Paphiopedilum insigne Morphological and Physiological Features during In Vitro Rooting and Ex Vitro Acclimatization Depending on the Types of Auxin and Substrate
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of NAA, IAA and IBA Auxins on In Vitro Rooting of Paphiopedilum insigne Plantlets
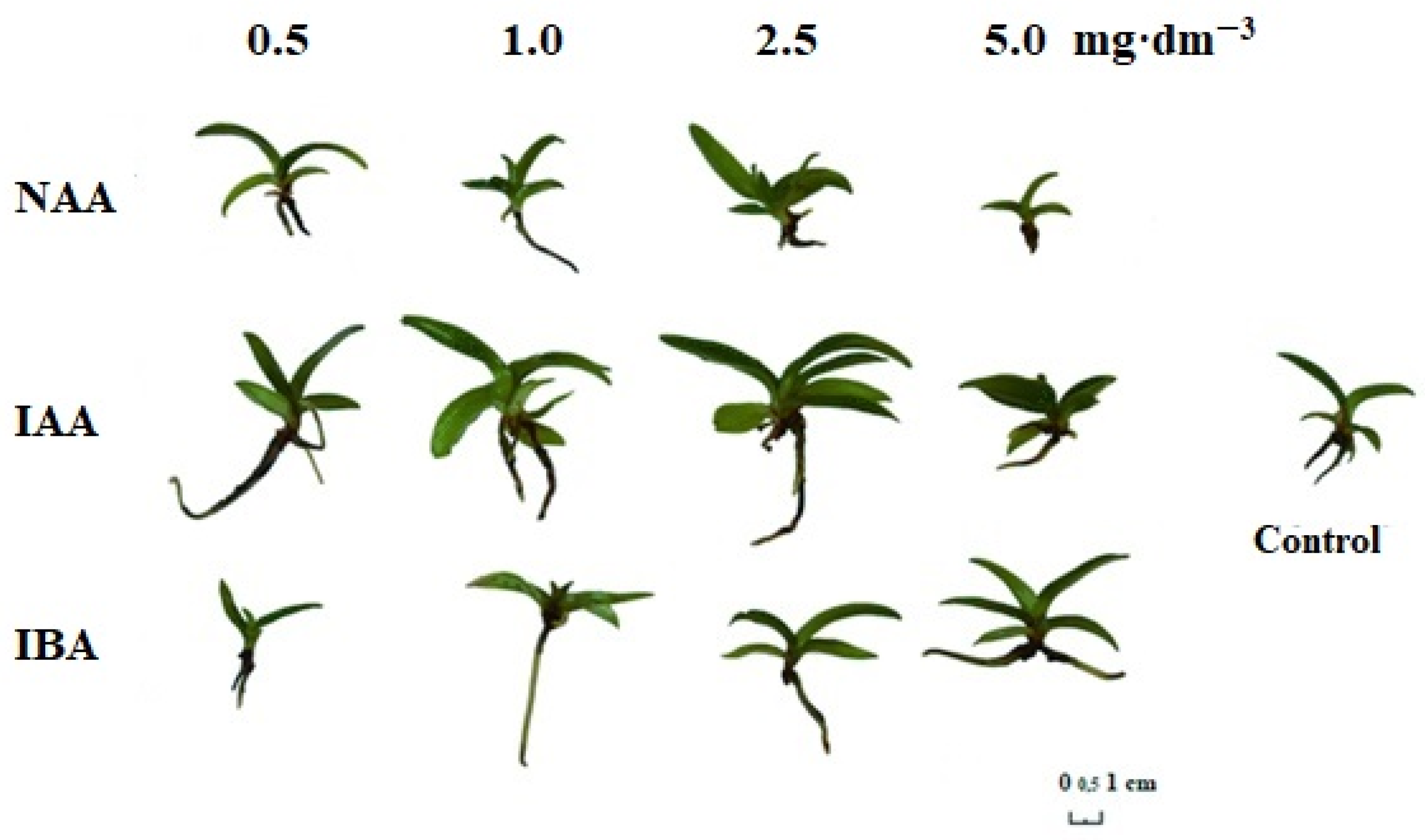
2.1.1. The Morphological Features of P. insigne Explants during In Vitro Rooting, Depending on the Presence of Auxins in the Media
2.1.2. The Paphiopedilum insigne Rooting System In Vitro Depending on the Presence of Auxins in the Media
2.2. The Subsequent Effect of IAA or IBA and a Type of Substrate on the Acclimatization of P. insigne Plantlets to Ex Vitro Conditions
2.2.1. The Survival Rate of Plantlets during the Acclimatization of P. insigne Depending on the Auxins used In Vitro and the Type of Substrate
2.2.2. The Morphological Features of Rosettes during the Acclimatization of P. insigne Depending on the Auxins Used In Vitro and the Type of Substrate
2.2.3. A Rooting of P. insigne Plantlets during Acclimatization Depending on the Auxins Used In Vitro and the Type of Substrate
2.3. The Physiological Response of P. insigne Plantlets during Acclimatization Depending on the Auxins Used In Vitro and the Type of Substrate
2.3.1. The Influence of IAA or IBA Used In Vitro and Substrate Type on Chlorophyll Fluorescence of P. insigne Plantlets during Acclimatization
2.3.2. The Influence of IAA or IBA Used In Vitro and Substrate Type on the Water Balance of Paphiopedilum insigne Plantlets during Acclimatization
2.3.3. The Influence of IAA or IBA Used In Vitro and Substrate Type on Stress Enzyme Activity in P. insigne Plantlets during Acclimatization
3. Material and Methods
3.1. The Influence of Auxins on the Paphiopedilum insigne Plantlets’ In Vitro Rooting
3.2. The Subsequent Effect of Auxins and a Type of Soil on the Acclimatization of Paphiopedilum insigne Plantlets to Ex Vitro Conditions
3.3. The Physiological Reaction of Plants to Stress during Acclimatization Depending on the In Vitro Pretreatment with Auxins and the Type of Substrate
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, Y.-J.; Tsai, Y.-C.; Sun, Y.-W.; Lin, R.-S.; Wu, F.-S. In vitro shoot induction and plant regeneration from flower buds in Paphiopedilum orchids. Vitr. Cell. Dev. Biol. Anim. 2011, 47, 702–709. [Google Scholar] [CrossRef][Green Version]
- Ng, C.-Y.; Saleh, N.M. In vitro propagation of Paphiopedilum orchid through formation of protocorm-like bodies. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 105, 193–202. [Google Scholar] [CrossRef]
- Dewir, Y.H.; El-Mahrouk, M.E.; Murthy, H.N.; Paek, K.Y. Micropropagation of Cattleya: Improved in vitro rooting and acclimatization. Hortic. Environ. Biotechnol. 2015, 56, 89–93. [Google Scholar] [CrossRef]
