Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley
Abstract
1. Introduction
2. Results
2.1. Plant Growth, Root and Shoot Dry Weight
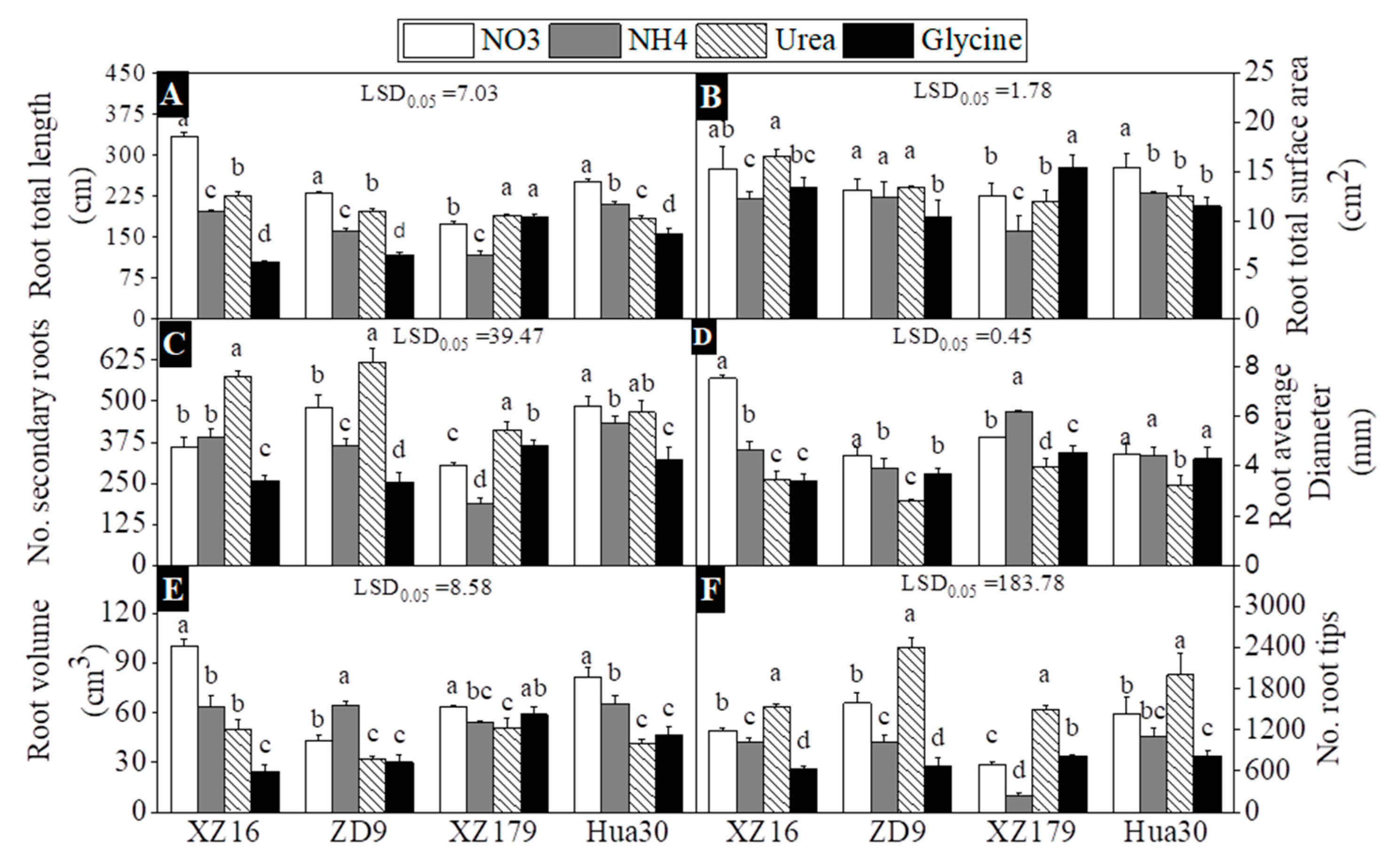
2.2. Root Architecture
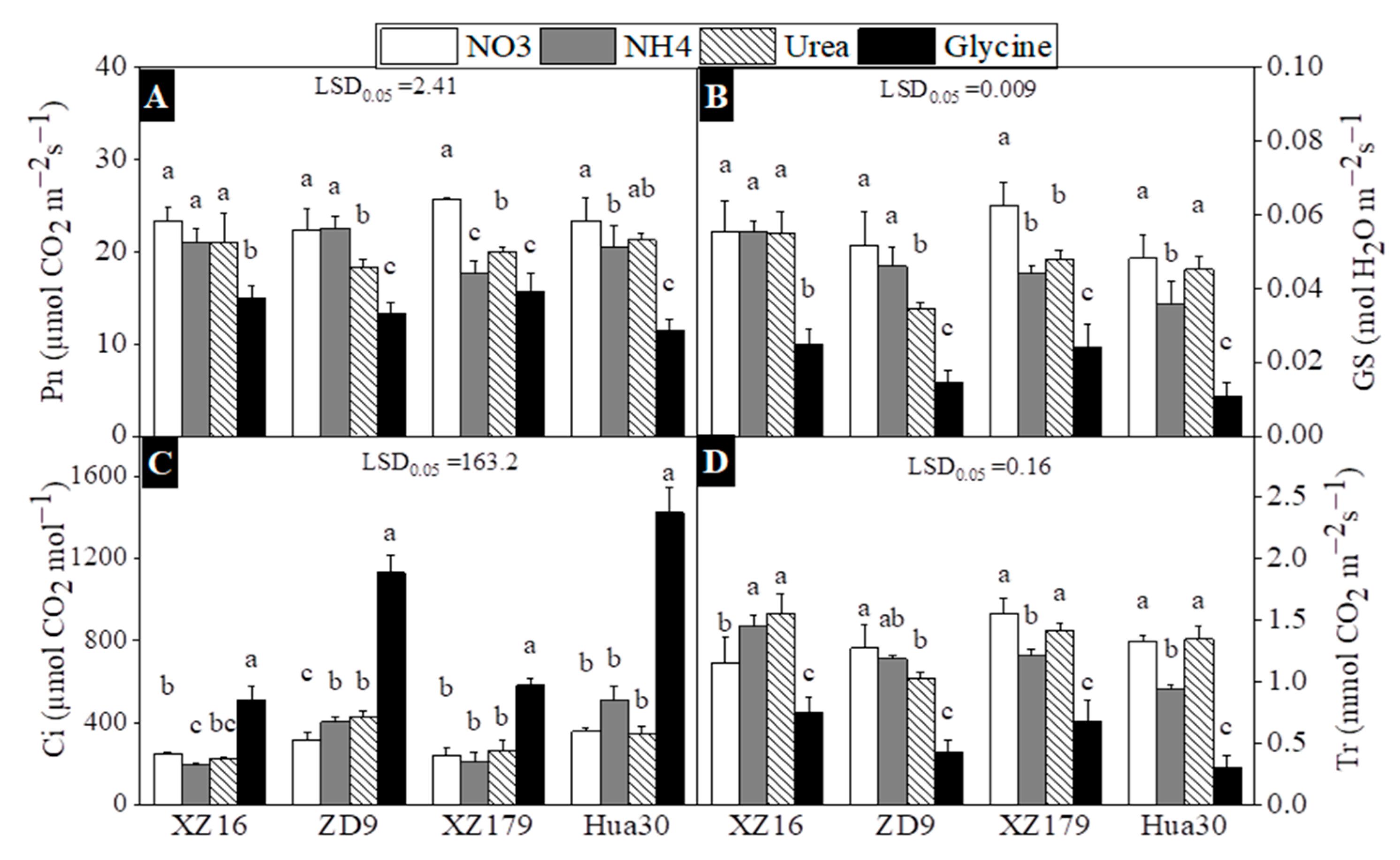
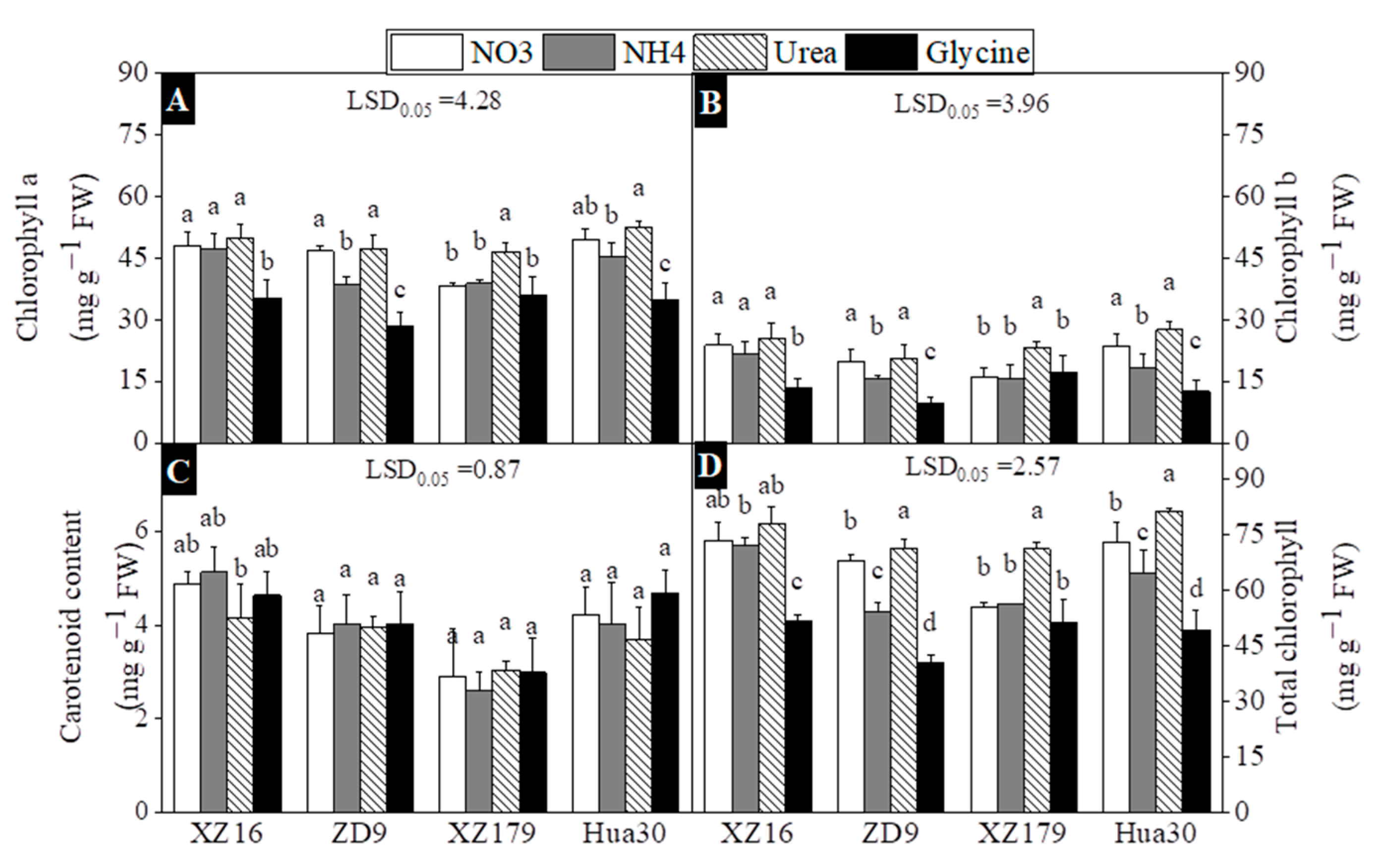
2.3. Photosynthetic Parameters, Chlorophyll and Carotenoid Content
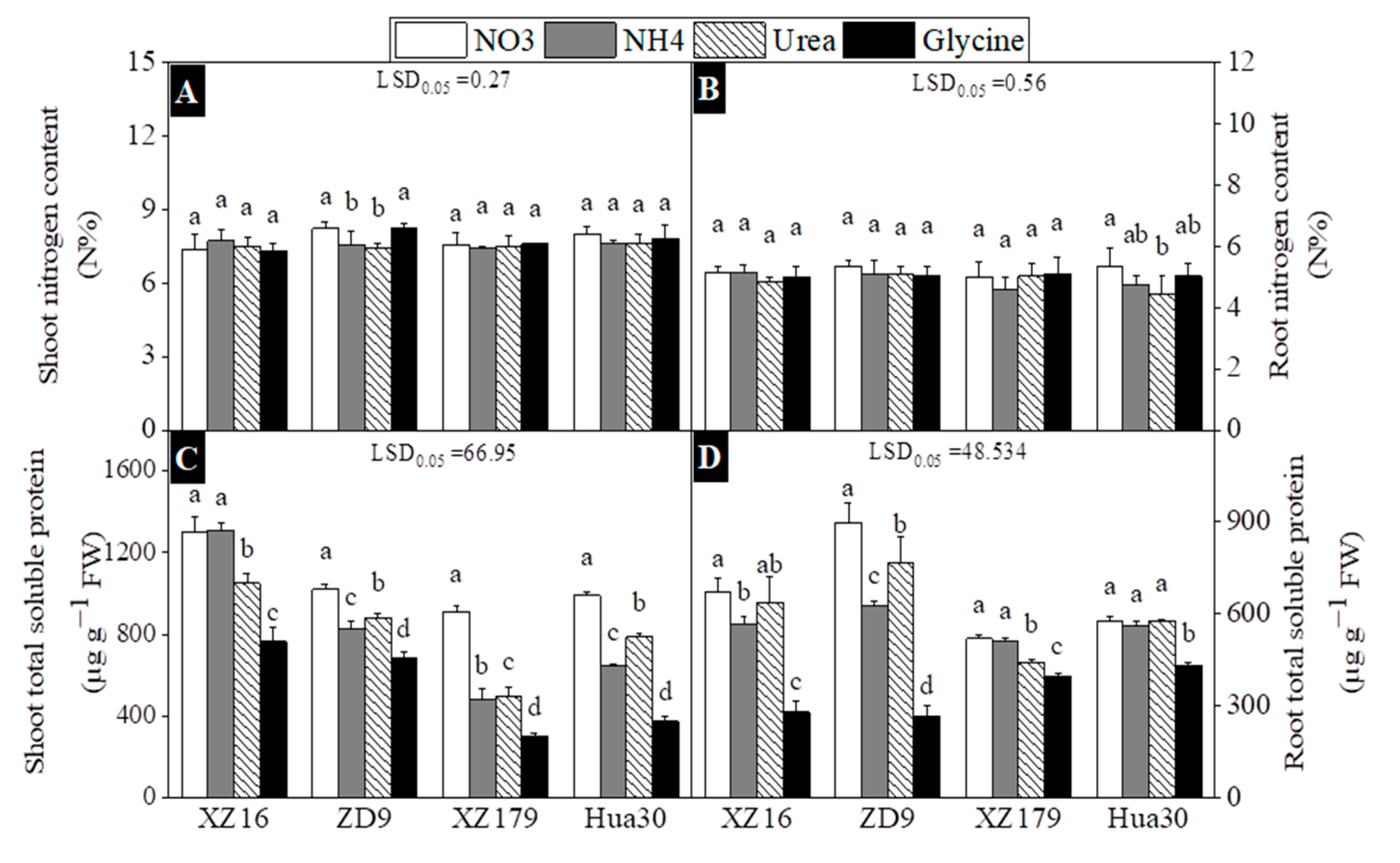
2.4. Tissue Nitrogen and Soluble Protein Concentrations
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Experimental Design
4.2. Measurement of Morphological Parameters
4.3. Gas Exchange Analysis
4.4. Chlorophyll and Carotenoids Content Determination
4.5. Analysis of Nitrogen Concentration and Total Soluble Protein
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, J.; Zhang, Q.; Li, L.; Kuang, T. Modification of photosystem II photochemistry in nitrogen deficient maize and wheat plants. J. Plant Physiol. 2001, 158, 1423–1430. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Pandey, R.K.; Maranville, J.W.; Admou, A. Deficit irrigation and nitrogen effects on maize in a Sahelian environment: I. Grain yield and yield components. Agric. Water Manag. 2000, 46, 1–13. