Pratylenchus penetrans Parasitizing Potato Crops: Morphometric and Genetic Variability of Portuguese Isolates
Abstract
1. Introduction
2. Results
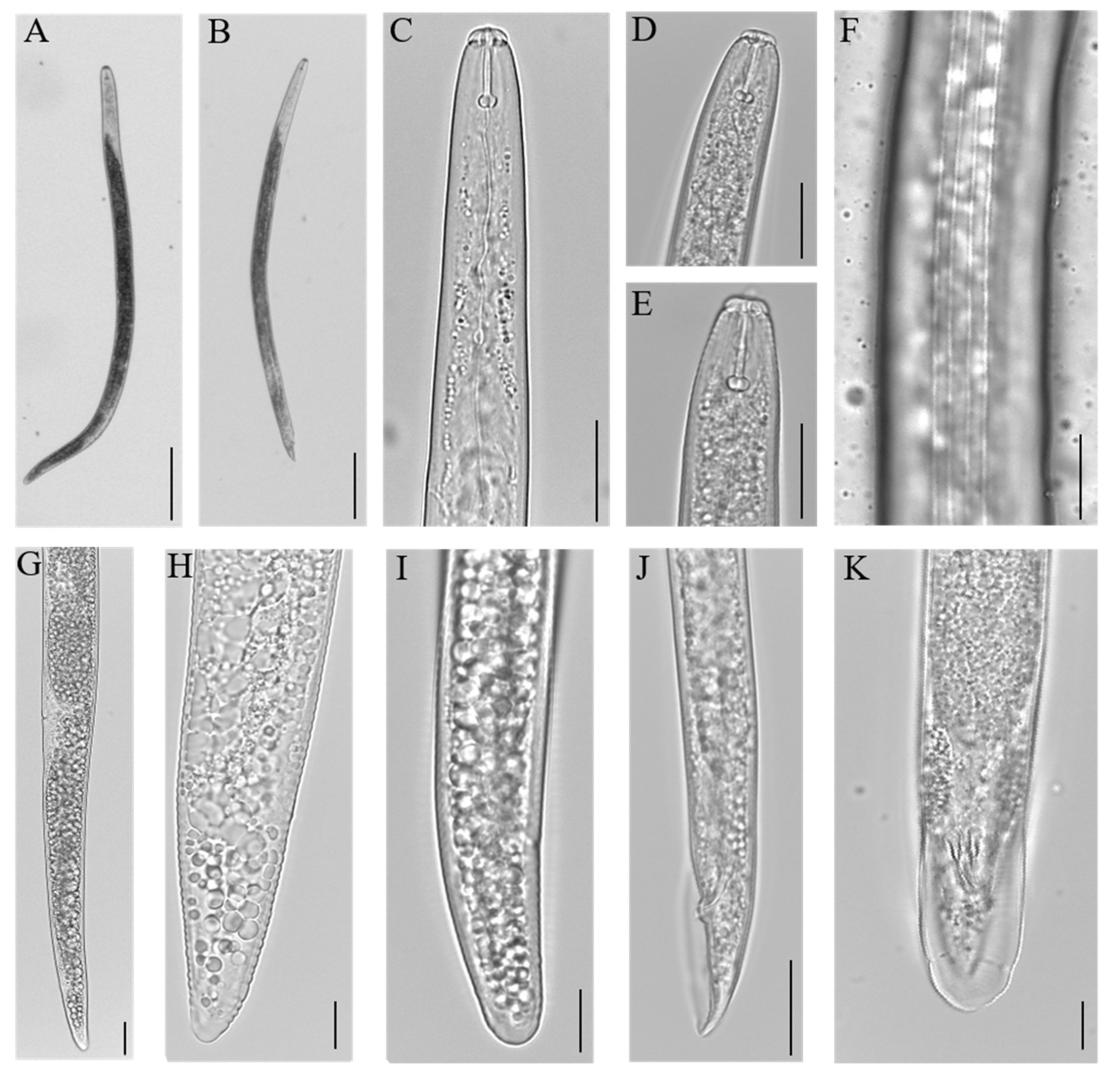
2.1. Morphology of P. penetrans Portuguese Isolates
2.1.1. Female
2.1.2. Male
2.2. Morphometry of P. penetrans Portuguese Isolates
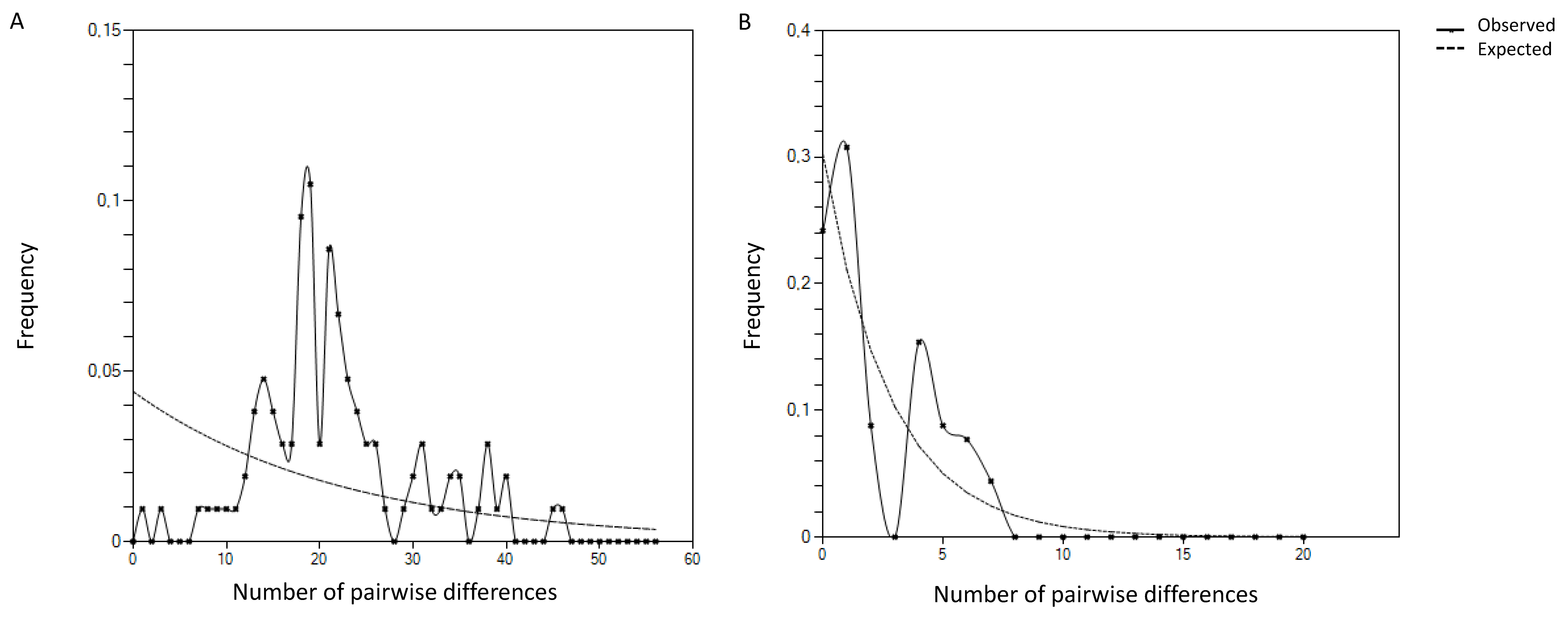
2.3. Genetic Diversity of P. penetrans Portuguese Isolates
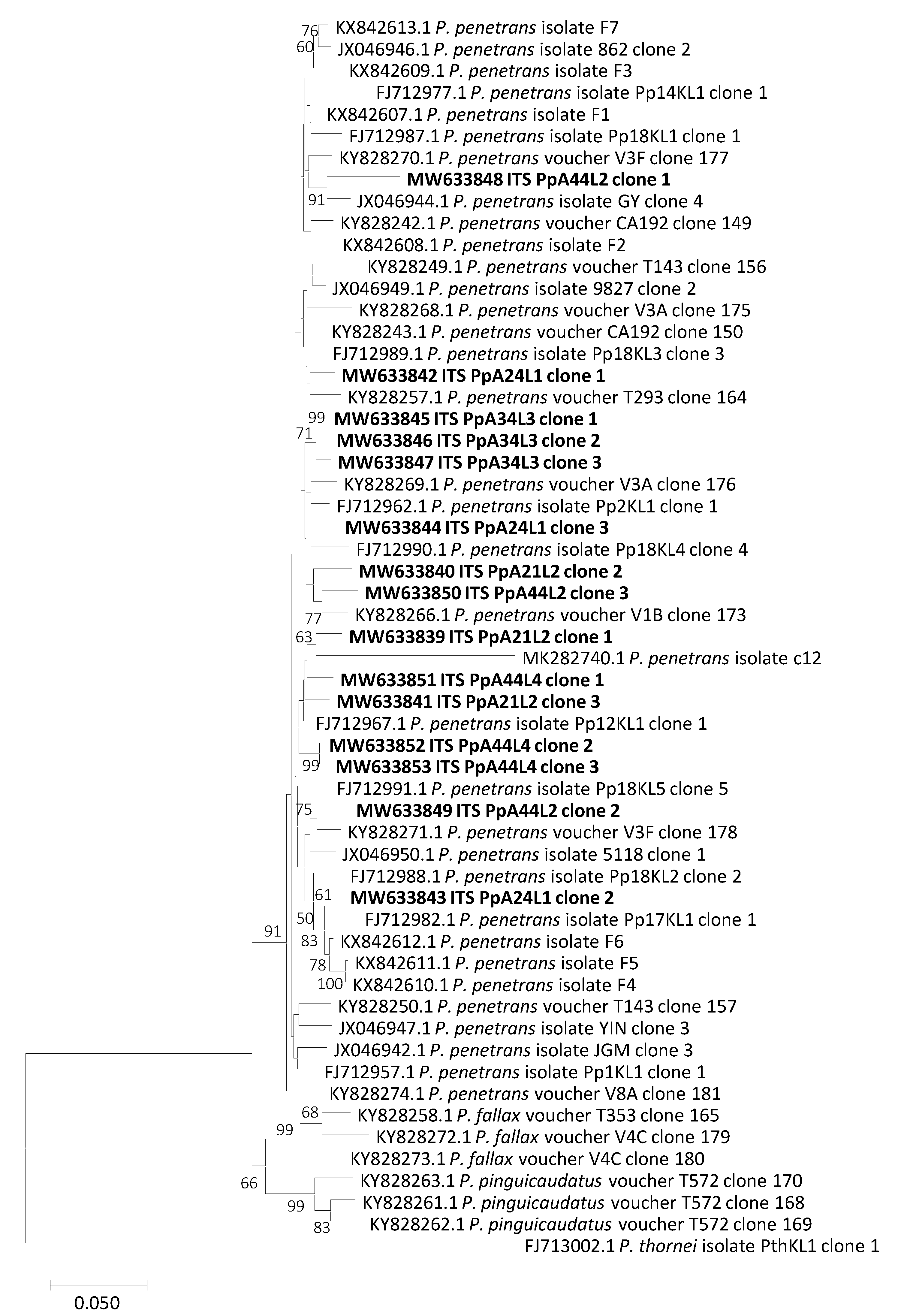
2.4. Phylogenetic and Molecular Evolution Relationships
3. Discussion
4. Materials and Methods
4.1. Pratylenchus penetrans Isolates
4.2. Morphometrical Analyses
4.3. DNA Extraction, PCR, Cloning and Sequencing
4.4. Sequence Analysis
4.5. Phylogenetic and Molecular Evolutionary Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cobb, N.A. A new parasitic nema found infesting cotton and potatoes. J. Agric. Res. 1917, 11, 27–33. [Google Scholar]
- Filipjev, I.N.; Stekhoven, J.H.S. A Manual of Agricultural Helminthology; Brill: Leiden, The Netherlands, 1941. [Google Scholar]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Rowe, R.C.; Riedel, R.M.; Martin, M.J. Synergistic interactions between Verticillium dahliae and Pratylenchus penetrans in potato early dying disease. Phytopathology 1985, 75, 412–418. [Google Scholar] [CrossRef]
- Kimpinski, J. Root lesion nematodes in potatoes. Am. Potato J. 1979, 56, 79–86. [Google Scholar] [CrossRef]
- Brown, M.J.; Riedel, R.M.; Rowe, R.C. Species of Pratylenchus associated with Solanum tuberosum cv. Superior in Ohio. J. Nematol. 1980, 12, 189–192. [Google Scholar] [PubMed]
- Olthof, T.H.A.; Anderson, R.V.; Squire, S. Plant-parasitic nematodes associated with potatoes (Solanum tuberosum L.) in Simcoe County, Ontario. Can. J. Plant Pathol. 1982, 4, 389–391. [Google Scholar] [CrossRef]
- MacGuidwin, A.E.; Rouse, D.I. Role of Pratylenchus penetrans in potato early dying disease of Russet Burbank potato. Phytopathology 1990, 80, 1077–1082. [Google Scholar] [CrossRef]
- Ingham, R.E.; Hamm, P.B.; Riga, E.; Merrifield, K.J. First report of stunting and root rot of potato associated with Pratylenchus penetrans in the Columbia Basin of Washington. Plant Dis. 2005, 89, 207. [Google Scholar] [CrossRef]