- Díaz, L.P.; Namur, J.J.; Bollati, S.A.; Arce, O.E.A. Acclimatization of Phalaenopsis and Cattleya obtained by micropropagation. Rev. Colomb. Biotecnol. 2010, 12, 27–40. [Google Scholar]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Novak, S.D.; Luna, L.J.; Gamage, R.N. Role of Auxin in Orchid Development. Plant. Signal. Behav. 2014, 9, e972277. [Google Scholar] [CrossRef]
- Paek, K.Y.; Yeung, E.C. The effects of 1-naphthaleneacetic acid and N6-benzyladenine on the growth of Cymbidium forrestii rhizomes in vitro. Plant. Cell Tissue Organ. Cult. (PCTOC) 1991, 24, 65–71. [Google Scholar] [CrossRef]
- Sana, A.; Touqeer, A.; Ishfaq, A.H.; Mehwish, Y.; Asghar, S.; Ahmad, T.; Hafiz, I.A.; Yaseen, M. In vitro propagation of orchid (Dendrobium nobile) var. Emma white. Afr. J. Biotechnol. 2011, 10, 3097–3103. [Google Scholar] [CrossRef]
- Khatun, H.; Khatun, M.M.; Biswas, M.S.; Kabir, M.R.; Al-Amin, M. In vitro growth and development of Dendrobium hybrid orchid. Bangladesh J. Agric. Res. 2010, 35, 507–514. [Google Scholar] [CrossRef][Green Version]
- Aktar, S.; Nasiruddin, K.; Huq, H. In Vitro Root Formation in Dendrobium Orchid Plantlets with IBA. J. Agric. Rural. Dev. 2007, 5, 48–51. [Google Scholar] [CrossRef]
- Pant, B. In vitro mass propagation of an epiphytic orchid, Dendrobium primulinum Lindl. through shoot tip culture. Afr. J. Biotechnol. 2012, 11, 9970–9974. [Google Scholar] [CrossRef]
- Kabir, M.F.; Rahman, M.S.; Jamal, A.; Rahman, M.; Khalekuzzaman, M. Multiple shoot regeneration in Dendrobium fim-briatum Hook an ornamental orchid. J. Anim. Plant Sci. 2013, 23, 1140–1145. [Google Scholar]
- Pradhan, S.; Paudel, Y.P.; Pant, B. Efficient regeneration of plants from shoot tip explants of Dendrobium densiflorum Lindl., a medicinal orchid. Afr. J. Biotechnol. 2013, 12, 1378–1383. [Google Scholar] [CrossRef]
- Mhopatra, A.; Rout, G.R. In vitro micropropagation of Geoderum purpureum P.BR. Indian J. Biotechnol. 2005, 4, 568–570. Available online: http://nopr.niscair.res.in/bitstream/123456789/5779/1/IJBT%204(4)%20568-570.pdf (accessed on 19 March 2021).
- Rahman, M.; Hasan, M.; Das, R.; Hossain, M.; Rahman, M. In Vitro Micropropagation of Orchid (Vanda tessellata L.) from Shoot Tip Explant. J. Bio Sci. 2011, 17, 139–144. [Google Scholar] [CrossRef]
- Chowdhury, I. Effects of Plant Growth Regulators on Callus Proliferation, Plantlet Regeneration and Growth of Plantlets of Doritaenopsis Orchid. Biotechnology 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Baker, A.; Behzad, K.; Ghorbanali, N.; Naser, N. Micropropagation of Orchis catasetum-a rare and endangered orchid. ACTA Sci. Pol. Hortorum Cultus 2014, 13, 197–205. [Google Scholar]
