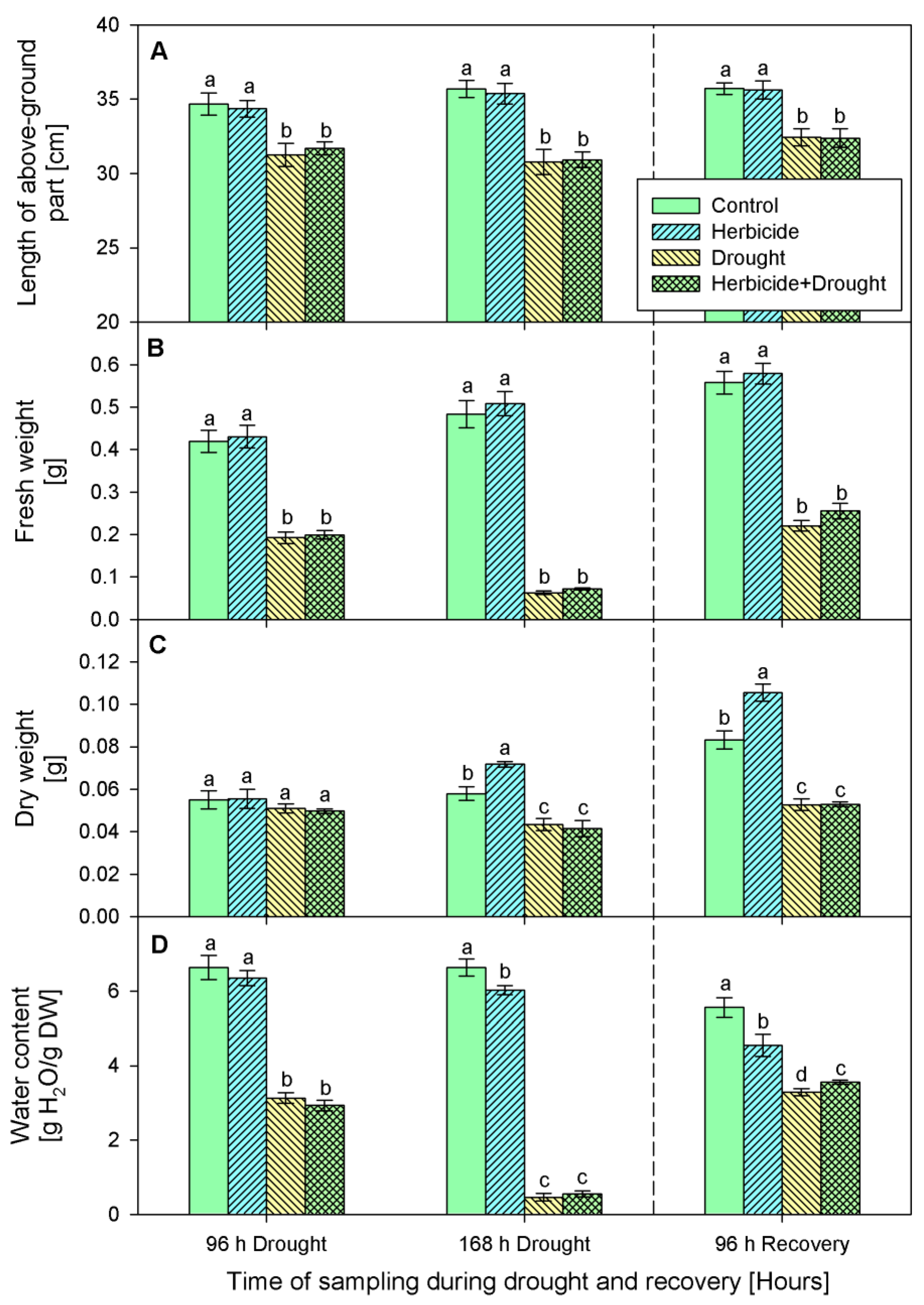
2.1. Growth Parameters
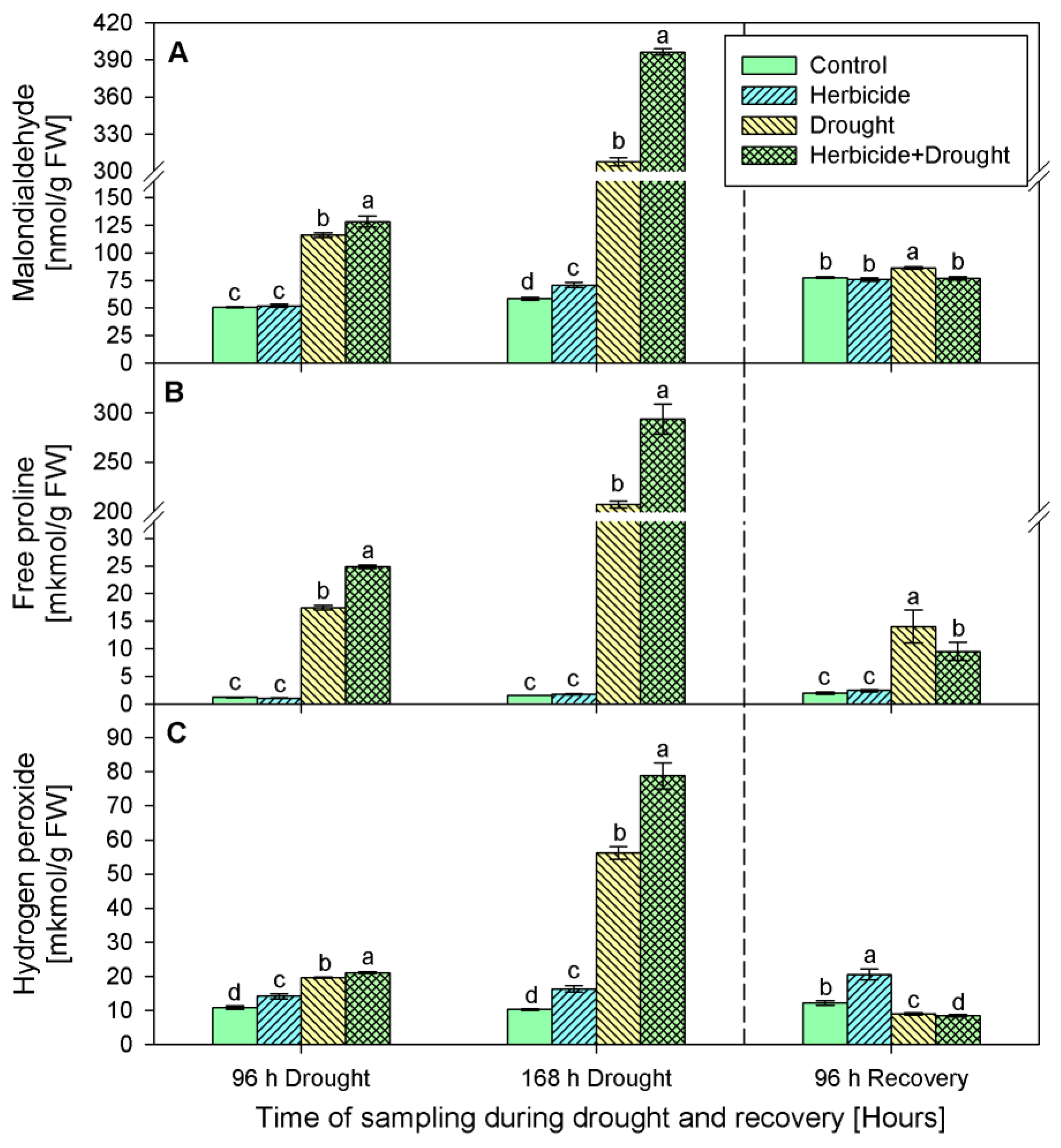
2.2. Stress Markers Content
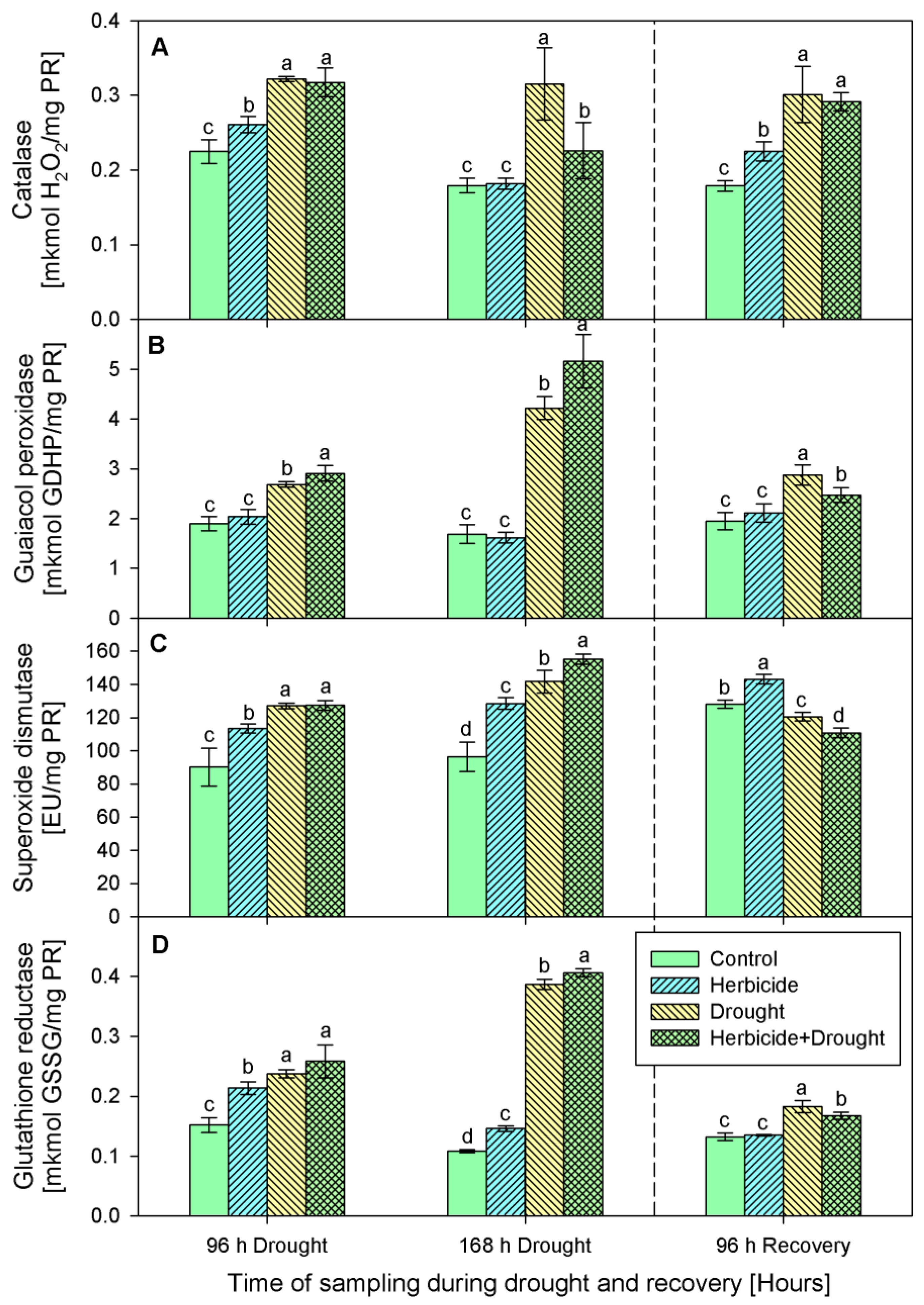
2.3. Activity of Antioxidant Enzymes