- Holgado, R.; Skau, K.A.O.; Magnusson, C. Field damage in potato by lesion nematode Pratylenchus penetrans, its association with tuber symptoms and its survival in storage. Nematol. Mediterr. 2009, 37, 25–39. [Google Scholar]
- Hafez, S.L.; Sundararaj, P.; Handoo, Z.A.; Siddiqi, M.R. Occurrence and distribution of nematodes in Idaho crops. Int. J. Nematol. 2010, 20, 91–98. [Google Scholar]
- Esteves, I.; Maleita, C.; Abrantes, I. Root-lesion and root-knot nematodes parasitizing potato. Eur. J. Plant Pathol. 2015, 141, 397–406. [Google Scholar] [CrossRef]
- Roman, J.; Hirschmann, H. Morphology and morphometrics of six species of Pratylenchus. J. Nematol. 1969, 1, 363–386. [Google Scholar] [PubMed]
- Tarte, R.; Mai, W.F. Morphological variation in Pratylenchus penetrans. J. Nematol. 1976, 8, 185–195. [Google Scholar] [PubMed]
- Janssen, T.; Karssen, G.; Orlando, V.; Subbotin, S.A.; Bert, W. Molecular characterization and species delimiting of plant-parasitic nematodes of the genus Pratylenchus from the Penetrans group (Nematoda: Pratylenchidae). Mol. Phylogenet. Evol. 2017, 117, 30–48. [Google Scholar] [CrossRef]
- Waeyenberge, L.; Ryss, A.; Moens, M.; Pinochet, J.; Vrain, T. Molecular characterisation of 18 Pratylenchus species using rDNA restriction fragment length polymorphism. Nematology 2000, 2, 135–142. [Google Scholar] [CrossRef]
- De Luca, F.; Reyes, A.; Troccoli, A.; Castillo, P. Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. Eur. J. Plant Pathol. 2011, 130, 415–426. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Gu, J.; Zhang, R.; Huang, G.; Wang, X.; Li, H.M.; Sun, J. Phylogenetic analysis of Pratylenchus (Nematoda, Pratylenchidae) based on ribosomal internal transcribed spacers (ITS) and D2-D3 expansion segments of 28S rRNA gene. Acta Zootaxonomica Sin. 2012, 37, 687–693. [Google Scholar]
- Subbotin, S.A.; Ragsdale, E.J.; Mullens, T.; Roberts, P.A.; Mundo-Ocampo, M.; Baldwin, J.G. A phylogenetic framework for root lesion nematodes of the genus Pratylenchus (Nematoda): Evidence from 18S and D2–D3 expansion segments of 28S ribosomal RNA genes and morphological characters. Mol. Phylogenet. Evol. 2008, 48, 491–505. [Google Scholar] [CrossRef]
- Van Megen, H.; van den Elsen, S.; Holterman, M.; Karssen, G.; Mooyman, P.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. A phylogenetic tree of nematodes based on about 1200 full-length small subunit ribosomal DNA sequences. Nematology 2009, 11, 927–950. [Google Scholar] [CrossRef]