- Lo, S.-F.; Nalawade, S.M.; Kuo, C.-L.; Chen, C.-L.; Tsay, H.-S. Asymbiotic germination of immature seeds, plantlet development and ex vitro establishment of plants of Dendrobium tosaense makino—A medicinally improrant orchid. Vitr. Cell. Dev. Biol. Anim. 2004, 40, 528–535. [Google Scholar] [CrossRef]
- Michałojć, Z.M.; Nurzyński, J. Przewodnik do Ćwiczeń z Nawożenia Roślin Ogrodniczych; Wyd AR: Lublin, Poland, 2006. [Google Scholar]
- Zandoná, A.P.; de Faria, R.T.; Lone, A.B.; Hoshino, R.T. Alternative substrates to the sphagnum moss in the acclimatization of Arundina graminifolia “alba” (Orchidaceae). Ornam. Hortic. 2014, 20, 7–12. [Google Scholar] [CrossRef]
- Oszkinis, K. Storczyki (Orchids); PWRiL: Warszawa, Poland, 2004; pp. 132–135. [Google Scholar]
- Borowski, E.; Nurzyński, J. Effect of different growing substrates on the plant water relations and marketable fruit yield greenhouse-grown tomato (Lycopersicon esculentum Mill.). ACTA Agrobot. 2012, 65, 49–56. [Google Scholar] [CrossRef][Green Version]
- Trelka, T.; Breś, W.; Kozłowska, A. Phalaenopsis cultivation in different media. Part, I. Growth and flowering. ACTA Sci. Pol. Hortorum Cultus 2010, 9, 85–94. [Google Scholar]
- Hsu, B.-D. On the possibility of using a chlorophyll fluorescence parameter as an indirect indicator for the growth of Phalaenopsis seedlings. Plant. Sci. 2007, 172, 604–608. [Google Scholar] [CrossRef]
- Sailo, N.; Rai, D.; De, L.C. Physiology of temperate and tropical orchids-an overview. Int. J. Sci. Res. 2014, 3, 3–8. [Google Scholar]
- Rahmah, S.; Mubbarakh, S.A.; Ping, K.S.; Subramaniam, S. Effects of Droplet-Vitrification Cryopreservation Based on Physiological and Antioxidant Enzyme Activities of Brassidium Shooting Star Orchid. Sci. World J. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Stancato, G.C.; Mazzafera, P.; Buckeridge, M.S. Effect of a drought period on the mobilisation of non-structural carbohydrates, photosynthetic efficiency and water status in an epiphytic orchid. Plant. Physiol. Biochem. 2001, 39, 1009–1016. [Google Scholar] [CrossRef]
- Kishor, R.; Sharma, G.J. Intergeneric hybrid of two rare and endangered orchids, Renanthera imschootiana Rolfe and Vanda coerulea Griff. ex L. (Orchidaceae): Synthesis and characterization. Euphytica 2008, 165, 247–256. [Google Scholar] [CrossRef]
- Talukder, S.; Nasiruddin, K.; Yasmin, S.; Hassan, L.; Begum, R. Shoot Proliferation of Dendrobium Orchid with BAP and NAA. J. Biol. Sci. 2003, 3, 1058–1062. [Google Scholar] [CrossRef]
- Jitsopakul, N.; Thammasiri, K.; Ishikawa, K. Efficient adventitious shoot regeneration from shoot tip culture of Vanda coerulea, a Thai orchid. Science 2013, 39, 449. [Google Scholar] [CrossRef][Green Version]
