Genetic Diversity of Symbiotic Green Algae of Paramecium bursaria Syngens Originating from Distant Geographical Locations
Abstract
1. Introduction
2. Results
2.1. Syngen Identification
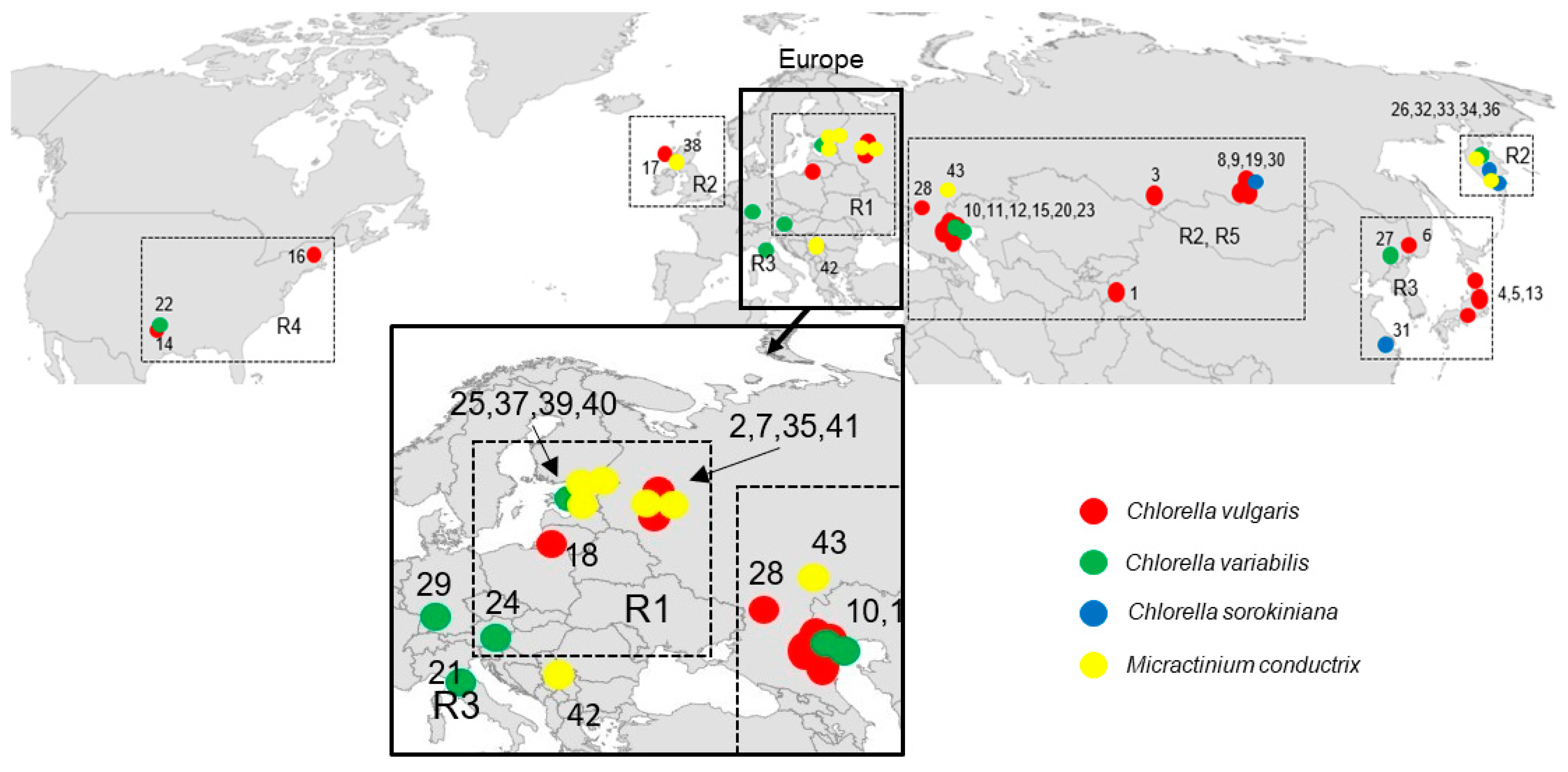
2.2. Geographical Distribution of Paramecium Bursaria Symbionts
2.3. Molecular Results
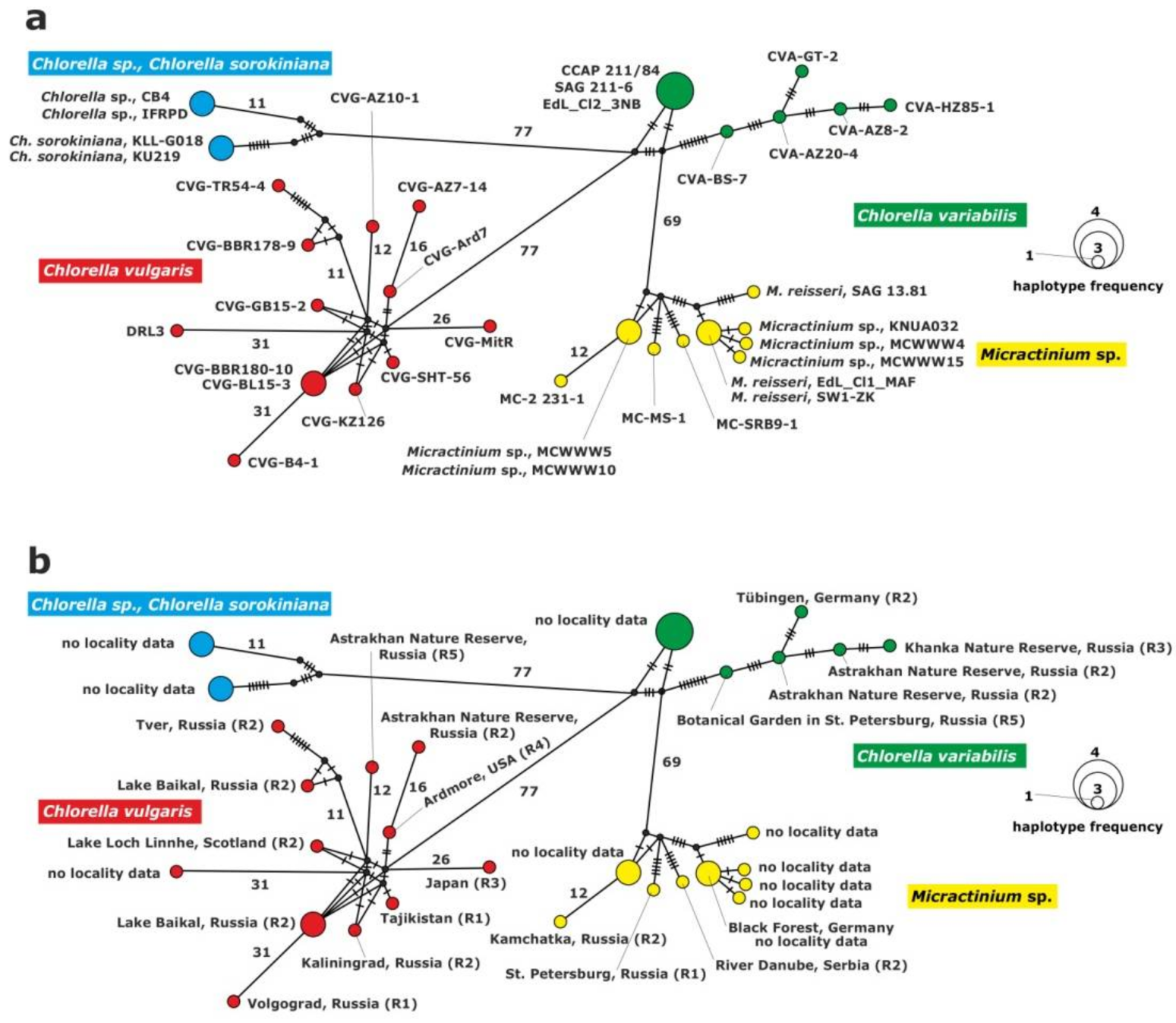
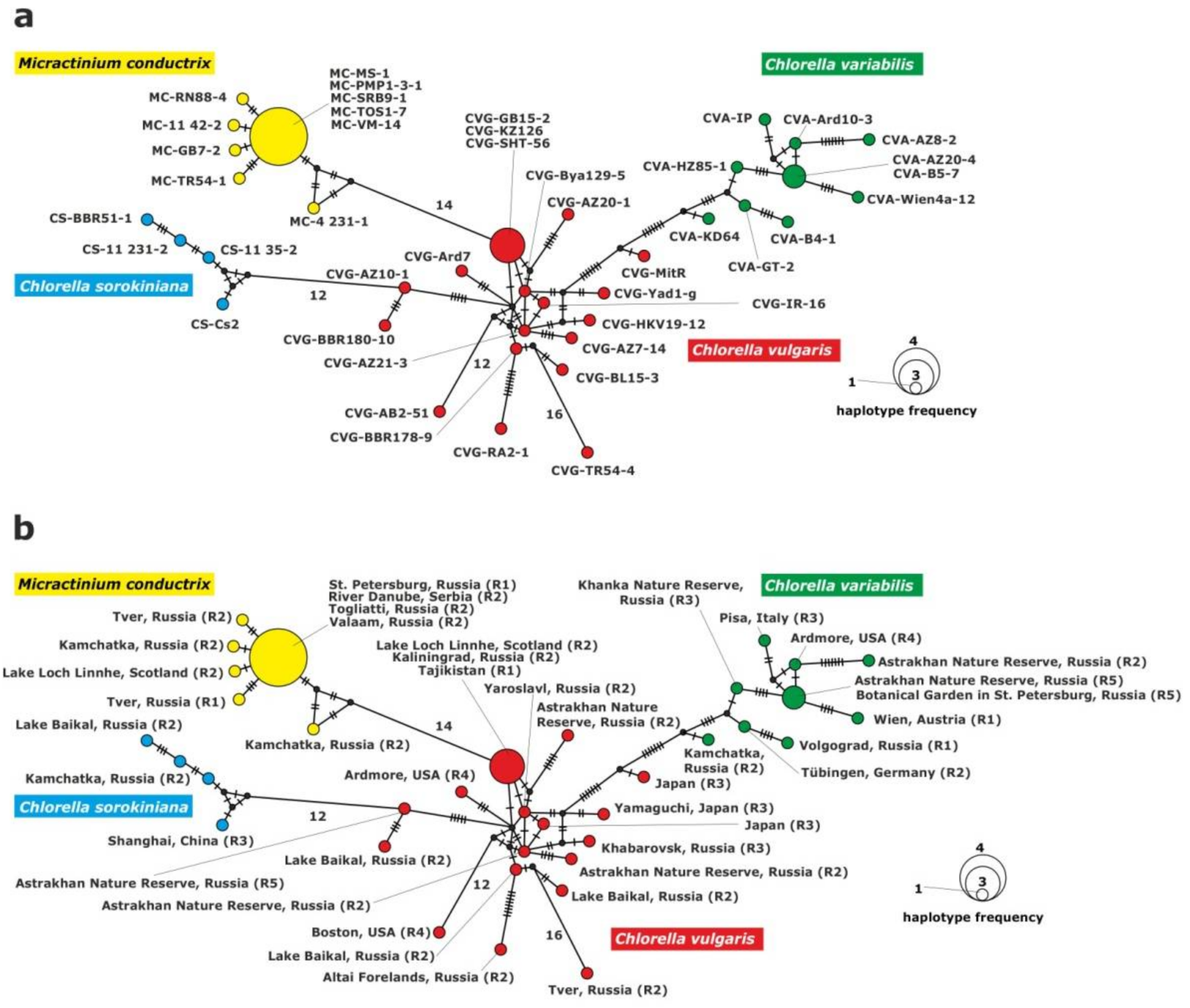
2.3.1. Analysis of the ITS1-5.8S rDNA-ITS2 Fragment
2.3.2. Analysis of the 28S rDNA Fragment
2.3.3. Analysis of the rpl36-infA Genes Fragment
3. Discussion
4. Materials and Methods
4.1. Strain Cultivation and Strain Crosses
4.2. Molecular Methods
4.3. Data Analyzes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doebeli, M.; Knowlton, N. The evolution of interspecific mutualisms. Proc. Natl. Acad. Sci. USA 1998, 95, 8676–8680. [Google Scholar] [CrossRef] [PubMed]
- Bomford, B. The syngens of Paramecium bursaria: New mating types and intersyngenic mating reactions. J. Protozool. 1966, 13, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Witcherman, R. The Biology of Paramecium, 2nd ed.; Plenum Press: New York, NY, USA, 1985. [Google Scholar]
- Greczek-Stachura, M.; Potekhin, A.; Przyboś, E.; Rautian, M.; Skoblo, I.; Tarcz, S. Identification of Paramecium bursaria syngens through molecular markers—Comparative analysis of three loci in the nuclear and mitochondrial DNA. Protist 2012, 163, 671–685. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Gapanova, I.N.; Andronov, E.E.; Migunova, A.V.; Vorobyev, K.P.; Chizhevskaja, E.P.; Kvitko, K.V. Genomic dactyloscopy of Chlorella sp., symbionts of Paramecium bursaria. Protistology 2007, 4, 311–317. [Google Scholar]