- Al-Banna, L.; Ploeg, A.T.; Williamson, V.M.; Kaloshian, I. Discrimination of six Pratylenchus species using PCR and species-specific primers. J. Nematol. 2004, 36, 142–146. [Google Scholar]
- Handoo, Z.A.; Carta, L.K.; Skantar, A.M. Morphological and molecular characterisation of Pratylenchus arlingtoni n. sp., P. convallariae and P. fallax (Nematoda: Pratylenchidae). Nematol. 2001, 3, 607–618. [Google Scholar] [CrossRef]
- Palomares-Rius, J.E.; Guesmi, I.; Horrigue-Raouani, N.; Cantalapiedra-Navarrete, C.; Liébanas, G.; Castillo, P. Morphological and molecular characterisation of Pratylenchus oleae n. sp. (Nematoda: Pratylenchidae) parasitizing wild and cultivated olives in Spain and Tunisia. Eur. J. Plant Pathol. 2014, 140, 53–67. [Google Scholar] [CrossRef]
- Troccoli, A.; Subbotin, S.A.; Chitambar, J.J.; Janssen, T.; Waeyenberge, L.; Stanley, J.D.; Duncan, L.; Agudelo, P.; Uribe, G.; Franco, J.; et al. Characterisation of amphimictic and parthenogenetic populations of Pratylenchus bolivianus Corbett, 1983 (Nematoda: Pratylenchidae) and their phylogenetic relationships with closely related species. Nematology 2016, 18, 651–678. [Google Scholar] [CrossRef]
- Singh, P.R.; Nyiragatare, A.; Janssen, T.; Couvreur, M.; Decraemer, W.; Bert, W. Morphological and molecular characterisation of Pratylenchus rwandae n. sp. (Tylenchida: Pratylenchidae) associated with maize in Rwanda. Nematology 2018, 20, 781–794. [Google Scholar] [CrossRef]
- Divsalar, N.; Shokoohi, E.; Hoseinipour, A.; Mashela, P. Molecular and morphological variation of the root-lesion nematode Pratylenchus neglectus. Biologia 2019, 74, 257–267. [Google Scholar] [CrossRef]
- Mirghasemi, S.N.; Fanelli, E.; Troccoli, A.; Jamali, S.; Sohani, M.M.; De Luca, F. Molecular variability of the root-lesion nematode, Pratylenchus loosi (Nematoda: Pratylenchidae), from tea in Iran. Eur. J. Plant Pathol. 2019, 155, 557–569. [Google Scholar] [CrossRef]
- De Luca, F.; Troccoli, A.; Duncan, L.W.; Subbotin, S.A.; Waeyenberge, L.; Coyne, D.L.; Brentu, F.C.; Inserra, R.N. Pratylenchus speijeri n. sp. (Nematoda: Pratylenchidae), a new root-lesion nematode pest of plantain in West Africa. Nematology 2012, 14, 987–1004. [Google Scholar] [CrossRef]