- Das, M.C.; Kumaria, S.; Tandon, P. Protocorm Regeneration, Multiple Shoot Induction and ex vitro Establishment of Cymbidium devonianum Paxt. Asian J. Plant. Sci. 2007, 6, 349–353. [Google Scholar] [CrossRef][Green Version]
- Zahara, M.; Datta, A.; Boonkorkaew, P.; Mishra, A. The Effects of Different Media, Sucrose Concentrations and Natural Additives on Plantlet Growth of Phalaenopsis Hybrid ‘Pink’. Braz. Arch. Biol. Technol. 2017, 60. [Google Scholar] [CrossRef]
- Sunitibala, H.; Kishor, R. Micropropagation of Dendrobium transparens L. from axenic pseudobulb segments. Indian J. Biotechnol. 2009, 8, 448–452. [Google Scholar]
- Parthibhan, S.; Rao, M.V.; Kumar, T.S. In vitro regeneration from protocorms in Dendrobium aqueum Lindley-An imperiled orchid. J. Genet. Eng. Biotechnol. 2015, 13, 227–233. [Google Scholar] [CrossRef]
- Thomas, T.D.; Michael, A. High-frequency plantlet regeneration and multiple shoot induction from cultured immature seeds of Rhynchostylis retusa Blume., an exquisite orchid. Plant. Biotechnol. Rep. 2007, 1, 243–249. [Google Scholar] [CrossRef]
- Chyuam-Yih, N.G.; Saleh, N.M.; Zaman, F.Q. In vitro multiplication of the rare and endangered slipper orchid, Paphiopedilum rothschildianum (Orchidaceae). Afr. J. Biotechnol. 2010, 9, 2062–2068. [Google Scholar]
- Alves, S.M.R.; Smozinski, C.V. Evaluation of different substrates on the acclimatization of Epidendrum ibaguense kunth plantlets. Saber Científico 2015, 4, 39–45. [Google Scholar]
- Suradinata, Y.R.; Suminar, E.; Nuraini, A.; Hamdani, J.S.; Mubarok, S. Data on the vegetative growth at post acclimatization stage of two dendrobium genotypes as an effect of different growing media. Data Brief. 2019, 26, 104493. [Google Scholar] [CrossRef]
- Juras, M.C.R.; Jorge, J.; Pescador, R.; Ferreira, W.D.M.; Tamaki, V.; Suzuki, R.M. In vitro culture and acclimatization of Cattleya xanthina (Orchidaceae), an endangered orchid of the Brazilian Atlantic Rainforest. Rodriguésia 2019, 70. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, K.; Da Silva, J.A.T.; Zhang, J.; Chen, Z.; Xia, N.; Duan, J. Asymbiotic seed germination, seedling development and reintroduction of Paphiopedilum wardii Sumerh., an endangered terrestrial orchid. Sci. Hortic. 2012, 138, 198–209. [Google Scholar] [CrossRef]
- Deb, C.R.; Jakha, H.Y. Factors affecting asymbiotic immature seed culture and in vitro propagation of Paphiopedilum insigne (Wall. Ex. Lindl.) Pfitzer, a horticultural important vulnerable orchid. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 129–141. [Google Scholar]
- Diengdoh, R.V.; Kumaria, S.; Tandon, P.; Das, M.C. Asymbiotic germination and seed storage of Paphiopedilum insigne, an endangered lady’s slipper orchid. S. Afr. J. Bot. 2017, 112, 215–224. [Google Scholar] [CrossRef]