- Pröschold, T.; Darienko, T.; Silva, P.C.; Reisser, W.; Krienitz, L. The systematics of Zoochlorella revisited employing an integrative approach. Environ. Microbiol. 2011, 13, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, R.; Imamura, N. Multiple origins of the symbioses in Paramecium bursaria. Protist 2008, 159, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, R.; Imamura, N. Origins of algal symbionts of Paramecium bursaria. In Endosymbionts in Paramecium. Microbiology Monographs; Fujishima, M., Ed.; Springer GmbH: Berlin/Heidelberg, Germany, 2009; Volume 12, pp. 1–29. [Google Scholar] [CrossRef]
- Hoshina, R.; Imamura, N. Phyogenetically close group I introns with difefrent positions among Paramecium bursaria pfotobionts imply a primitive stage of intron diversification. Mol. Biol. Evol. 2009, 26, 1309–1319. [Google Scholar] [CrossRef]
- Nakahara, M.; Handa, S.; Watanabe, S.; Deguchi, H. Choricystis minor as a new symbiont of simultaneous two-species association with Paramecium bursaria and implications for its phylogeny. Symbiosis 2004, 36, 127–151. [Google Scholar]
- Pröschold, T.; Pitsch, G.; Darienko, T. Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a new endosymbiont isolated from ciliates. Diversity 2020, 12, 200. [Google Scholar] [CrossRef]
- Zagata, P.; Greczek-Stachura, M.; Tarcz, S.; Rautian, M. The evolutionary relationships between endosymbiotic green algae of Paramecium bursaria syngens originating from different geographical locations. Folia Biol. 2016, 64, 47–54. [Google Scholar] [CrossRef]