- Fanelli, E.; Troccoli, A.; Capriglia, F.; Lucarelli, G.; Vovlas, N.; Greco, N.; De Luca, F. Sequence variation in ribosomal DNA and in the nuclear hsp90 gene of Pratylenchus penetrans (Nematoda: Pratylenchidae) populations and phylogenetic analysis. Eur. J. Plant Pathol. 2018, 152, 355–365. [Google Scholar] [CrossRef]
- Carta, L.K.; Skantar, A.M.; Handoo, Z.A. Molecular, morphological and thermal characters of 19 Pratylenchus spp. and relatives using the D3 segment of the nuclear LSU rRNA gene. Nematropica 2001, 31, 193–208. [Google Scholar]
- Loof, P.A. Taxonomic studies on the genus Pratylenchus (Nematoda). Tijdschrift Plantenziekten 1960, 66, 29–90. [Google Scholar]
- Rusinque, L.C.M.; Vicente, C.; Inácio, M.L.S.; Nóbrega, F.; Camacho, M.J.; Lima, A.; Ramos, A.P. First report of Pratylenchus penetrans (Nematoda: Pratylenchida) associated with Amaryllis (Hippeastrum × hybridum), in Portugal. Plant Dis. 2020, 1–5. [Google Scholar] [CrossRef]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Andaloussi, F.A.; Moens, M. Diversity of root-lesion nematodes (Pratylenchus spp.) associated with wheat (Triticum aestivum and T. durum) in Morocco. Nematology 2016, 18, 781–801. [Google Scholar] [CrossRef]
- Machado, A.C.Z.; Siqueira, K.M.S.; Ferraz, L.C.C.B.; Inomoto, M.M.; Bessi, R.; Harakava, R.; Oliveira, C.M.G. Characterization of Brazilian populations of Pratylenchus brachyurus using morphological and molecular analyses. Trop. Plant Pathol. 2015, 40, 102–110. [Google Scholar] [CrossRef]
- Townshend, J.L. Morphological observations of Pratylenchus penetrans from celery and strawberry in southern Ontario. J. Nematol. 1991, 23, 205. [Google Scholar] [PubMed]
- Boisseau, M.; Sarah, J.L. In vitro rearing of Pratylenchidae nematodes on carrot discs. Fruits 2008, 63, 307–310. [Google Scholar] [CrossRef]
- Derycke, S.; Remerie, T.; Vierstraete, A.; Backeljau, T.; Vanfleteren, J.; Vincx, M.; Moens, T. Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Mar. Ecol. Prog. Ser. 2005, 300, 91–103. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Wessa, P. Kendall tau Rank Correlation (v1.0.13) in Free Statistics Software (v1.2.1). Office for Research Development and Education, 2017. Available online: https://www.wessa.net/rwasp_kendall.wasp/ (accessed on 10 January 2021).