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Fernández, I.; De Diego, N.; Sampedro, M.C.; Mena-Petite, A.; Ortiz-Barredo, A.; Lacuesta, M. High nitrate supply reduces growth in maize, from cell to whole plant. J. Plant Physiol. 2015, 173, 120–129. [Google Scholar] [CrossRef]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J.; Cui, Z.L.; Yin, B.; Christie, P.; Zhu, Z.L.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef]
- Hirel, B.; Le Gouis, J.; Ney, B.; Gallais, A. The challenge of improving nitrogen use efficiency in crop plants: Towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 2007, 58, 2369–2387. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I.; Gamage, H.K.; Carroll, B.J.; Schenk, P.M.; Schmidt, S. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef]
- Öhlund, J.; Näsholm, T. Growth of conifer seedlings on organic and inorganic nitrogen sources. Tree Physiol. 2001, 21, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Gerendhs, J.; Zhu, Z.; Bendixen, R.; Ratcliffe, R.G.; Sattelrnacher, B. physiological and biochemical processes related to ammonium toxicity in higher plants. Z. Pflanz. Bodenkd. 1997, 160, 239–251. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Bloom, A.J.; Caldwell, R.M.; Finazzo, J.; Warner, R.L.; Weissbart, J. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol. 1989, 91, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Kafkafi, U. Root temperature, concentration and the ratio NO3−/NH4+ effect on plant development. J. Plant Nutr. 1990, 13, 1291–1306. [Google Scholar] [CrossRef]
- Cramer, M.D.; Lewis, O.A.M. The influence of NO3− and NH4+ nutrition on the carbon and nitrogen partitioning characteristics of wheat (Triticum aestivum L.) and maize (Zea mays L.) plants. Plant Soil. 1993, 154, 289–300. [Google Scholar] [CrossRef]
- Li, B.; Xin, W.; Sun, S.; Shen, Q.; Xu, G. Physiological and molecular responses of nitrogen-starved rice plants to re-supply of different nitrogen sources. Plant Soil. 2006, 287, 145–159. [Google Scholar] [CrossRef]
- Roosta, H.R.; Schjoerring, J.K. Effects of ammonium toxicity on nitrogen metabolism and elemental profile of cucumber plants. J. Plant Nutr. 2007, 30, 1933–1951. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Y.L.; Wang, X.M.; Cui, J.X.; Xia, X.J.; Shi, K.; Yu, J.Q. Effects of nitrogen form on growth, CO2 assimilation, chlorophyll fluorescence, and photosynthetic electron allocation in cucumber and rice plants. J. Zhejiang Univ. Sci. B 2011, 12, 126–134. [Google Scholar] [CrossRef]