- Rosenberg, J.N.; Kobayashi, N.; Barnes, A.; Noel, E.A.; Btenbaugh, M.J.; Oyler, G.A. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the microalga C. sorokiniana. PLoS ONE 2014, 9, e92460. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Jo, S.W.; Cho, H.W.; Nam, S.W.; Shin, W.; Park, K.M.; Lee, K.I.; Yoon, H.S. Phylogeny, morphology, and physiology of Micractinium strains isolated from shallow ephemeral freshwater in Antarctica. Phycol. Res. 2015, 63, 212–218. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.G.; Kozera, C.; O’Leary, S.J.; McGinn, P.J. Seasonal isolation of microalgae from municipal wastewater for remediation and biofuel applications. J. App. Microbiol. 2015, 119, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Pröschold, T.; Bock, C.; Krienitz, L. Generic concept in Chlorella-related coccoid green algae (Chlorophyta, Trebouxiophyceae). Plant Biol. 2010, 12, 545–553. [Google Scholar] [CrossRef]
- Kaplan-Levy, R.N.; Alster-Gloukhovski, A.; Benyamini, Y.; Zohary, T. Lake Kinneret phytoplankton: Integrating classical and molecular taxonomy. Hydrobiologia 2016, 764, 283–302. [Google Scholar] [CrossRef]
- Darienko, T.; Gustavs, L.; Eggert, A.; Wolf, W.; Pröschold, T. Evaluating the species boundaries of green microalgae (Coccomyxa, Trebouxiophyceae, Chlorophyta) using integrative taxonomy and DNA barcoding with further implications for the species identification in environmental samples. PLoS ONE 2015, 10, e0127838. [Google Scholar] [CrossRef]
- Caisova, L.; Marin, B.; Melkonian, M. A consensus secondary structure of ITS2 in the Chlorophyta identified by phylogenetic reconstruction. Protist 2013, 164, 482–496. [Google Scholar] [CrossRef]
- Ustinova, I.; Krienitz, L.; Huss, V.A.R. Closteriopsis acicularis (G.M. Smith) Belcher et Swale is a fusiform alga closely related to Chlorella kessleri Fott et Novakova (Chlorophyta, Trebouxiophyceae). Eur. J. Phycol. 2001, 36, 341–351. [Google Scholar] [CrossRef]