| Character | Isolate | Loof (1960) | ||||
|---|---|---|---|---|---|---|
| PpA21L2 | PpA24L1 | PpA34L3 | PpA44L2 | PpA44L4 | ||
| L * | 630.3 ± 59.4 a,b,c (527.5–720.0) | 723.5 ± 93.9 c,d (603.3–858.8) | 600.8 ± 86.3 a,b (522.1–812.1) | 712.2 ± 61.0 c,d (615.5–802.5) | 695.6 ± 82.4 b,c,d (605.3–869.5) | 343–811 |
| Stylet length | 16.9 ± 0.8 a (16.0–17.5) | 16.2 ± 0.6 a (15.4–17.2) | 16.9 ± 1.2 a (16.7–17.9) | 16.2 ± 0.6 a (15.2–17.3) | 16.5 ± 0.7 a (15.9–17.6) | 15–17 |
| Anterior end to medium bulb | 57.2 ± 3.6 a (50.7–62.3) | 63.5 ± 7.6 a (49.4–71.8) | 61.8 ± 5.1 a (53.7–70.5) | 61.9 ± 2.7 a (56.0–66.2) | 60.9 ± 5.3 a (49.4–67.9) | |
| Anterior end to esophageal gland lobe | 125.4 ± 15.6 a (103.4–147.7) | 136.2 ± 15.3 a (108.4–156.7) | 137.3 ± 10.5 a (126.2–155.2) | 134.0 ± 9.2 a (120.7–151.9) | 124.7 ± 21.7 a (82.4–148.0) | |
| Anterior end to excretory pore | 89.0 ± 9.1 a,b (78.4–105.6) | 98.7 ± 9.3 b,c,d (81.6–113.3) | 91.4 ± 9.7 a,b,c (83.9–116.8) | 104.7 ± 4.3 c,d (96.0–111.7) | 92.9 ± 15.3 a,b,c (71.7–114.5) | |
| Anterior end to vulva | 510.6 ± 45.5 a (423.7–575.9) | 586.1 ± 77.5 a (485.6–695.8) | 496.0 ± 77.8 a (427.0–697.0) | 574.5 ± 53.9 a (474.2–637.5) | 558.9 ± 77.6 a (475.9–724.8) | |
| Maximum body width | 28.2 ± 5.1 a (21.9–39.2) | 34.1 ± 6.7 a (23.5–43.8) | 30.0 ± 3.7 a (23.6–34.2) | 31.2 ± 4.5 a (23.2–36.4) | 33.0 ± 5.9 a (24.2–43.4) | |
| Body width at anus | 14.8 ± 1.7 a,b (11.6–17.3) | 17.5 ± 2.5 b,c,d (13.2–21.0) | 15.6 ± 1.8 a,b,c (13.5–19.2) | 18.2 ± 2.8 c,d (13.2–24.0) | 16.9 ± 2.3b,c,d (13.9–20.2) | |
| Vulva-anus | 87.6 ± 16.4 a,b,c (64.9–118.7) | 103.5 ± 17.2 c,d (72.7–127.7) | 79.6 ± 14.6 a,b (58.9–111.0) | 104.6 ± 18.0 c,d (80.9–141.7) | 94.1 ± 11.3 b,c,d (72.9–109.0) | |
| Tail | 29.4 ± 4.9 a,b (19.0–35.8) | 31.8 ± 2.6 a,b,c (27.7–35.6) | 23.7 ± 3.9 d (19.5–31.4) | 34.0 ± 4.2 b,c (26.6–41.0) | 32.8 ± 3.9 a,b,c (25.9–38.4) | |
| V * | 81.1 ± 2.2 a (75.7–83.7) | 81.0 ± 1.7 a (77.7–83.9) | 82.5 ± 2.2 a (78.3–83.9) | 80.6 ± 2.0 a (77.0–83.5) | 80.3 ± 4.0 a (79.8–83.4) | 75–84 |
| a * | 22.8 ± 3.5 a (17.4–28.3) | 21.6 ± 3.0 a (18.5–26.9) | 20.1 ± 2.3 a (16.1–23.8) | 23.1 ± 3.1 a (20.1–29.3) | 21.4 ± 2.4 a (16.5–25.0) | 19–32 |
| b’ * | 5.1 ± 0.6 a,b,c (4.3–6.4) | 5.3 ± 0.5 c (4.8–6.3) | 4.4 ± 0.4 a (3.8–5.2) | 5.3 ± 0.5 c (4.6–6.0) | 5.9 ± 2.0 c (4.3–10.5) | |
| c * | 21.9 ± 3.3 a (18.1–29.6) | 22.9 ± 3.2 a (17.6–27.2) | 25.6 ± 2.8 b (21.1–28.9) | 21.2 ± 2.3 a (18.5–25.6) | 21.3 ± 2.3 a (18.2–24.6) | 15–24 |
| c’ * | 2.0 ± 0.3 a (1.4–2.5) | 1.9 ± 0.4 a (1.5–2.7) | 1.5 ± 0.3 b (1.2–1.9) | 1.9 ± 0.4 a (1.4–2.4) | 2.0 ± 0.3 a (1.5–2.4) | |
| Character | Isolate | Loof (1960) | ||||
|---|---|---|---|---|---|---|
| PpA21L2 | PpA24L1 | PpA34L3 | PpA44L2 | PpA44L4 | ||
| L * | 535.0 ± 30.7 a (483.3–582.4) | 582.6 ± 35.0 b (529.0–629.4) | 522.0 ± 32.2 a (470.5–580.5) | 602.6 ± 36.0 b (531.5–670.1) | 536.0 ± 17.9 a (508.2–570.0) | 305–574 |
| Stylet length | 15.8 ± 0.4 a (15.3–16.7) | 15.3 ± 0.4 a (14.9–15.7) | 15.7 ± 0.6 a (15.0–16.5) | 15.6 ± 0.6 a (14.8–16.7) | 15.9 ± 1.1 a (14.4–17.5) | |
| Anterior end to medium bulb | 57.5 ± 3.1 a,b (52.7–62.9) | 53.6 ± 4.6 b,c (45.7–59.2) | 59.5 ± 3.4 a,b (54.6–63.7) | 63.7 ± 4.4 d (54.9–71.5) | 56.0 ± 3.9 a,b,c (49.5–60-5) | |