- Claussen, W.; Lenz, F. Effect of ammonium and nitrate on net photosynthesis, flower formation, growth and yield of eggplants (Solanum melongena L.). Plant Soil. 1995, 171, 267–274. [Google Scholar] [CrossRef]
- Setién, I.; Fuertes-mendizabal, T.; González, A.; Aparicio-tejo, P.M. High irradiance improves ammonium tolerance in wheat plants by increasing N assimilation. J. Plant Physiol. 2013, 170, 758–771. [Google Scholar] [CrossRef]
- Chen, G.L.; Gao, X.R.; Zhang, X.B. Effect of partial replacement of nitrate by amino acid and urea on nitrate content of non-heading chinese cabbage and lettuce in hydroponic condition. Agric. Sci. China 2002, 1, 444–449. [Google Scholar]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Wang, X.; Tang, D.; Huang, D. Proteomic analysis of pakchoi leaves and roots under glycine-nitrogen conditions. Plant Physiol. Biochem. 2014, 75, 96–104. [Google Scholar] [CrossRef]
- Rubio-Asensio, J.S.; Bloom, A.J. Inorganic nitrogen form: A major player in wheat and Arabidopsis responses to elevated CO2. J. Exp. Bot. 2017, 68, 2611–2625. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Wong, J.; Su, T.; Beatty, P.H.; Good, A.G. Identification of nitrogen use efficiency genes in barley: Searching for QTLs controlling complex physiological traits. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Cao, F.; Chen, X.; Zhang, M.; Ahmed, I.M.; Chen, Z.H.; Li, C.; Zhang, G.; Wu, F. Comparative proteomic analysis of aluminum tolerance in tibetan wild and cultivated barleys. PLoS ONE 2013, 8, e63428. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, H.; Zhu, B.; Jin, X.; Wu, F.; Zhang, G. Genotypic variations of N use efficiency in Tibetan wild and cultivated barleys. J. Zhejiang Univ. (Agric. Life Sci.) 2014, 40, 155–164. [Google Scholar]
- Quan, X.; Zeng, J.; Chen, G.; Zhang, G. Transcriptomic analysis reveals adaptive strategies to chronic low nitrogen in Tibetan wild barley. BMC Plant Biol. 2019, 19, 68. [Google Scholar] [CrossRef]
- Quan, X.; Zeng, J.; Han, Z.; Zhang, G. Ionomic and physiological responses to low nitrogen stress in Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2017, 111, 257–265. [Google Scholar] [CrossRef]