- Nyberg, D. The species concept and breeding systems. In Paramecium; Görtz, H.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988. [Google Scholar] [CrossRef]
- Jennings, H.S. Sex reaction types and their interrelations in Paramecium bursaria: I. Proc. Natl. Acad. Sci. USA 1938, 24, 112–177. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-T. Varieties and mating types in Paramecium bursaria. II. Variety and mating types found in China. J. Exp. Zool. 1956, 132, 266–268. [Google Scholar] [CrossRef]
- Hoshina, R.; Hayashi, S.; Imamura, N. Intraspecific genetic divergnce of Paramecium bursaria and re-construction of paramecian phylogentic tree. Acta Protozool. 2006, 45, 377–386. [Google Scholar]
- Zagata, P.; Greczek-Stachura, M.; Tarcz, S.; Rautian, M. Molecular identification of Paramecium bursaria syngens and studies on geographic distribution using mitochondrial cytochrome C oxidase subunit I (COI). Folia Biol. 2015, 63, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Spanner, C.; Darienko, T.; Biehler, T.; Sonntag, B.; Pröschold, T. Endosymbiotic green algae in Paramecium bursaria: A new isolation method and a simple diagnostic PCR approach for the identification. Diversity 2020, 12, 240. [Google Scholar] [CrossRef]
- Luo, W.; Pflugmacher, S.; Pröschold, T.; Walz, N.; Krienitz, L. Genotype versus phenotype variability in Chlorella and Micractinium (Chlorophyta, Trebouxiophyceae). Protist 2006, 157, 315–333. [Google Scholar] [CrossRef]
- Weis, D.S. Correlation of infectivity and concanavalin a agglutinability of algae exsymbiotic from Paramecium bursaria. J. Protozool. 1978, 25, 366–370. [Google Scholar] [CrossRef]