- Cetner, M.D.; Dąbrowski, P.; Samborska, I.A.; Łukasik, I.; Swoczyna, T.; Pietkiewicz, S.; Bąba, W.; Kalaji, H.M. Zastosowanie pomiarów fluorescencji chlorofilu w badaniach środowiskowych. Kosmos 2016, 2, 197–205. [Google Scholar]
- Kalaji, M.H.; Łoboda, T. Fluorescencja chlorofilu w badaniach stanu fizjologicznego roślin (The Chlorophyll Fluorescence in the Research of the Physiological State of Plants); SGGW: Warszawa, Poland, 2010; p. 116. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 2000, 19, 29–58. [Google Scholar]
- Matysiak, B. Wpływ natężenia światła na wzrost i fluorescencję chlorofilu mikrosadzonek różaneczników w czasie aklimatyzacji. Folia Hortic. Supl. 2003, 2, 59–61. [Google Scholar]
- Johnson, G.N.; Young, A.J.; Scholes, J.D.; Horton, P. The dissipation of excess excitation energy in British plant species. Plant. Cell Environ. 1993, 16, 673–679. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Ebrahimi, F.; Ahmadizadeh, M. Effect of different substrates on herbaceous pigments and chlorophyll amount of strawberry in hydroponic cultivation system American-Eurasian. J. Agric. Environ. Sci. 2012, 12, 154–158. [Google Scholar]
- De La Rosa-Manzano, E.; Andrade, J.L.; Zotz, G.; Reyes-García, C. Physiological plasticity of epiphytic orchids from two contrasting tropical dry forests. Acta Oecologica 2017, 85, 25–32. [Google Scholar] [CrossRef]
- Yen, W.-Y.; Chang, Y.-C.A.; Wang, Y.-T. The Acidification of Sphagnum Moss Substrate during Phalaenopsis Cultivation. HortScience 2011, 46, 1022–1026. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Faisal, M.; Anis, M. Changes in photosynthetic activity, pigment composition, electrolyte leakage, lipid peroxidation, and antioxidant enzymes during ex vitro establishment of micropropagated Rauvolfia tetraphylla plantlets. Plant. Cell Tissue Organ. Cult. (PCTOC) 2009, 99, 125–132. [Google Scholar] [CrossRef]
- Sudhakar, C.; Lakshmi, A.; Giridarakumar, S. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant. Sci. 2001, 161, 613–619. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Chai, Y.Y.; Jiang, C.D.; Shi, L.; Shi, T.S.; Gu, W.B. Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol. Plant. 2010, 54, 145–148. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant. Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Poniewozik, M.; Parzymies, M.; Szot, P. The influence of disinfection methods and liquid phase media on Paphiopedilum insigne seeds germination and media supplements on morphological features of protocorms in tissue culture. ACTA Sci. Pol. Hortorum Cultus 2020, 19, 7–14. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Barrs, H.D. Determination of water deficits in plant tissue. In Water Deficits and Plant Growth; Kozłowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; pp. 235–368. [Google Scholar]