- Quan, X.; Zeng, J.; Ye, L.; Chen, G.; Shah, J.M.; Zhang, G. Transcriptome profiling analysis for two Tibetan wild barley genotypes in responses to low nitrogen. BMC Plant Biol. 2016, 16, 30. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Zhang, M.; Chen, X.; Zhang, G.; Wu, F. Difference in yield and physiological features in response to drought and salinity combined stress during anthesis in Tibetan Wild and Cultivated barleys. PLoS ONE 2013, 8, e77869. [Google Scholar] [CrossRef]
- Shah, J.M.; Asgher, Z.; Zeng, J.; Quan, X.; Ali, E.; Shamsi, I.H.; Zhang, G. Growth and physiological characterization of low nitrogen responses in Tibetan wild barley (Hordeum spontaneum) and cultivated barley (Hordeum vulgare). J. Plant Nutr. 2017, 40, 861–868. [Google Scholar] [CrossRef]
- Erol, N.O. Genetic analysis of nitrogen use efficiency in Arabidopsis thaliana. J. Exp. Bot. 2019, 68, 2477–2488. [Google Scholar]
- Brechner, M.; Both, A.J. Hydroponik Lettuce Handbook Cornell Controlled Environment Agriculture; Cornell University: New York, NY, USA, 2013; Available online: https://cpb-us-e1.wpmucdn.com/blogs.cornell.edu/dist/8/8824/files/2019/06/Cornell-CEA-Lettuce-Handbook-.pdf (accessed on 8 May 2020).