- Reisser, W.; Vietze, S.; Widowski, M. Taxonomic studies on endocytobiotic chlorophycean algae isolated from different American and European strains of Paramecium bursaria. Symbiosis 1988, 6, 253–270. [Google Scholar]
- Meier, R.; Wiessner, W. Infection of algae-free Paramecium bursaria with symbiotic Chlorella sp. isolated from green paramecia II: A time study. J. Cell Sci. 1989, 93, 571–579. [Google Scholar] [CrossRef]
- Summerer, M.; Sonntag, B.; Sommaruga, R. An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environ. Microbiol. 2007, 9, 2117–2122. [Google Scholar] [CrossRef]
- Summerer, M.; Sonntag, B.; Sommaruga, R. Ciliate-symbiont specificity of freshwater endosymbiotic Chlorella (Trebouxiophyceae, Chlorophyta). J. Phycol. 2008, 44, 77–84. [Google Scholar] [CrossRef]
- Fan, W.; Guo, W.; Van Etten, J.L.; Mower, J.P. Multiple origins of endosymbionts in Chlorellaceae with no reductive effects on the plastid or mitochondrial genomes. Sci. Rep. 2017, 7, 10101. [Google Scholar] [CrossRef]
- Sonneborn, T.M. Methods in Paramecium research. In Methods in Cell Biology; Prescott, E.D.M., Ed.; Academic Press: New York, NY, USA, 1970; Volume 3, pp. 241–339. [Google Scholar] [CrossRef]
- Provan, J.; Murphy, S.; Maggs, C.A. Universal plastid primers for Chlorophyta and Rodophyta. Eur. J. Phycol. 2004, 39, 43–50. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.M., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hoshina, A.R.; Kamako, S.I.; Imamura, N. Phylogenetic position of endosymbiotic green algae in Paramecium bursaria Ehrenberg from Japan. Plant Biol. 2004, 6, 447–453. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Expertise in Software for Genetics aand Engineering. Available online: http://www.fluxus-engineering.com/ (accessed on 2 October 2020).