- Stocker, O. Das Wasserdefizit von Gefässplanzen in verschiedenen klimazinen. Planta 1929, 7, 382–387. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Wiloch, U.; Mioduszewska, H.; Banaś, A. The influence of alloxydim on the antioxidant enzymatic activity in the roots maize (Zea mays L.). ACTA Physiol. Plant. Suppl. 1999, 21, 535–541. [Google Scholar]

| Treatment | Mn 1 Rate (pcs) | Width of Rosettes (mm) | Number of Leaves per Rosette | Length of Leaves (mm) | Width of Leaves (mm) | Weight of Leaf Rosettes (mg) | |
|---|---|---|---|---|---|---|---|
| Auxin (mg·dm−3) | |||||||
| Control | 0 | 1.3 a 2 | 55.8 a | 4.3 ab | 28.0 ab | 9.2 b | 496.4 a |
| NAA | 0.5 | 1.1 a | 44.3 ab | 3.4 b | 26.6 a–c | 13.0 a | 399.9 a |
| 1 | 1.5 a | 40.5 ab | 5.0 a | 23.7 bc | 8.1 b | 375.1 a | |
| 2.5 | 1.2 a | 42.4 ab | 4.5 a | 26.4 a–c | 8.9 b | 463.6 a | |
| 5 | 1.5 a | 34.5 b | 3.8 b | 22.0 c | 7.2 b | 439.0 a | |
| IAA | 0.5 | 1.2 a | 44.5 ab | 4.3 ab | 28.1 ab | 8.3 b | 451.1 a |
| 1 | 1.0 a | 45.3 ab | 3.9 ab | 28.1 ab | 8.4 b | 475.8 a | |
| 2.5 | 1.2 a | 45.4 ab | 4.6 ab | 29.8 a | 8.7 b | 553.8 a | |
| 5 | 1.3 a | 43.2 ab | 5.1 a | 27.5ab | 8.3 b | 480.0 a | |
| IBA | 0.5 | 1.2 a | 39.9 ab | 3.8 b | 24.4 a–c | 9.6 b | 407.1 a |
| 1 | 1.2 a | 44.2 ab | 4.1 ab | 27.9 ab | 9.3 b | 475.5 a | |
| 2.5 | 1.1 a | 44.2 ab | 3.6 b | 25.7 a–c | 9.2 b | 456.9 a | |
| 5 | 1.2 a | 37.9 ab | 4.3 ab | 25.7 a–c | 9.1 b | 420.7 a | |
| Treatment | Rooting Frequency (%) | Number of Roots/Explant | Length of Roots (mm) | Weight of Roots (mg) | |
|---|---|---|---|---|---|
| Auxin | Concentration (mg·dm−3) | ||||
| Control | 0 | 94 ab 1 | 2.9 ab | 20.8 ab | 179.8 a |
| NAA | 0.5 | 69 c | 2.6 ab | 21.5 ab | 214.3 a |
| 1 | 91 ab | 2.5 ab | 19.8 ab | 170.5 a | |
| 2.5 | 84 a–c | 2.9 ab | 18.3 ab | 208.1 a | |
| 5 | 82 a–c | 2.2 b | 9.3 c | 113.5 a | |
| IAA | 0.5 | 100 a | 2.6 ab | 17.9 ab | 147.7 a |
| 1 | 100 a | 2.3 ab | 16.3 a–c | 295.6 a | |
| 2.5 | 73 bc | 3.3 a | 21.0ab | 144.4 a | |
| 5 | 89 a–c | 2.9 ab | 23.7 a | 220.3 a | |
| IBA | 0.5 | 82 a–c | 2.6 ab | 23.1 a | 228.0 a |
| 1 | 100 a | 2.4 ab | 25.1 a | 208.6 a | |
| 2.5 | 100 a | 2.7 ab | 18.5 ab | 156.3 a | |
| 5 | 94 ab | 2.3 ab | 9.8 bc | 128.7 a | |
| Type of Substrate | Type of Auxin (1mg·dm−3) | Mean for Substrate | |
|---|---|---|---|
| IAA | IBA | ||
| sphagnum moss | 53.7 ab 1 | 47.3 b | 50.5 AB |
| sphagnum moss + substrate for orchids (1:1) | 56.8 ab | 33.3 c | 45.1 B |
| substrate for orchids | 62.0 a | 49.6 b | 55.8 A |
| substrate for orchids + acid peat (1:1) | 49.0 b | 53.7 ab | 51.4 A |
| Mean for auxins | 55.4 A | 46.0 B | |
| Auxin (1 mg·dm−3) | Type of Substrate | Number of Leaves | Mean for Substrate | Length of Leaves (mm) | Mean for Substrate | Width of Leaves (mm) | Mean for Substrate |