- Kronzucker, H.J.; Britto, D.T.; Davenport, R.J.; Tester, M. Ammonium toxicity and the real cost of transport. Trends Plant Sci. 2001, 6, 335–337. [Google Scholar] [CrossRef]
- Finkemeier, I.; Kluge, C.; Metwally, A.; Georgi, M.; Grotjohann, N.; Dietz, K.J. Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare. Plant Cell Environ. 2003, 26, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Bačkor, M.; Repčák, M. Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci. 2007, 172, 393–399. [Google Scholar] [CrossRef]
- Scheible, W.R.; Morcuende, R.; Czechowski, T.; Fritz, C.; Osuna, D.; Paacios-Rojas, N.; Schindelasch, D.; Thimm, O.; Udvardi, M.K.; Stitt, M. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of arabidopsis in response to nitrogen. Genome Anal. 2004, 136, 2483–2499. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Babula, P.; Jarošová, M. Variation of antioxidants and secondary metabolites in nitrogen-deficient barley plants. J. Plant Physiol. 2014, 171, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-May, Á.V.; Carrillo-Pech, M.; Barredo-Pool, F.A.; Martínez-Estévez, M.; Us-Camas, R.Y.; Moreno-Valenzuela, O.A.; Echevarría-Machado, A. A novel effect for glycine on root system growth of habanero pepper. J. Am. Soc. Hortic. Sci. 2013, 138, 433–442. [Google Scholar] [CrossRef]
- Balkos, K.D.; Britto, D.T.; Kronzucker, H.J. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant Cell Environ. 2010, 33, 23–34. [Google Scholar]
- Schortemeyer, M.; Stamp, P.; Feil, B. Ammonium tolerance and carbohydrate status in maize cultivars. Ann. Bot. 1997, 79, 25–30. [Google Scholar] [CrossRef]
- Mohammad, N.; Heidari, R. Effects of water stress on respiration, photosynthetic pigments and water content in two maize cultivars. Pak. J. Biol. Sci. 2007, 10, 4022–4028. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Jahangir, M.M.; Abbas, F.; Bharwana, S.A.; Zhang, G.P. Effect of chromium and nitrogen form on photosynthesis and anti-oxidative system in barley. Biol. Plant. 2013, 57, 758–763. [Google Scholar] [CrossRef]
- Mobini, M.; Khoshgoftarmanesh, A.H.; Ghasemi, S. The effect of partial replacement of nitrate with arginine, histidine, and a mixture of amino acids extracted from blood powder on yield and nitrate accumulation in onion bulb. Sci. Hortic. 2014, 176, 232–237. [Google Scholar] [CrossRef]
- Mishra, S.N.; Srivastava, H.S. Role of inorganic nitrogen in synthesis and degradation of protein in maize leaves. Indian J. Physiol. 1985, XXVIII, 43–52. [Google Scholar]
- Holger, B.; Shiwei, G. Influence of N form on growth photosynthesis of Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 2006, 169, 849–856. [Google Scholar]
- Guo, S.; Brück, H.; Sattelmacher, B. Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil. 2002, 239, 267–275. [Google Scholar] [CrossRef]
- Cechin, I.; Fumis, T. Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse. Plant Sci. 2004, 166, 1379–1385. [Google Scholar] [CrossRef]
- Ciompi, S.; Gentili, E.; Guidi, L.; Soldatini, G.F. The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci. 1996, 118, 177–184. [Google Scholar] [CrossRef]
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Karimzadeh, G.; Sharifi-Sirchi, G.; Jalali-Javaran, M.; Dehghani, H.; Francis, D. Soluble proteins induced by low temperature treatment in the leaves of spring and winter wheat cultivars. Pak. J. Bot. 2006, 38, 1015. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naz, S.; Shen, Q.; Lwalaba, J.L.W.; Zhang, G. Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley. Plants 2021, 10, 595. https://doi.org/10.3390/plants10030595
Naz S, Shen Q, Lwalaba JLW, Zhang G. Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley. Plants. 2021; 10(3):595. https://doi.org/10.3390/plants10030595
Chicago/Turabian StyleNaz, Shama, Qiufang Shen, Jonas Lwalaba Wa Lwalaba, and Guoping Zhang. 2021. "Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley" Plants 10, no. 3: 595. https://doi.org/10.3390/plants10030595
APA StyleNaz, S., Shen, Q., Lwalaba, J. L. W., & Zhang, G. (2021). Genotypic Difference in the Responses to Nitrogen Fertilizer Form in Tibetan Wild and Cultivated Barley. Plants, 10(3), 595. https://doi.org/10.3390/plants10030595