- Bandelt, H.J.; Forster, P.; Röthl, A. Median-Joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]

| Endosymbiont Species | Syngen of Paramecium bursaria | ||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| Chlorella vulgaris | 2 | 10 | 4 | 1 | 1 |
| Chlorella variabilis | 1 | 4 | 2 | 1 | 1 |
| Chlorella sorokiniana | 0 | 3 | 1 | 0 | 0 |
| Micractinium conductrix | 3 | 7 | 0 | 0 | 0 |
| No. | Algal (Endosymbiont) Species | Algal (Endosymbiont) Strain | Paramecium bursaria (Host) Strain | Taxonomic Designation of the Host | Origin of the Host | GenBank Accession Number | References | ||
|---|---|---|---|---|---|---|---|---|---|
| 28S rDNA | 3′rpl36-5′infA | ITS1-5.8S-ITS2 | |||||||
| 1. | Chlorella vulgaris | CVG-SHT-56 | SHT-56 | R1 | Tajikistan | KX639563 | KX639603 | KX639535 | This study |
| 2. | Chlorella vulgaris | CVG-TR54-4 | TR54-4 | R2 | Tver, Russia | KX639564 | KX639604 | KX639536 | This study |
| 3. | Chlorella vulgaris | CVG-RA2-1 | RA2-1 | R2 | Altai Forelands, Russia | KX639562 | KX639602 | nd | This study |
| 4. | Chlorella vulgaris | CVG-MitR | MitR | R3 | Japan | KX639561 | KX639601 | KX639534 | This study |
| 5. | Chlorella vulgaris | CVG-JR-16 | JR-16 | R3 | Japan | KX639560 | KX639600 | nd | This study |
| 6. | Chlorella vulgaris | CVG-HKV19-12 | HKV19-12 | R3 | Khabarovsk, Russia | KM203671 | KM203663 | nd | [13] |
| 7. | Chlorella vulgaris | CVG-Bya129-5 | Bya129-5 | R2 | Yaroslavl, Russia | KX639559 | KX639598 | nd | This study |
| 8. | Chlorella vulgaris | CVG-BBR180-10 | BBR180-10 | R2 | Lake Baikal, Russia | KX639557 | KX639596 | KX639531 | This study |
| 9. | Chlorella vulgaris | CVG-BBR178-9 | BBR178-9 | R2 | Lake Baikal, Russia | KX639556 | KX639595 | KX639530 | This study |
| 10. | Chlorella vulgaris | CVG-AZ21-3 | AZ21-3 | R2 | Astrakhan Nature Reserve, Russia | KX639555 | KX639594 | nd | This study |
| 11. | Chlorella vulgaris | CVG-AZ20-1 | AZ20-1 | R5 | Astrakhan Nature Reserve, Russia | KX639554 | KX639593 | nd | This study |
| 12. | Chlorella vulgaris | CVG-AZ10-1 | AZ10-1 | R5 | Astrakhan Nature Reserve, Russia | KM203670 | KM203662 | KX639528 | [13], this study (ITS1-5.8S-ITS2) |
| 13. | Chlorella vulgaris | CVG-Yad1-g | Yad1-g | R3 | Yamaguchi, Japan | KX639565 | KX639605 | nd | This study |
| 14. | Chlorella vulgaris | CVG-Ard7 | Ard7 | R4 | Ardmore, USA | KX639552 | KX639591 | KX639526 | This study |
| 15. | Chlorella vulgaris | CVG-AZ7-14 | AZ7-14 | R2 | Astrakhan Nature Reserve, Russia | KX639553 | KX639592 | KX639527 | This study |
| 16. | Chlorella vulgaris | CVG-AB2-51 | AB2-51 | R4 | Boston, USA | KM203673 | KM203661 | nd | [13] |
| 17. | Chlorella vulgaris | CVG-GB15-2 | GB15-2 | R2 | Lake Loch Linnhe, Scotland | KX639551 | KX639599 | KX639525 | This study |
| 18. | Chlorella vulgaris | CVG-KZ-126 | KZ-126 | R2 | Kaliningrad, Russia | KM203672 | KM203660 | KX639533 | [13], this study (ITS1-5.8S-ITS2) |
| 19. | Chlorella vulgaris | CVG-BL15-3 | BL15-3 | R2 | Lake Baikal, Russia | KX639558 | KX639597 | KX639532 | This study |
| 20 | Chlorella vulgaris | CVG-B4-1 | B4-1 | R1 | Volgograd, Russia | KX639546 | KX639586 | KX639529 | This study |
| 21. | Chlorella variabilis | CVA-AZ8-2 | AZ8-2 | R2 | Astrakhan Nature Reserve, Russia | KX639544 | KX639584 | KX639520 | This study |