| Anterior end to esophageal gland lobe | 124.0 ± 12.9 a (105.2–149.2) | 122.3 ± 6.6 a (110.9–131.2) | 122.5 ± 8.0 a (110.7–137.9) | 127.7 ± 10.2 a (108.5–144.0) | 127.9 ± 9.5 a (112.0–146.2) | |
| Anterior end to excretory pore | 85.0 ± 5.0 a (79.0–92.6) | 83.2 ± 6.9 a (71.7–94.2) | 84.5 ± 7.7 a (72.3–97.6) | 94.9 ± 6.0 b (81.7–104.1) | 87.1 ± 5.7 a (77.0–95.9) | |
| Maximum body width | 21.9 ± 2.4 a (18.2–27.1) | 20.8 ± 1.7 a (17.7–23.5) | 21.3 ± 1.8 a (19.1–25.0) | 21.2 ± 1.7 a (19.2–24.4) | 19.8 ± 4.3 a (8.6–23.2) | |
| Body width at anus | 14.0 ± 1.4 a (12.0–15.7) | 13.3 ± 0.7 a (12.4–14.6) | 13.3 ± 0.7 a (12.2–14.9) | 14.3 ± 1.2 a (12.3–15.8) | 13.8 ± 0.7 a (12.9–14.8) | |
| Spicule | 16.2 ± 0.8 a (15.2–17.5) | 15.8 ± 1.5 a (13.8–18.0) | 19.1 ± 1.6 b (16.6–21.6) | 18.6 ± 1.7 b (15.9–21.9) | 16.7 ± 1.4 a (14.4–18.5) | 14–17 |
| Tail | 25.6 ± 2.9 a,b (22.4–32.7) | 29.0 ± 4.1 b,c (21.7–35.4) | 21.9 ± 2.6 d (16.1–24.9) | 28.2 ± 3.8 a,b,c (22.5–36.0) | 27.4 ± 2.8 a,b,c (23.6–31.8) | |
| a * | 24.6 ± 2.5 a (19.8–29.4) | 28.2 ± 2.6 b (25.3–34.4) | 24.6 ± 2.4 a (19.9–27.1) | 28.5 ± 1.6 b (26.8–30.5) | 29.3 ± 11.9 b (22.8–62.6) | 23–34 |
| b’ * | 4.4 ± 0.5 a (3.4–4.9) | 4.8 ± 0.4 b (4.2–5.5) | 4.3 ± 0.2 a (4.0–4.6) | 4.7 ± 0.2 b (4.4–5.1) | 4.2 ± 0.4 a (3.7–4.8) | |
| c * | 21.1 ± 2.5 a (16.4–24.3) | 20.4 ± 2.5 a (15.6–24.4) | 24.2 ± 3.4 b (20.7–31.4) | 21.6 ± 1.9 a (18.6–24.1) | 19.7 ± 1.9 a (16.9–22.8) | 16–22 |
| c’ * | 1.8 ± 0.2 a,b (1.6–2.2) | 2.2 ± 0.4 b,c (1.5–2.7) | 1.6 ± 0.2 a (1.2–2.0) | 2.0 ± 0.3 a,b,c (1.5–2.4) | 2.0 ± 0.3 a,b,c (1.7–2.5) | |
| Character | Isolate | Inter-Isolate Coefficient of Variability (%) | ||||
|---|---|---|---|---|---|---|
| PpA21L2 | PpA24L1 | PpA34L3 | PpA44L2 | PpA44L4 | ||
| L * | 9.4 | 13.0 | 14.4 | 8.6 | 11.8 | 8.0 |
| Stylet length | 4.6 | 3.9 | 7.2 | 3.9 | 4.5 | 2.2 |
| Anterior end to medium bulb | 6.4 | 11.9 | 8.2 | 4.4 | 8.7 | 3.8 |
| Anterior end to esophageal gland lobe | 12.5 | 11.2 | 7.6 | 6.9 | 17.4 | 4.6 |
| Anterior end to excretory pore | 10.3 | 9.5 | 10.6 | 4.1 | 16.5 | 6.6 |
| Anterior end to vulva | 8.9 | 13.2 | 15.7 | 9.4 | 13.9 | 7.3 |
| Maximum body width | 18.2 | 19.8 | 12.4 | 14.4 | 17.9 | 7.5 |
| Body width at anus | 11.2 | 14.5 | 11.3 | 15.3 | 13.4 | 8.4 |
| Vulva-anus | 18.7 | 16.6 | 18.3 | 17.2 | 12.1 | 11.3 |
| Tail | 16.6 | 8.3 | 16.5 | 12.4 | 12.0 | 13.5 |
| V * | 2.8 | 2.0 | 2.7 | 2.5 | 5.0 | 1.0 |
| a * | 15.3 | 13.8 | 11.3 | 13.2 | 11.4 | 5.5 |
| b’ * | 12.7 | 8.5 | 9.1 | 9.6 | 33.5 | 10.5 |
| c * | 14.9 | 13.9 | 10.8 | 10.9 | 10.6 | 8.1 |
| c’ * | 16.2 | 19.2 | 17.1 | 19.2 | 14.3 | 10.1 |
| Character | Isolate | Inter-Isolate Coefficient of Variability (%) | ||||
|---|---|---|---|---|---|---|
| PpA21L2 | PpA24L1 | PpA34L3 | PpA44L2 | PpA44L4 | ||
| L * | 5.7 | 6.0 | 6.2 | 6.0 | 3.3 | 6.3 |
| Stylet length | 2.7 | 2.8 | 3.6 | 3.9 | 6.7 | 1.4 |
| Anterior end to medium bulb | 5.4 | 8.5 | 5.7 | 6.9 | 7.0 | 6.6 |
| Anterior end to esophageal gland lobe | 10.4 | 5.4 | 6.5 | 8.0 | 7.5 | 2.2 |
| Anterior end to excretory pore | 5.8 | 8.3 | 9.1 | 6.3 | 6.5 | 5.4 |
| Maximum body width | 11.0 | 8.1 | 8.3 | 8.1 | 21.7 | 3.7 |
| Body width at anus | 9.9 | 5.6 | 5.3 | 8.4 | 5.0 | 3.3 |
| Spicule | 4.9 | 9.3 | 8.3 | 8.9 | 8.4 | 8.7 |
| Tail | 11.3 | 14.2 | 12.0 | 13.4 | 10.2 | 10.7 |
| a * | 10.3 | 9.2 | 9.6 | 5.6 | 40.7 | 8.3 |
| b’ * | 10.7 | 8.1 | 4.5 | 4.4 | 8.7 | 5.9 |
| c * | 11.6 | 12.2 | 13.9 | 8.9 | 9.6 | 8.0 |