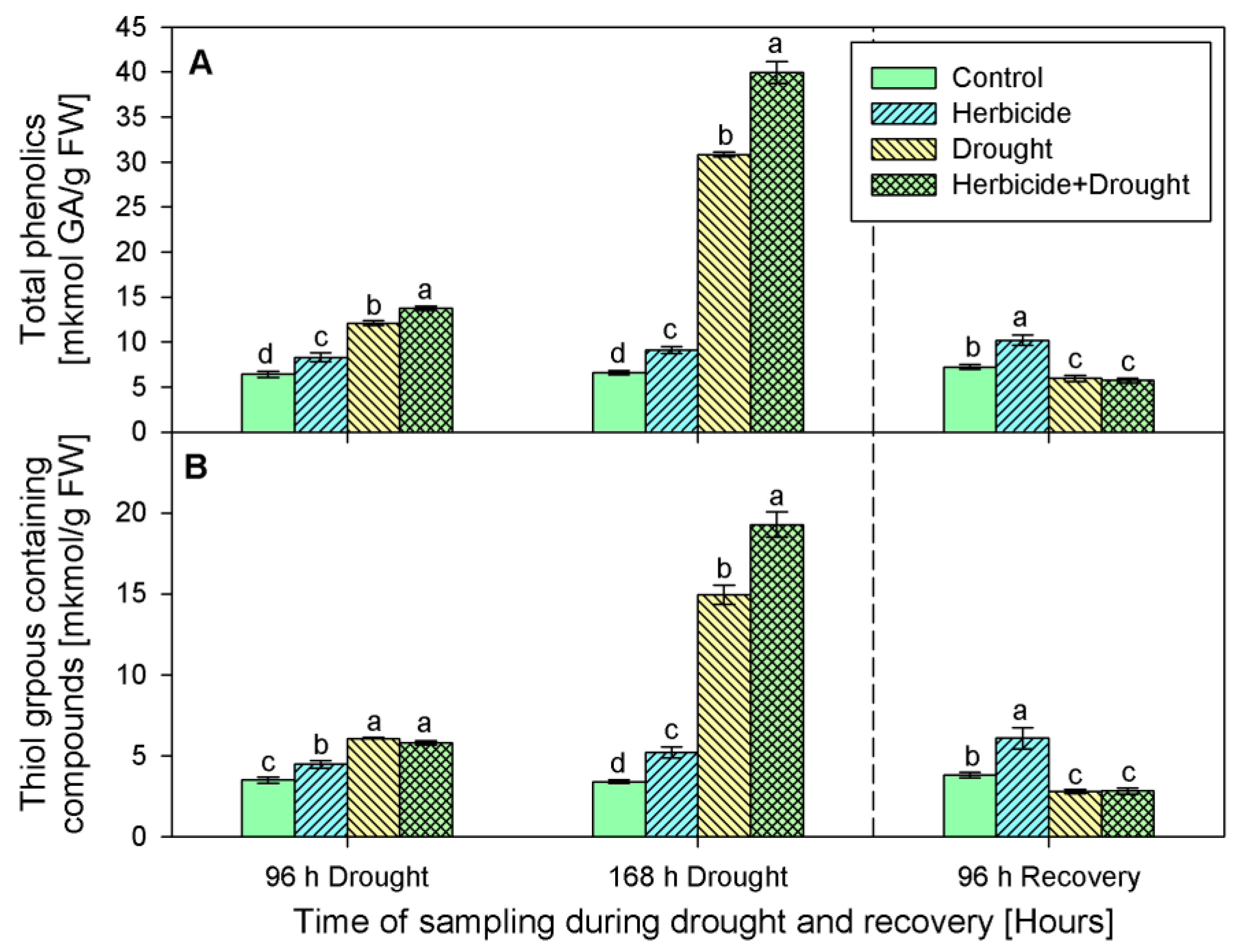
2.4. Content of Non-Enzymatic Antioxidants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
- Normally irrigated control plants.
- Normally irrigated plants treated with herbicide.
- Drought stressed plants.
- Plants treated with herbicide and subsequently exposed to drought stress.
4.2. Biochemical Analyses
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NASA, World of Change: Global Temperatures. Available online: https://earthobservatory.nasa.gov/Features/WorldOfChange/decadaltemp.php (accessed on 19 March 2021).