| 22. | Chlorella variabilis | CVA-IP | IP | R3 | Pisa, Italy | KX639549 | KX639589 | nd | This study |
| 23. | Chlorella variabilis | CVA-Ard10-3 | Ard10-3 | R4 | Ardmore, USA | KM203667 | KM203658 | nd | [13] |
| 24. | Chlorella variabilis | CVA-AZ20-4 | AZ20-4 | R2 | Astrakhan Nature Reserve, Russia | KX639545 | KX639585 | KX639521 | This study |
| 25. | Chlorella variabilis | CVA-Wien4a-12 | Wien4a-12 | R1 | Wien, Austria | KX639550 | KX639590 | nd | This study |
| 26. | Chlorella variabilis | CVA-B5-7 | B5-7 | R5 | Botanical Garden in St. Petersburg, Russia | KM203669 | KM203659 | KX639522 | [13], this study (ITS1-5.8S-ITS2) |
| 27. | Chlorella variabilis | CVA-KD64 | KD64 | R2 | Kamchatka, Russia | KM203668 | KM203657 | nd | [13] |
| 28. | Chlorella variabilis | CVA-HZ85-1 | HZ85-1 | R3 | Khanka Nature Reserve, Russia | KX639548 | KX639587 | KX639524 | This study |
| 29. | |||||||||
| 30. | Chlorella variabilis | CVA-GT-2 | GT-2 | R2 | Tübingen, Germany | KX639547 | KX639587 | KX639523 | This study |
| 31. | Chlorella sorokiniana | CS-BBR51-1 | BBR51-1 | R2 | Lake Baikal, Russia | KX639542 | KX639582 | nd | This study |
| 32. | Chlorella sorokiniana | CS-Cs2 | Cs2 | R3 | Shanghai, China | KX639543 | KX639583 | nd | This study |
| 33. | Chlorella sorokiniana | CS-11 231-2 | 11 231-2 | R2 | Kamchatka, Russia | KX639540 | KX639580 | nd | This study |
| 34. | Chlorella sorokiniana | CS-11 35-2 | 11 35-2 | R2 | Kamchatka, Russia | KX639541 | KX639581 | nd | This study |
| 35. | Micractinium conductrix | MC-11 42-2 | 11 42-2 | R2 | Kamchatka, Russia | KX639567 | KX639574 | nd | This study |
| 36. | Micractinium conductrix | MC-RN88-4 | RN88-4 | R2 | Tver, Russia | KX639570 | KX639577 | nd | This study |
| 37. | Micractinium conductrix | MC-4 231-1 | 4 231-1 | R2 | Kamchatka, Russia | KX639566 | KX639573 | KX639537 | This study |
| 38. | Micractinium conductrix | MC-MS-1 | MS-1 | R1 | St. Petersburg, Russia | KM203675 | KM203666 | KX639538 | [13], this study (ITS1-5.8S-ITS2) |
| 39. | Micractinium conductrix | MC-GB7-2 | GB7-2 | R2 | Lake Loch Linnhe, Scotland | KX639568 | KX639575 | nd | This study |
| 40. | Micractinium conductrix | MC-VM-14 | VM-14 | R2 | Valaam, Russia | KM203674 | KM203664 | nd | [13] |
| 41. | Micractinium conductrix | MC-PMP1-3-1 | PMP1-3-1 | R1 | St. Petersburg, Russia | KX639569 | KX639576 | nd | This study |
| 42. | Micractinium conductrix | MC-TR54-1 | TR54-1 | R1 | Tver, Russia | KX639572 | KX639579 | nd | This study |
| 43. | Micractinium conductrix | MC-SRB9-1 | SRB9-1 | R2 | River Danube, Serbia | KX639571 | KX639578 | KX639539 | This study |
| 44. | Micractinium conductrix | MC-TOS1-7 | TOS1-7 | R2 | Togliatti, Russia | KM203676 | KM203665 | nd | [13] |
| 45. | Micractinium inermum | NLP-F014 | nd | nd | nd | KF597304.1 | nd | nd | Unpublished data |
| 46. | Chlorella sorokiniana | UTEX 1665 | nd | nd | nd | KJ676113.1 | nd | nd | [14] |
| 47. | Micractinium sp. | KNUA029 | nd | nd | nd | KM243321.1 | nd | nd | [15] |
| 48. | Micractinium reisseri (conductrix) | SW1-ZK, (SW1) | nd | nd | Black Forest, Germany | AB437256.1 | nd | AB437244.1 | [10] |
| 49. | Micractinium sp. | MCWWW15 | nd | nd | nd | nd | nd | KP204593.1 | [16] |
| 50. | Micractinium sp. | MCWWW4 | nd | nd | nd | nd | nd | KP204582.1 | [16] |