| c’ * | 10.2 | 16.4 | 13.3 | 13.6 | 12.5 | 10.5 |
| Isolate | Genomic Region | No. of Clones | Sequences Length (bp) | S * | Eta * | No. of Haplotypes | Hd * (Standard Deviation) | Pi * (Standard Deviation) | K * |
|---|---|---|---|---|---|---|---|---|---|
| PpA21L2 | ITS | 3 | 677; 683; 673 | 35 | 35 | 3 | 1.000 (0.272) | 0.03488 (0.00254) | 23.333 |
| COI | 2 | 393 | 2 | 2 | 2 | 1.000 (0.500) | 0.00509 (0.00964) | 2.000 | |
| PpA24L1 | ITS | 3 | 676; 673; 671 | 38 | 42 | 3 | 1.000 (0.272) | 0.03992 (0.01097) | 26.667 |
| COI | 3 | 393 | 1 | 1 | 2 | 0.667 (0.314) | 0.00170 (0.00080) | 0.667 | |
| PpA34L3 | ITS | 3 | 671; 671; 673 | 10 | 10 | 3 | 0.667 (0.314) | 0.00997 (0.00425) | 6.667 |
| COI | 3 | 393 | 1 | 1 | 2 | 1.000 (0.272) | 0.00170 (0.00080) | 0.667 | |
| PpA44L2 | ITS | 3 | 673; 675; 678 | 60 | 62 | 3 | 1.000 (0.272) | 0.06115 (0.01759) | 40.667 |
| COI | 3 | 393 | 0 | 0 | 1 | 0.000 (0.000) | 0.0000 (0.0000) | 0.000 | |
| PpA44L4 | ITS | 3 | 676; 674; 674 | 20 | 20 | 3 | 1.000 (0.272) | 0.00339 (0.00160) | 13.333 |
| COI | 3 | 393 | 2 | 2 | 2 | 0.667 (0.314) | 0.01990 (0.00765) | 1.333 | |
| All 5 isolates | ITS | 15 | - | 99 | 109 | 15 | 1.000 (0.024) | 0.03350 (0.00414) | 21.743 |
| COI | 14 | - | 10 | 10 | 7 | 0.758 (0.116) | 0.00587 (0.00164) | 2.308 |
| Isolate | GPS Coordinates | Locality | Accession (ITS) | Accession (COI) |
|---|---|---|---|---|
| PpA21L2 | 41°16′18″ N 8°41′23″ W | Aveleda, Maia, Portugal | MW633839 MW633840 MW633841 | MW660605 MW660606 - |
| PpA24L1 | 41°15′27″ N 8°40′30″ W | Vila Nova da Telha, Maia, Portugal | MW633842 MW633843 MW633844 | MW660607 MW660608 MW660609 |
| PpA34L3 | 40°37′28″ N 8°38′19″ W | Aveiro, Portugal | MW633845 MW633846 MW633847 | MW660610 MW660611 MW660612 |
| PpA44L2 | 40°23′25.2″ N 8°30′07.7″ W | Coimbra, Portugal | MW633848 MW633849 MW633850 | MW660613 MW660614 MW660615 |
| PpA44L4 | 40°23′25.2″ N 8°30′07.7″ W | Coimbra, Portugal | MW633851 MW633852 MW633853 | MW660616 MW660617 MW660618 |
| Species | Isolate | Region | Host | Acession Number | |
|---|---|---|---|---|---|
| ITS | COI | ||||
| Pratylenchus fallax | T353 | The Netherlands, Doornenburg | Malus pumila | KY828258 | KY816988 |
| P. fallax | V4 C | The Netherlands, Ysbrechtum | Vitis vinifera | KY828272;KY828273 | KY816938 |
| P. penetrans | V3 A | The Netherlands, Baarlo | M. pumila | KY828268;KY828269 | KY816941 |
| P. penetrans | V8 A | The Netherlands, Baarlo | M. pumila | KY828274 | KY816936 |
| P. penetrans | V1B | The Netherlands, Meijel | M. pumila | KY828266 | KY816942 |
| P. penetrans | V3 F | The Netherlands, Nagele | M. pumila | KY828270; KY828271 | KY816940 |
| P. penetrans | N3678 | USA, Minnesota | Zea mays | - | MK877982 |
| P. penetrans | N6260 | USA, Fairbanks County | Paeonia sp. | - | MK877984 |
| P. penetrans | N7126 | USA, Otoe County | Malus sp. | - | MK877987 |
| P. penetrans | N7198 | USA, Idaho | Solanum tuberosum | - | MK877988 |
| P. penetrans | N7199 | USA, Idaho | S. tuberosum | - | MK877989 |
| P. penetrans | N7200 | USA, Idaho | S. tuberosum | - | MK877990 |
| P. penetrans | N7201 | USA, Idaho | S. tuberosum | - | MK877991 |
| P. penetrans | N7202 | USA, Idaho | S. tuberosum | - | MK877992 |
| P. penetrans | P148032 | USA, Portage County | S. tuberosum | - | MK877998 |
| P. penetrans | P147033 | USA, Portage County | S. tuberosum | - | MK877995 |