|---|---|---|---|---|---|---|---|
| IAA | sphagnum moss | 4.8 ab 1 | 4.3 A | 30.3 a | 30.8 A | 7.9 ab | 8.0 A |
| IBA | 3.7 cd | 31.3 a | 8.1 ab | ||||
| IAA | sphagnum moss + substrate for orchids (1:1) | 4.6 a–c | 4.3 A | 24.2 a | 26.1 B | 7.4 b | 8.1 A |
| IBA | 4.9 bc | 28.1 a | 8.9 a | ||||
| IAA | substrate for orchids | 3.0 d | 4.3 A | 28.2 a | 26. 8 AB | 7.9 ab | 8.2 A |
| IBA | 5.1 a | 25.3 a | 8.4 ab | ||||
| IAA | substrate for orchids + acid peat (1:1) | 4.3 a–c | 4.0 A | 28.8 a | 29.7 AB | 8.0 ab | 8.2 A |
| IBA | 4.2 a–c | 30.6 a | 8.3 ab | ||||
| Mean for auxins | IAA | 4.2 A | 27.9 A | 8.1 A | |||
| IBA | 4.0 A | 28.8 A | 7.8 A | ||||
| Auxin (1 mg·dm−3) | Type of Substrate | Number of Roots/Plant | Mean for Substrate | Length of Roots (mm) | Mean for Substrate |
|---|---|---|---|---|---|
| IAA | sphagnum moss | 2.9 bc 1 | 2.8 A | 25.2 a | 25.5 A |
| IBA | 2.7 bc | 25.7 a | |||
| IAA | sphagnum moss + substrate for orchids (1:1) | 1.4 d | 2.3 A | 24.8 a | 23.1 AB |
| IBA | 3.1 b | 22.0 a | |||
| IAA | substrate for orchids | 2.9 bc | 3.4 A | 17.0 a | 19.3 B |
| IBA | 3.9 a | 21.7 a | |||
| IAA | substrate for orchids + acid peat (1:1) | 2.7 bc | 2.6 A | 16.2 a | 19.3 B |
| IBA | 2.4 c | 22.4 a | |||
| Mean for auxins | IAA | 2.8 A | 20.6 A | ||
| IBA | 2.5 A | 23.0 A | |||
| Auxin (1 mg·dm−3) | Type of Substrate | Fo | Mean for Substrate | Fm | Mean for Substrate | Fv/Fm | Mean for Substrate |
|---|---|---|---|---|---|---|---|
| IAA | sphagnum moss | 243.75 a 1 | 252.88 A | 917.50 bc | 870.50 B | 0.735 a–c | 0.708 B |
| IBA | 262.00 a | 823.50 d | 0.681 c | ||||
| IAA | sphagnum moss + substrate for orchids (1:1) | 213.75 ab | 228.12 AB | 879.50 cd | 916.38 AB | 0.755 a–c | 0.750 AB |
| IBA | 242.50 a | 953.25 a–c | 0.743 a–c | ||||
| IAA | substrate for orchids | 246.50 a | 214.38 B | 893.25 cd | 937.63 A | 0.773 ab | 0.788 A |
| IBA | 182.25 b | 982.00 ab | 0.802 a | ||||
| IAA | substrate for orchids + acid peat (1:1) | 260.75 a | 236.00 AB | 911.75 bc | 958.88 A | 0.707 bc | 0.748 AB |
| IBA | 211.25 ab | 1006.00 a | 0.790 a | ||||
| Mean for auxins | IAA | 241.19 A | 900.50 B | 0.742 A | |||
| IBA | 224.50 A | 941.19 A | 0.754 A | ||||
| Auxin (1 mg·dm−3) | Type of Substrate | RWC (%) | Mean for Substrate | WSD (%) | Mean for Substrate |
|---|---|---|---|---|---|
| IAA | sphagnum moss | 83.6 bc 1 | 81.3 C | 16.4 ab | 18.7 A |
| IBA | 79.0 c | 21.0 a | |||
| IAA | sphagnum moss + substrate for orchids (1:1) | 86.3 b | 87.1 B | 13.7 b | 13.0 B |
| IBA | 87.8 ab | 12.3 bc | |||
| IAA | substrate for orchids | 88.1 ab | 90.2 A | 11.9 bc | 9.8 C |
| IBA | 92.4 a | 7.6 c | |||
| IAA | substrate for orchids + acid peat (1:1) | 86.3 b | 86.9 B | 12.4 bc | 13.1 B |