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather. Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mahmud, J.A.; Anee, T.I.; Nahar, K.; Islam, M.T. Drought stress tolerance in wheat: Omics approaches in understanding and enhancing antioxidant defense. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Zargar, S., Zargar, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 267–307. [Google Scholar]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Jallouli, S.; Ayadi, S.; Landi, S.; Capasso, G.; Santini, G.; Chamekh, Z.; Zouari, I.; Azaiez, F.E.B.; Trifa, Y.; Esposito, S. Physi-ological and molecular osmotic stress responses in three durum wheat (Triticum turgidum ssp durum) genotypes. Agronomy 2019, 9, 550. [Google Scholar] [CrossRef]
- Landi, S.; Hausman, J.-F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Kataria, S.; Jain, M.; Kanungo, M.; Sharma, S. Wheat responses and tolerance to UV-B radiation: An overview. In Wheat Pro-Duction in Changing Environments; Hasanuzzaman, M., Nahar, K., Hossain, M., Eds.; Springer: Singapore, 2019; pp. 175–196. [Google Scholar]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Kalhoro, N.A.; Rajpar, I.; Kalhoro, S.A.; Ali, A.; Raza, S.; Ahmed, M.; Kalhoro, F.A.; Ramzan, M.; Wahid, F. Effect of Salts Stress on the Growth and Yield of Wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 2257–2271. [Google Scholar] [CrossRef]
- Cohen, I.; Rapaport, T.; Chalifa-Caspi, V.; Rachmilevitch, S. Synergistic effects of abiotic stresses in plants: A case study of nitrogen limitation and saturating light intensity in Arabidopsis thaliana. Physiol. Plant. 2018, 165, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Chojak-Koźniewska, J.; Kuźniak, E.; Zimny, J. The Effects of Combined Abiotic and Pathogen Stress in Plants: Insights from Salinity and Pseudomonas syringae pv lachrymans Interaction in Cucumber. Front. Plant Sci. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Stefanovic, L.; Zaric, L. Effect of herbicides and low temperatures on certain maize genotypes. Plant Protection 1991, 42, 345–356. [Google Scholar]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Cvikrová, М.; Gemperlová, L.; Martincová, O.; Vankova, R. Effect of drought and combined drought and heat stress on pol-yamine metabolism in proline-over-producing tobacco plants. Plant Physiol. Biochem. 2013, 73, 7–15. [Google Scholar] [CrossRef]
- Shah, A.H.; Shah, S.H.; Ahmad, H.; Swati, Z.A.; Shah, A.H.; Afzal, M.; Aiman, U.; Khalid, Q. The phenomenon of cross tolerance in osmotically and ionically adapted rice (Oryza sativa L.) cell lines. Afr. J. Biotech. 2012, 11, 713–717. [Google Scholar]
- Talanova, V.V.; Topchieva, L.V.; Titov, A.F. Effect of abscisic acid on the resistance of cucumber seedlings to combined exposure to high temperature and chloride. Biol. Bull. 2006, 33, 619–622. [Google Scholar] [CrossRef]
- Doupis, G.; Chartzoulakis, K.; Beis, A.; Patakas, A. Allometric and biochemical responses of grapevines subjected to drought and enhanced ultraviolet-B radiation. Aust. J. Grape Wine Res. 2010, 17, 36–42. [Google Scholar] [CrossRef]
- Sakalauskiene, S.; Šabajeviene, G.; Lazauskas, S.; Brazaityte, A.; Samuoliene, G.; Urbonavičiute, A.; Sakalauskaite, J.; Ulinskaite, R.; Duchovskis, P. Complex influence of different humidity and temperature regime on pea photosynthetic indices in VI-VII organogenesis stages. Sodinink. Darzinink. 2008, 27, 199–207. [Google Scholar]
- Radyukina, N.L.; Toaima, V.I.M.; Zaripova, N.R. The involvement of low-molecular antioxidants in cross-adaptation of medicine plants to successive action of UV-B radiation and salinity. Russ. J. Plant Physiol. 2012, 59, 71–78. [Google Scholar] [CrossRef]