| 51. | Micractinium sp. | MCWWW5 | nd | nd | nd | nd | nd | KP204583.1 | [16] |
| 52. | Micractinium sp. | MCWWW10 | nd | nd | nd | nd | nd | KP204588.1 | [16] |
| 53. | Micractinium sp. | MCWWW11 | nd | nd | nd | nd | nd | KP204589.1 | [16] |
| 54. | Micractinium sp. | KNUA032 | nd | nd | nd | nd | nd | KM243324.1 | [15] |
| 55. | Micractinium reisseri (conductrix) | EdL_Cl1_MAF | nd | nd | nd | nd | nd | KF887345.1 | Unpublished data |
| 56. | SAG 13.81 | nd | nd | nd | nd | nd | FM205866.1 | [17] | |
| 57. | Chlorella sp. | CB4 | nd | nd | nd | nd | nd | JQ710683.1 | Unpublished data |
| 58. | Chlorella sp. | IFRPD | nd | nd | nd | nd | nd | AB260898.1 | [8] |
| 59. | Chlorella sorokiniana | KLL-G018 | nd | nd | nd | nd | nd | KP726221.1 | [18] |
| 60. | Chlorella sorokiniana | KU219 | nd | nd | nd | nd | nd | KM061463.1 | Unpublished data |
| 61. | Chlorella variabilis | CCAP 211/84 | nd | nd | nd | nd | nd | FN298923.1 | [7] |
| 62. | Chlorella variabilis | SAG 211-6 | nd | nd | nd | nd | nd | FM205849.1 | [17] |
| 63. | Chlorella variabilis | EdL_Cl2_3NB | nd | nd | nd | nd | nd | KF887350.1 | Unpublished data |
| 64. | Chlorella vulgaris | DRL3 | nd | nd | nd | nd | nd | JX139000.1 | Unpublished data |
| 65. | Coccomyxa chodatii | SAG: 216-2 | nd | nd | nd | HG972989.1 | nd | nd | [19] |
| 66. | Stigeoclonium tenue | CCAP 477/11A | nd | nd | nd | HF920680.1 | nd | nd | [20] |
| 67. | Stigeoclonium variabile | CCAP 477/13 | nd | nd | nd | HF920679.1 | nd | nd | [20] |
| 68. | Parachlorella kessleri | SAG: 211-11g | nd | nd | nd | nd | X65099.1 | nd | [21] |
| 69. | Actinastrum hantzschii | SAG 2015 | nd | nd | nd | nd | nd | FM205841.1 | [18] |
| DNA Fragment | Primer | Sequence 5′-3′ | References |
|---|---|---|---|
| ITS1-5.8S rDNA-ITS2 | ITS1 | TCCGTAGGTGAACCTGCGG | [33] |
| ITS1F | AATCTATCGAATCCACTTTGGTAAC | Designed in the present study | |
| ITS2R | CTGCTAGGTCTCCAGCAAAG | Designed in the present study | |
| 28S rDNA frgment | HLR0F | GGCAAGACTACCCGCTGAA | [8] |
| HLR4R | TTTCAAGACGGGCCGATT | [8] | |
| 3′rpl36-5′infA genes | UCP2F | CCTTGWCKTTGTTTATGTTTKGG | [36] |
| UCP2R | GCTCATGTYTCHGGBAAAATWCG | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greczek-Stachura, M.; Leśnicka, P.Z.; Tarcz, S.; Rautian, M.; Możdżeń, K. Genetic Diversity of Symbiotic Green Algae of Paramecium bursaria Syngens Originating from Distant Geographical Locations. Plants 2021, 10, 609. https://doi.org/10.3390/plants10030609
Greczek-Stachura M, Leśnicka PZ, Tarcz S, Rautian M, Możdżeń K. Genetic Diversity of Symbiotic Green Algae of Paramecium bursaria Syngens Originating from Distant Geographical Locations. Plants. 2021; 10(3):609. https://doi.org/10.3390/plants10030609
Chicago/Turabian StyleGreczek-Stachura, Magdalena, Patrycja Zagata Leśnicka, Sebastian Tarcz, Maria Rautian, and Katarzyna Możdżeń. 2021. "Genetic Diversity of Symbiotic Green Algae of Paramecium bursaria Syngens Originating from Distant Geographical Locations" Plants 10, no. 3: 609. https://doi.org/10.3390/plants10030609
APA StyleGreczek-Stachura, M., Leśnicka, P. Z., Tarcz, S., Rautian, M., & Możdżeń, K. (2021). Genetic Diversity of Symbiotic Green Algae of Paramecium bursaria Syngens Originating from Distant Geographical Locations. Plants, 10(3), 609. https://doi.org/10.3390/plants10030609