| IBA | 87.6 ab | 13.7 b | |||
| Mean for auxins | IAA | 86.1 A | 13.6 A | ||
| IBA | 86.7 A | 13.7 A | |||
| Auxin (1 mg·dm−3) | Type of Substrate | Catalase (U mg−1 FW) | Mean for Substrate | Ascorbate Peroxidase (U mg−1 FW) | Mean for Substrate |
|---|---|---|---|---|---|
| IAA | sphagnum moss | 0.480 cd 1 | 0.400 D | 26.087 a | 22.062 A |
| IBA | 0.320 d | 18.037 b | |||
| IAA | sphagnum moss + substrate for orchids (1:1) | 0.540 c | 0.753 C | 18.877 b | 17.13 B |
| IBA | 0.967 b | 15.150 d | |||
| IAA | substrate for orchids | 0.640 c | 0.970 B | 12.867 e | 15.210 C |
| IBA | 1.300 a | 17.553 bc | |||
| IAA | substrate for orchids + acid peat (1:1) | 1.287 a | 1.127 A | 15.547 b–d | 17.480 B |
| IBA | 0.967 b | 19.413 b | |||
| Mean for auxins | IAA | 0.737 B | 18.344 A | ||
| IBA | 0.888 A | 15.210 B | |||
| Substrate for Orchids | |
| COMPO SANA | Ready-to-use substrate for all species of orchids. Prepared on the basis of peat, contains all necessary nutrients and pine bark. Contents: peat (decomposition H3–H5), pine bark, calcium, NPK fertilizer, pH 5.0–6.5. |
| Sphagnum moss | Prepared from various species of sphagnum moss. It is the least decomposed peat with plants still visible. Decomposes slowly so that there is no risk of too high a level of nitrogen. It is very porous (82–85%) with high water-holding capacity. pH 3.0–4.5. |
| Acid peat | Fine decomposed, fine texture, lower pore space and air-filled poor space than sphagnum moss. A total pore space of around 80%. pH 3.5–4.5. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poniewozik, M.; Parzymies, M.; Szot, P.; Rubinowska, K. Paphiopedilum insigne Morphological and Physiological Features during In Vitro Rooting and Ex Vitro Acclimatization Depending on the Types of Auxin and Substrate. Plants 2021, 10, 582. https://doi.org/10.3390/plants10030582
Poniewozik M, Parzymies M, Szot P, Rubinowska K. Paphiopedilum insigne Morphological and Physiological Features during In Vitro Rooting and Ex Vitro Acclimatization Depending on the Types of Auxin and Substrate. Plants. 2021; 10(3):582. https://doi.org/10.3390/plants10030582
Chicago/Turabian StylePoniewozik, Monika, Marzena Parzymies, Paweł Szot, and Katarzyna Rubinowska. 2021. "Paphiopedilum insigne Morphological and Physiological Features during In Vitro Rooting and Ex Vitro Acclimatization Depending on the Types of Auxin and Substrate" Plants 10, no. 3: 582. https://doi.org/10.3390/plants10030582
APA StylePoniewozik, M., Parzymies, M., Szot, P., & Rubinowska, K. (2021). Paphiopedilum insigne Morphological and Physiological Features during In Vitro Rooting and Ex Vitro Acclimatization Depending on the Types of Auxin and Substrate. Plants, 10(3), 582. https://doi.org/10.3390/plants10030582