- Juknys, R.; Račaitė, M.; Vitkauskaitė, G. Cross-adaptation of spring barley (Hordeum vulgare L.) to environmental stress induced by heavy metals. Ekologija 2010, 56, 1–9. [Google Scholar] [CrossRef]
- McCarthy-Suárez, I. Role of reactive oxygen species in auxin herbicide phytotoxicity: Current information and hormonal im-plications—Are gibberellins, cytokinins, and polyamines involved? Botany 2017, 95, 369–385. [Google Scholar] [CrossRef]
- Langaro, A.C.; Agostinetto, D.; Ruchel, Q.; Garcia, J.R.; Perboni, L.T. Oxidative stress caused by the use of preemergent herb-icides in rice crops. Rev. Ciênc. Agron. 2017, 48, 358–364. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D Past, Present, and Future: A Review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Soolanayakanahally, R.; Ogawa, S.; Uga, Y.; Selvaraj, M.G.; Kagale, S. Drought Response in Wheat: Key Genes and Regulatory Mechanisms Controlling Root System Architecture and Transpiration Efficiency. Front. Chem. 2017, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Simova-Stoilova, L.; Kirova, E.; Pecheva, D. Drought stress response in winter wheat varieties–changes in leaf proteins and proteolytic activities. Acta Bot. Croat. 2020, 79. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Xu, P.; Zhang, Z. Overexpression of a WRKY transcription factor TaWRKY2 enhances drought stress tol-erance in transgenic wheat. Front. Plant Sci. 2018, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Kirova, E.; Pecheva, D.; Simova-Stoilova, L. Drought response in winter wheat: Protection from oxidative stress and mutagenesis effect. Acta Physiol. Plant. 2021, 43, 1–11. [Google Scholar] [CrossRef]
- Kartseva, T.; Dobrikova, A.; Kocheva, K.; Alexandrov, V.; Georgiev, G.; Brestič, M.; Misheva, S. Optimal Nitrogen Supply Ameliorates the Performance of Wheat Seedlings under Osmotic Stress in Genotype-Specific Manner. Plants 2021, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Ad-vances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Marcińska, I.; Czyczyło-Mysza, I.; Skrzypek, E.; Filek, M.; Grzesiak, S.; Grzesiak, M.T.; Janowiak, F.; Hura, T.; Dziurka, M.; Dziurka, K.; et al. Impact of osmotic stress on physiological and biochemical characteristics in drought-susceptible and drought-resistant wheat genotypes. Acta Physiol. Plant. 2012, 35, 451–461. [Google Scholar] [CrossRef]
- Schwachtje, J.; Whitcomb, S.J.; Firmino, A.A.P.; Zuther, E.; Hincha, D.K.; Kopka, J. Induced, Imprinted, and Primed Responses to Changing Environments: Does Metabolism Store and Process Information? Front. Plant Sci. 2019, 10, 106. [Google Scholar] [CrossRef]
- Hamada, S.; Abdel-Lateef, M.; Abdelmonem, A.; El-Kholy, R.; Helalia, A. Efficiency of certain clodinafop-propargyl formulations in controlling annual grassy weeds in wheat. Ann. Agric. Sci. 2013, 58, 13–18. [Google Scholar] [CrossRef]
- Guan, W.; Ma, Y.; Zhang, H. Dissipation of Clodinafop-Propargyl and Its Metabolite in Wheat Field Ecosystem. Bull. Environ. Contam. Toxicol. 2013, 90, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Lukatkin, A.S.; Gar’Kova, A.N.; Bochkarjova, A.S.; Nushtaeva, O.V.; da Silva, J.A.T. Treatment with the herbicide TOPIK induces oxidative stress in cereal leaves. Pestic. Biochem. Physiol. 2013, 105, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Hassannejad, S.; Ghafarbi, S.P. Assessment of some chlorophyll a fluorescence parameters of different corn cultivars in response to clodinafop-propagrgyl herbicide and salicylic acid. J. Plant Physiol. Breed. 2018, 8, 47–57. [Google Scholar]
- Zobiole, L.; Gast, R.; Masters, R.; Pereira, G.; Rubin, R. Pyroxsulam: Sulfonamide Herbicide for Weed Control in Wheat in Brazil. Planta Daninha 2018, 36, 018155253. [Google Scholar] [CrossRef]