| P. penetrans | P147034 | USA, Portage County | S. tuberosum | - | MK877996 |
| P. penetrans | P147035 | USA, Portage County | S. tuberosum | - | MK877997 |
| P. penetrans | c12 | Canada, Kentville | Prunus sp. | MK282740 | - |
| P. penetrans | 862 | Chile | Lillium sp. | JX046946 | - |
| P. penetrans | GY | France | Prunus sp. | JX046944 | - |
| P. penetrans | JGM | France | Sambucus sp. | JX046942 | - |
| P. penetrans | CA192 | France, Britany | M. pumila | KY828242;KY828243 | - |
| P. penetrans | Pp18KL1 | Long Island, USA | S. tuberosum | FJ712987 | - |
| P. penetrans | Pp18KL2 | Long Island, USA | S. tuberosum | FJ712988 | - |
| P. penetrans | Pp18KL3 | Long Island, USA | S. tuberosum | FJ712989 | - |
| P. penetrans | Pp18KL4 | Long Island, USA | S. tuberosum | FJ712990 | - |
| P. penetrans | Pp18KL5 | Long Island, USA | S. tuberosum | FJ712991 | - |
| P. penetrans | F1 | MN, USA | S. tuberosum | KX842607 | - |
| P. penetrans | F2 | MN, USA | S. tuberosum | KX842608 | - |
| P. penetrans | F3 | MN, USA | S. tuberosum | KX842609 | - |
| P. penetrans | F4 | MN, USA | S. tuberosum | KX842610 | - |
| P. penetrans | F5 | MN, USA | S. tuberosum | KX842611 | - |
| P. penetrans | F6 | MN, USA | S. tuberosum | KX842612 | - |
| P. penetrans | F7 | MN, USA | S. tuberosum | KX842613 | - |
| P. penetrans | Pp17KL1 | Monroe County, USA | Prunus cerasus | FJ712982 | - |
| P. penetrans | Pp12KL1 | Rennes, France | Malus sp. | FJ712967 | - |
| P. penetrans | T143 | Rwanda, Nyakiriba | Allium. cepa | KY828249;KY828250 | KY817013 |
| P. penetrans | Pp14KL1 | Spain | Malus sp. | FJ712977 | - |
| P. penetrans | 9827 | The Netherlands | Iris sp. | JX046949 | - |
| P. penetrans | 5118 | The Netherlands | Lillium sp. | JX046950 | - |
| P. penetrans | T293 | The Netherlands, Apeldoorn | Pyrus sp. | KY828257 | KY816992 |
| P. penetrans | Pp1KL1 | Tongeren, Belgium | Rubus sp. | FJ712957 | - |
| P. penetrans | YIN | USA | Acer x freemanii | JX046947 | - |
| P. penetrans | Pp2KL1 | Zandhoven, Belgium | Z. mays | FJ712962 | - |
| P. pinguicaudatus | T572 | UK, England, Rothemstadt | Triticum sp. | KY828261;KY828262;KY828263 | KY816984 |
| P. thornei | N3786 | California, USA | V. vinifera | - | MK878270 |
| P. thornei | PthKL1 | Santaella, Spain | Cicer arietinum | FJ713002 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, D.; Cardoso, J.M.S.; Abrantes, I.; Esteves, I. Pratylenchus penetrans Parasitizing Potato Crops: Morphometric and Genetic Variability of Portuguese Isolates. Plants 2021, 10, 603. https://doi.org/10.3390/plants10030603
Gil D, Cardoso JMS, Abrantes I, Esteves I. Pratylenchus penetrans Parasitizing Potato Crops: Morphometric and Genetic Variability of Portuguese Isolates. Plants. 2021; 10(3):603. https://doi.org/10.3390/plants10030603
Chicago/Turabian StyleGil, Diogo, Joana M.S. Cardoso, Isabel Abrantes, and Ivânia Esteves. 2021. "Pratylenchus penetrans Parasitizing Potato Crops: Morphometric and Genetic Variability of Portuguese Isolates" Plants 10, no. 3: 603. https://doi.org/10.3390/plants10030603
APA StyleGil, D., Cardoso, J. M. S., Abrantes, I., & Esteves, I. (2021). Pratylenchus penetrans Parasitizing Potato Crops: Morphometric and Genetic Variability of Portuguese Isolates. Plants, 10(3), 603. https://doi.org/10.3390/plants10030603