- Zainulabdeen, J.A.; Ibrahim, S.A. Effect of herbicide pyroxsulam on nickel, cadmium, lead and total flavonoid levels of two Iraqi wheat. EurAsian J. BioSci. 2020, 14, 2817–2821. [Google Scholar]
- Hossain, M.A.; Li, Z.-G.; Hoque, T.S.; Burritt, D.J.; Fujita, M.; Munné-Bosch, S. Heat or cold priming-induced cross-tolerance to abiotic stresses in plants: Key regulators and possible mechanisms. Protoplasma 2018, 255, 399–412. [Google Scholar] [CrossRef]
- Carvalho, F.E.L.; Silveira, J.A.G. H2O2-retrograde signaling as a pivotal mechanism to understand priming and cross stress tolerance in plants. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 57–78. [Google Scholar]
- Niienments, U. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. Forest Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.-L.; Jiang, D. Priming: A promising strategy for crop production in response to future climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Alagna, F.; Balestrini, R.; Chitarra, W.; Marsico, A.D.; Nerva, L. Getting ready with the priming: Innovative weapons against biotic and abiotic crop enemies in a global changing scenario. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 35–56. [Google Scholar]
- Llorens, Е.; González-Hernández, A.I.; Scalschi, L.; Fernández-Crespo, E.; Camañes, G.; Vicedo, B.; García-Agustín, P. Priming mediated stress and cross-stress tolerance in plants: Concepts and opportunities. In Priming-Mediated Stress and Cross-Stress Tolerance in Crop Plants; Hossain, M.A., Liu, F., Burritt, D., Fujita, M., Huang, B., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–20. [Google Scholar]
- Shahbaz, M.; Masood, Y.; Perveen, S.; Ashraf, M. Is foliar-applied glycine betaine effective in mitigating the adverse effects of drought stress on wheat (Triticum aestivum L.)? J. Appl. Bot. Food Qual. 2011, 84, 192–199. [Google Scholar]
- Tounekti, T.; Hernández, I.; Müller, M.; Khemira, H.; Munné-Bosch, S. Kinetin applications alleviate salt stress and improve the antioxidant composition of leaf extracts in Salvia officinalis. Plant Physiol. Biochem. 2011, 49, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Kramer, G.F.; Norman, H.A.; Krizek, D.T.; Mirecki, R.M. influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Swain, T.; Goldstein, L. Methods in Polyphenol Chemistry; Pridham, J.B., Ed.; Pergamon Press: Oxford, UK, 1964; pp. 131–146. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Aebi, M. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Dias, I.; Costa, M. Efect of low salt concentration on nitrate reductase and peroxidase of sugar beet leaves. J. Exp. Bot. 1983, 34, 537–543. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase. Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, D.; Sergiev, I.; Katerova, Z.; Shopova, E.; Dimitrova, L.; Brankova, L. Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants 2021, 10, 733. https://doi.org/10.3390/plants10040733
Todorova D, Sergiev I, Katerova Z, Shopova E, Dimitrova L, Brankova L. Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants. 2021; 10(4):733. https://doi.org/10.3390/plants10040733
Chicago/Turabian StyleTodorova, Dessislava, Iskren Sergiev, Zornitsa Katerova, Elena Shopova, Ljudmila Dimitrova, and Liliana Brankova. 2021. "Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide" Plants 10, no. 4: 733. https://doi.org/10.3390/plants10040733
APA StyleTodorova, D., Sergiev, I., Katerova, Z., Shopova, E., Dimitrova, L., & Brankova, L. (2021). Assessment of the Biochemical Responses of Wheat Seedlings to Soil Drought after Application of Selective Herbicide. Plants, 10(4), 733. https://doi.org/10.3390/plants10040733






